Abstract
Minimal information is available on the incidence of Crimean–Congo hemorrhagic fever (CCHF) virus and hantavirus infections in Georgia. From 2008 to 2011, 537 patients with fever ≥ 38°C for ≥ 48 hours without a diagnosis were enrolled into a sentinel surveillance study to investigate the incidence of nine pathogens, including CCHF virus and hantavirus. Of 14 patients with a hemorrhagic fever syndrome, 3 patients tested positive for CCHF virus immunoglobulin M (IgM) antibodies. Two of the patients enrolled in the study had acute renal failure. These 2 of 537 enrolled patients were the only patients in the study positive for hantavirus IgM antibodies. These results suggest that CCHF virus and hantavirus are contributing causes of acute febrile syndromes of infectious origin in Georgia. These findings support introduction of critical diagnostic approaches and confirm the need for additional surveillance in Georgia.
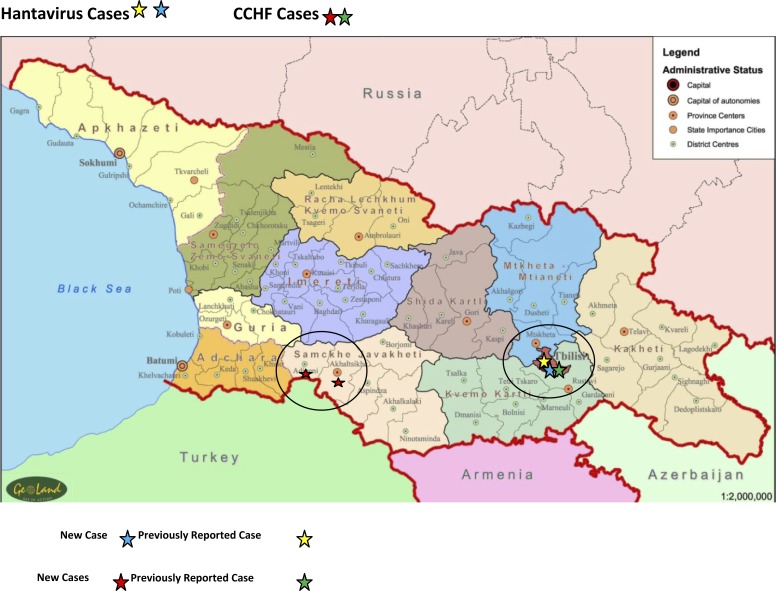
A variety of viruses can induce hemorrhagic manifestations during infection and are often categorized as viral hemorrhagic fever (VHF) viruses. Members of the family of Bunyaviridae are included in the VHF viruses and cover a wide geographic area.1 In this report, we describe cases of Crimean–Congo hemorrhagic fever (CCHF) and hemorrhagic fever with renal syndrome caused by hantaviruses detected through an Acute Febrile Illness (AFI) Surveillance Study carried out in the country of Georgia from 2008 to 2011 (Figure 1 and Table 1).
Figure 1.
Map of Georgia with the geographic distribution of CCHF and hantavirus cases.
Table 1.
Clinical symptoms and signs.
| Symptom | Hantavirus (N = 2) | CCHF (N = 3) |
|---|---|---|
| Fever | 2 (100%) | 3 (100%) |
| Shaking/rigors | 2 (100%) | 3 (100%) |
| Excessive sweating | 2 (100%) | 2 (67%) |
| Headache | 1 (50%) | 1 (33%) |
| Pallor | 2 (100%) | 3 (100%) |
| Shortness of breath | 2 (100) | 0 (0%) |
| Fatigue | 2 (100%) | 3 (100%) |
| Depressed mood | 2 (100%) | 2 (67%) |
| Difficulty sleeping | 1 (50%) | 1 (33%) |
| Pain in joints | 2 (100%) | 3 (100%) |
| Muscle soreness | 1 (50%) | 3 (100%) |
| Nausea/vomiting | 1 (50%) | 2 (67%) |
| Diarrhea | 1 (50%) | 1 (33%) |
| Rash | 0 (0%) | 2 (67%) |
| Unusual bleeding | 0 (0%) | 3 (100%) |
| Lymphadenopathy | 1 (50%) | 1 (33%) |
| Jaundice | 0 (0%) | 1 (33%) |
| Pharyngeal injection | 1 (50%) | 0 (0%) |
| Abdominal distention | 1 (50%) | 2 (67%) |
| Abdominal tenderness | 0 (0%) | 1 (33%) |
| Hepatomegaly | 1 (50%) | 3 (100%) |
| Spleenomegaly | 1 (50%) | 3 (100%) |
| Edema | 0 (0%) | 1 (33%) |
| Petechial rash | 0 (0%) | 2 (67%) |
| Bleeding | 0 (0%) | 3 (100%) |
CCHF virus is primarily transmitted to humans by ticks of the genus Hyalomma. Human-to-human transmission can occur by direct contact with the blood or tissue of viremic patients.2 For CCHF virus, case-fatality rates as high as 30% have been reported.3 For hantavirus cases, most human infections result from inhalation of aerosolized rodent excreta; rodent scratches and bites as well as contaminated food or water can also transmit the virus.4 In addition to fever, vascular leakage, and rapid development of shock typical for all the VHF viruses, including CCHF virus, Old World hantavirus infections may also cause significant impairment of renal function.1 The pathogenic hantaviruses detected in Europe and Russia include but are not restricted to Puumala (PUUV) and Dobrava–Belgrade (DOBV) viruses, which cause mild to moderate and moderate to severe clinical manifestations, respectively. Case-fatality rates of 0.2% and 12% have been reported for PUUV and DOBV infections, respectively, whereas the case-fatality rates for other hantaviruses have been reported to be as high as 50%.4–6 Of Georgia's neighboring countries, Russia has the highest number of reported hantavirus infection cases annually (10,000–12,000 cases per year). The district of Sochi located on the border with Georgia on the Black Sea coast is endemic for hantavirus.6 Turkey has reported an outbreak of hantavirus infection from the Black Sea region in 2009.7 Additionally, emergence of CCHF in southwest Russia and Turkey has been observed in the recent years.8
The first reports of CCHF and hantavirus cases from Georgia were published by our team in 2009.9,10 Herein, we report three additional CCHF cases and two hantavirus infection cases detected in patients participating in an AFI surveillance protocol. One of these hantavirus cases has been previously reported.9 Laboratory-based sentinel surveillance for AFI was conducted at six hospitals to establish the frequency of nine infectious causative agents of febrile illness in Georgia. Hospitalized patients ≥ 4 years of age with a temperature ≥ 38°C for ≥ 48 hours were asked to participate voluntarily. Epidemiologic information was collected through a questionnaire, and blood samples were obtained for laboratory determination of probable infectious agents, including common bacterial pathogens as well as Brucella, Salmonella typhi, and Leptospira. Serologic testing (enzyme-linked immunosorbent assay [ELISA]) was conducted for antibodies against Leptospira (PanBio, Brisbane, Australia), Brucella (US Naval Medical Research Unit 3 [NAMRU-3] Cairo, Egypt/ Naval Medical Research Center [NMRC] Silver Spring, MD, in-house ELISA11), West Nile virus (WNV; Focus Diagnostics, Cypress, CA), CCHF virus (Vector-Best, Novosibirsk, Russia), Coxiellaburnetii (PanBio), tick-borne encephalitis virus (TBEV; IBL International, Hamburg, Germany), hantavirus (Focus Diagnostics), S. typhi (NAMRU-3/NMRC in-house ELISA12), and Rickettsia typhi (Fuller Laboratories, Fullerton, CA), Leptospira ELISA results were confirmed by the microscopic agglutination test (MAT); C. burnetii and WNV results were confirmed by immunofluorescence assay (IFA; Focus Diagnostics), and hantavirus ELISA results were confirmed by immunoglobulin M (IgM) /IgG IFA (Euroimmun, Hamburg, Germany) and an immunoblotting assay (Mikrogen, Neuried, Germany).
Three of fourteen (21%) patients presenting with a hemorrhagic fever syndrome tested positive for CCHF virus. All three CCHF cases (two males and one female; mean age of 40 years) were from the southwest districts of Adigeni and Akhaltsikhe (bordered by Turkey) and occurred between May and July of 2009. One case reported an insect bite, two cases reported forest visits, and all cases reported exposure to cattle and engagement in agricultural work within the 1 month before the onset of illness. All CCHF cases presented with fever, rigors, arthralgia, myalgia, fatigue, unusual bleeding (epistaxis, hematemesis, bloody diarrhea, and/or gingival bleeding), pallor, and hepatosplenomegaly. Additionally, two of three CCHF cases presented with petechial rash and abdominal distention, and one case presented with abdominal tenderness. Laboratory results were available in two of three CCHF cases: decreased hematocrit, low white blood cell and platelet count, elevated liver enzymes, and high C-reactive protein level were observed. Initially, all CCHF cases were clinically diagnosed as fever of unknown origin (FUO) and started on antibiotic treatment. Two CCHF cases had improved on follow-up 2–6 weeks after discharge from the hospital. The third case was lost to follow-up.
Two patients presenting without a hemorrhagic fever syndrome but with acute renal failure tested positive for hantavirus. Two male patients from Tbilisi (mean age of 30 years) with acute renal failure and FUO as a preliminary hospital diagnosis were confirmed as hantavirus cases. Both cases had febrile illness with progressive deterioration of renal function without any hemorrhagic manifestation. Only one patient had known exposure to rodents before disease onset. Renal biopsy in one case revealed acute tubular necrosis with mild grade arteriolosclerosis.9
Clinical and epidemiological information on these confirmed CCHF and hantavirus cases in Georgia has direct and indirect public health implications.4,5,13 We observed improvement in two CCHF cases with standard supportive care treatment, which adds additional evidence of mild to moderate cases occurring in the region. A fourth case of CCHF occurred during this study but was not enrolled in the study, and information from this case is not included in this report. However, this patient fully recovered.10 The clinical presentation of the hantavirus-infected patients was also relatively mild: with renal failure and without apparent hemorrhage. Continuing education for laboratory and healthcare personnel in Georgia is a reasonable response to improve the detection and management of these infectious diseases in hospital settings. It is important to implement adequate medical and safety precautions during initial clinical evaluation, management of patients in intensive care units, and laboratory testing. It will also be important to develop appropriate public health preparedness strategies and improve response capacity to these zoonotic diseases. Additional comprehensive studies on the ecology of these zoonotic pathogens and characterization of circulating strains are needed to improve understanding of the risk factors for these infectious diseases in Georgia. In addition, targeted laboratory surveillance to screen and diagnose patients with compatible syndromes is needed to improve medical care of these patients. The cases of CCHF virus and hantavirus infection identified through this surveillance study highlight the important roles of laboratory-based surveillance systems in public health for disease monitoring and outbreak response.
ACKNOWLEDGMENTS
We acknowledge the contribution of all the participants in the clinical and laboratory network for Acute Febrile Illness Surveillance in Georgia.
Disclaimer: The opinions expressed by the authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention, the US Department of Defense, the Ministry of Health in Georgia, the German Ministry of Defense, or the institutions with which the authors are affiliated.
Footnotes
Financial support: This work was funded by the US Department of Defense Global Emerging Infections Surveillance (GEIS) Program and the Medical Biodefense Research Program of the Bundeswehr Joint Medical Service, and it was supported by the US Department of Defense Threat Reduction Agency.
Authors' addresses: Tinatin Kuchuloria, I. Javakhishvili Tbilisi State University, Tbilisi, Georgia, E-mail: drkuchuloria@yahoo.com. Paata Imnadze, I. Javakhishvili Tbilisi State University, Tbilisi, Georgia and National Center for Disease Control and Public Health, Tbilisi, Georgia, E-mail: pimnadze@ncdc.ge. Maiko Chokheli, National Center for Disease Control and Public Health, Tbilisi, Georgia, E-mail: chokhelimaiko@yahoo.com. Tengiz Tsertsvadze, I. Javakhishvili Tbilisi State University, Tbilisi, Georgia and Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia, E-mail: aids@gol.ge. Marina Endeladze and Ketevan Mshvidobadze, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia, E-mails: marinaendeladze@ymail.com and katemshvidobadze@yahoo.com. Danielle V. Clark and Christian T. Bautista, Walter Reed Army Institute of Research, Silver Spring, MD, E-mails: dvclark@gmail.com and Christian.Bautista@us.army.mil. Moustafa Abdel Fadeel, Guillermo Pimentel, and Brent House, Global Disease Detection and Response Program, US Naval Medical Research Unit No. 3, Cairo, Egypt, E-mails: Moustafa.AbdelFadeel.eg@med.navy.mil, gpiment@gmail.com, and Brent.House@med.navy.mil. Matthew J. Hepburn and Robert G. Rivard, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, E-mails: matthew.hepburn@yahoo.com and robert.g.rivard.mil@mail.mil. Silke Wölfel and Roman Wölfel, Bundeswehr Institute of Microbiology, Munich, Germany, E-mails: silkewoelfel@bundeswehr.org and romanwoelfel@bundeswehr.org.
References
- 1.Bray M. Hemorrhagic fever viruses. Encyclopedia of Microbiology. (3rd Ed.) 2009:339–353. [Google Scholar]
- 2.Maltezou HC, Papa A. Crimean-Congo hemorrhagic fever: risk for emergence of new endemic foci in Europe? Travel Med Infect Dis. 2010;8:139–143. doi: 10.1016/j.tmaid.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Aradaib IE, Erickson BR, Mustafa ME, Khristova ML, Saeed NS, Elageb RM, Nichol ST. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg Infect Dis. 2010;16:837–839. doi: 10.3201/eid1605.091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krüger DH, Ulrich R, Lundkvist A. Hantavirus infections and their prevention. Microbes Infect. 2001;3:1129–1144. doi: 10.1016/s1286-4579(01)01474-5. [DOI] [PubMed] [Google Scholar]
- 5.Papa A. Dobrava-Belgrade virus: phylogeny, epidemiology, disease. Antiviral Res. 2012;95:104–117. doi: 10.1016/j.antiviral.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Maltezou HC, Papa A. Crimean-Congo hemorrhagic fever: risk for emergence of new endemic foci in Europe? Travel Med Infect Dis. 2010;8:139–143. doi: 10.1016/j.tmaid.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Klempa B, Tkachenko EA, Dzagurova TK, Yunicheva YV, Morozov VG, Okulova NM, Slyusareva GP, Smirnov A, Kruger DH. Hemorrhagic fever with renal syndrome caused by 2 lineages of Dobrava hantavirus, Russia. Emerg Infect Dis. 2008;14:617–625. doi: 10.3201/eid1404.071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertek M, Buzgan T. Refik Saydam National Public Health Agency; Ministry of Health, Ankara, Turkey An outbreak caused by hantavirus in the Black Sea region of Turkey, January–May 2009. Euro Surveill. 2009;14:19214. doi: 10.2807/ese.14.20.19214-en. [DOI] [PubMed] [Google Scholar]
- 9.Kuchuloria T, Clark DV, Hepburn MJ, Tsertsvadze T, Pimentel G, Imnadze P. Hantavirus infection in the Republic of Georgia. 2009. Emerg Infect Dis. 2009;15:1489–1491. doi: 10.3201/eid1509.090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakhashivilli TN, Chikviladze T, Jghenti E, Bekaia M, Kuchuloria T, Hepburn MJ, Imnadze P, Nanuashvili A. Crimean-Congo hemorrhagic fever in man, Republic of Georgia, 2009. Emerg Infect Dis. 2010;16:1326–1328. doi: 10.3201/eid1608.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadeel MA, Wasfy MO, Pimentel G, Klena JD, Mahoney FJ, Hajjeh RA. Rapid enzyme-linked immunosorbent assay for the diagnosis of human brucellosis in surveillance and clinical settings in Egypt. Saudi Med J. 2006;27:975–981. [PubMed] [Google Scholar]
- 12.Fadeel MA, House BL, Wasfy MM, Klena JD, Habashy EE, Said MM, Maksoud MA, Rahman BA, Pimentel G. Evaluation of a newly developed ELISA against Widal, TUBEX-TF and Typhidot for typhoid fever surveillance. J Infect Dev Ctries. 2011;5:169–175. doi: 10.3855/jidc.1339. [DOI] [PubMed] [Google Scholar]
- 13.Vorou RM. Crimean-Congo hemorrhagic fever in southeastern Europe. Int J Infect Dis. 2009;13:659–662. doi: 10.1016/j.ijid.2009.03.028. [DOI] [PubMed] [Google Scholar]