Abstract
This study was designed to examine the height-for-age z-scores (HAZ), and the prevalence of intestinal inflammation, gastrointestinal infections with parasites, and enteroaggregative Escherichia coli (EAEC) in rural Panamanian children. Stool microscopy and polymerase chain reaction (PCR) testing for EAEC detected Giardia lamblia (32%, 32 of 100) and EAEC (13%, 11 of 87) in the study participants, respectively. Anthropometric analyses showed that those children who were > 12 months of age had lower HAZ scores (mean of −1.449) than the reference population. As a group, the children in the study 1 to 5 years of age did not show recovery from the previously mentioned decline in terms of their HAZ. The HAZ means of the children infected with G. lamblia, EAEC, and Ascaris lumbricoides were −1.49, −1.67, and −2.11, respectively. Furthermore, the study participants with A. lumbricoides and EAEC infections in the presence of lactoferrin showed another decrease of 0.19 and 0.13, respectively, in their HAZ means.
Introduction
Preschool child growth retardation is often an anthropometric indicator of the accumulated, long-term effects of repeated infections and food scarcity.1–3 Longitudinal studies in various parts of the world have convincingly shown that periods of growth faltering align with patterns of infection at least as much as changes in diet.1–3 In particular, enteric infections represent prominent causes of growth failure. Data from a prospective study of 45 Guatemalan highland village children, who were examined every week from birth to 3 years of age for intestinal infections, found weight loss and height arrest to be concomitant with infectious disease.4 The results from another investigation of 716 rural Guatemalan children ranging in age from 15 days to 7 years quantified an epidemiological association between diarrhea and reduced growth rates.5 Longitudinal investigations of 152 children from Keneba, The Gambia, who were seen once a month until 3 years of age, indicated that children suffering from gastroenteritis for a whole month would have had a weight gain of ∼750 gm/month less and an increase in height of slightly over 4 mm/month less than that of healthy children.2,3 Giardiasis, in particular, showed a significant negative effect on growth rate in these children.2,3 Furthermore, a surveillance study from 1989 to 1998 of 119 children in a Northeast Brazilian shantytown revealed that helminthiases at 0 to 2 years of age was significantly associated with linear growth faltering and a further 4.6 cm shortfall at age 7.6
In addition to possessing long-term effects on linear growth, enteric infections may also adversely affect cognitive development. A follow-up assessment of 143 Peruvian children, who had been previously examined longitudinally from birth to 2 years of age for anthropometric measurements, parasitic infections, and diarrheal illness, showed that the children with severe stunting by the second year of life scored 10 points lower on the Wechsler intelligence scale for children-revised (WISC-R) than children without severe stunting.7 Children with more than one episode of Giardia lamblia per year also scored 4.1 points lower than children with one episode or fewer per year.7 Another study of 46 children in a Northeast Brazilian shantytown showed that diarrhea in the first 2 years of life was associated with reduced cognitive function 4 to 7 years later as assessed by a battery of five cognitive tests.8
Of particular interest to this study are enteroaggregative Escherichia coli (EAEC) strains, which are increasingly recognized as a cause of watery diarrhea, occasionally with blood and mucus, in children and adults worldwide.9 The basic strategy of EAEC infection seems to comprise the adherence of self-aggregating bacteria, in association with a thick mucus layer, to the mucosal surface of human ileal and colonic tissues, followed by secretion of enterotoxins and cytotoxins, and induction of mucosal damage and inflammation.10,11 Several studies have suggested that persistent EAEC infection may produce an inflammatory enteritis in children that is associated with growth impairment and malnutrition.12,13 Fecal leukocytes are found in diarrhea patients with diffuse colonic inflammation but absent in non-inflammatory cases, and are most commonly identified in infectious diarrheas of invasive bacterial origin.14 Although it is not specific for a particular pathogen, the detection of elevated concentrations of fecal lactoferrin, an iron-based glycoprotein expressed by activated polymorphonuclear leukocytes, serves as a sensitive and specific diagnostic marker for the majority of inflammatory diarrheas.14–16
This investigation was undertaken to assess the prevalence of gastrointestinal infections with parasites and EAEC in children < 5 years of age in certain impoverished, rural communities within the Cañazas District of Veraguas Province, Panama. In addition, this study aimed to determine whether the children living in these villages experienced growth shortfalls in association with enteric infections and/or intestinal inflammation.
Materials and Methods
Study area and population.
On June 30, 2010, every household with young children in four rural communities, Alto y Bala, Calle Lourdes, Polo Sur, and San José, in Cañazas County (Cañazas District, Veraguas Province) was visited (Figure 1). All eligible members of each existing dwelling were invited to participate in the study, given a plastic container to collect a stool sample, and asked to deliver the stool specimen to the San Francisco Javier Hospital, a rural hospital in Cañazas District, by the next day. Information concerning age, sex, anthropometry, clinical symptoms, defecation habits, hygiene customs, and dwelling characteristics were collected with the use of a questionnaire administered by trained medical technologists and biologists. From the 174 children invited to join the study, 120 provided stool specimens. Of these, 100 were < 5 years of age and were selected to be the study population. Informed consent was obtained from all parents or legal guardians of minors, with the name of the appropriate institutional review board having approved the project. This study was approved by the National Review Board, Comité Nacional de Bioética de la Investigación, Instituto Conmemorativo Gorgas de Estudios de la Salud, Panamá City, Panamá (1985/CNBI/ICGES/).
Figure 1.
Map of the Cañazas District (projected region) showing the geographic location of the Cañazas County (darker zone).
Sample collection.
At the time of collection, 87 fresh samples contained enough stool to divide each sample into two portions. The first portion was frozen at −20°C without any additives in a small plastic container. The second portion was stored with a 5% formalin solution in preparation for the fecal parasite concentration processing. The remaining 13 samples were processed with a 5% formalin solution only.
Immunochromatography.
An immunochromatographic assay for the detection of Cryptosporidium spp. and G. lamblia was performed on all of the fresh samples using CERTEST CRYPTO-GIARDIA (Biotec S.L., Zaragoza, Spain), according to the manufacturer's instructions.
Fecal parasite concentration.
Within a week of collection, the 100 fresh samples were processed by means of a 5% formalin-ethyl acetate sedimentation procedure at the recommended centrifugation speed and time of 500 × g and 10 minutes, respectively.17
Kinyoun stain.
The dry slides were initially fixed and stained with the modified Kinyoun's acid-fast stain (cold method).17 Each slide was then left to air dry before being examined under the oil immersion objective lens at 100× magnification. This procedure stained the oocysts of coccidia pink to red to dark purple over a blue background.17
DNA extraction.
The DNA was extracted with QIAmp DNA Stool Minikit (Qiagen Inc., Germantown, MD) from 200 mg of each frozen stool sample, according to the manufacturer's instructions, with minor modifications as follows. Initially 650 μL of Buffer ASL were added to each sample, before vortexing and incubating it at 82.5°C for 5 minutes. After inhibitors had adsorbed to the InhibitEX matrix, 200 μL of nuclease-free water were added to each sample, and the samples were centrifuged for 2 minutes. The supernatant was pipetted into a new tube with 30 μL of proteinase K, to which 400 μL of Buffer AL were added. Each sample was vortexed and incubated according to the manufacturer's instructions. The 400 μL of ethanol (100%) were then added to each lysate. Subsequently, Qiagen's “Protocol: Isolation of DNA from Stool for Pathogen Detection” was followed to completion.
EAEC polymerase chain reaction (PCR) analysis.
One PCR method amplified aggR gene using the appropriate forward (5′-CTAATTGTACAATCGATGTA-3′) and reverse (5′-ATGAAGTAATTCTTGAAT-3′) primers.18 The other PCR assay targeted the aatA gene using the corresponding forward (5′-CTGGCGAAAGACTGTATCAT-3′) and reverse (5′-CAATGTATAGAAATCCGCTGTT-3′) primers.18 These two plasmid-borne loci are highly specific for EAEC and have been validated in several studies.18,19 Both PCR tests were performed in a final reaction volume of 25 μL. Each reaction tube was prepared by adding 4.3 μL of nuclease-free water, 1.6 μL (6.2 μM) of the forward primer, 1.6 μL (6.2 μM) of the reverse primer, 12.5 μL of Master Mix 2X (Promega M7502, Madison, WI), and 5 μL of DNA template. For aggR, the amplification was performed with an initial denaturation at 95°C for 5 minutes followed by 30 cycles of 95°C for 30 seconds, 43.6°C for 30 seconds, 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. The cycling conditions were the same for aatA with the exception of an annealing temperature of 53°C. An EAEC prototype strain 042 was included in each test as a positive control. Electrophoresis on 1.5% agarose gels and staining with 5 μL of ethidium bromide in 100 mL was used for visualization of amplified targets. A negative control, which consisted of the PCR mixture without the DNA template, was included in every run.
Fecal lactoferrin testing.
An enzyme-linked immunosorbent assay (ELISA) test for the qualitative detection of lactoferrin, a marker of fecal neutrophils and an indicator of intestinal inflammation, was performed on the previously frozen stool samples using IBD-CHECK (Techlab, Blacksburg, VA), according to the manufacturer's instructions. The samples with positive results for the original ELISA were then assayed using IBD-SCAN (Techlab), a quantitative ELISA for measuring concentrations of fecal lactoferrin, according to the manufacturer's instructions. The qualitative and quantitative assays included positive and negative controls as per the manufacturer's instructions. Because the presence of breast milk is considered to give false positive results for fecal lactoferrin tests,20,21 breastfeeding children who had positive lactoferrin tests were excluded from the data sets regarding presence or absence of intestinal inflammation.
Height-for-age z-scores (HAZ).
Algorithms provided by the World Health Organization (WHO) by an SPSS version (SPSS, Inc., Chicago, IL) were used to calculate the corresponding indicators of attained growth standards. These algorithms were based on the 2006 WHO international growth reference.
Statistical analysis.
The study participants' HAZ were grouped according to the presence or absence of fecal lactoferrin and enteric pathogens, and the detection of the three most common enteric pathogens, Giardia lamblia, Ascaris lumbricoides, and EAEC. To compare the HAZ means of the eight groups and test the null hypothesis that samples in these eight groups were drawn from populations with the same mean values, a one-way analysis of variance by GraphPad Prism (Charlottesville, VA), a two-dimensional graphing and statistics software published by GraphPad Software, Inc. (San Diego, CA), was used. A P value of less than or equal to 0.05 indicated statistical significance.
Results
From the 174 children surveyed and invited to join the study, 120 provided stool specimens. Out of these, 100 were < 5 years of age and were considered to be the study population. Nonetheless, only 87 specimens were frozen and used for detection of fecal lactoferrin and EAEC. Our study population included 52 female and 48 male children ranging from 4.4 to 59.4 months of age with a median age of 35.1 months. On questioning, 47% (47 of 100) of the participants' parents reported living in a house with an earth floor and 52% (52 of 100) indicated that they had no electricity in their homes. The survey revealed that 93% (93 of 100) of the participants had access to a latrine and only 10% (10 of 100) had a private toilet. The region's aqueduct with unchlorinated water served as the sole water source for 86% (86 of 100) of the participants. There was an average of six people per household.
On further questioning, 37% (37 of 100) of the parents reported having been aware of prior intestinal parasitic infections in their children. Out of these 37 children, 65% (24 of 37) had received medical treatment of their illness at the time. A health care provider, in the form of a nurse, a nutritionist, or a physician, had informed the parents of 42% (42 of 100) of the participants that these children were underweight. Overall, high numbers of gastrointestinal symptoms, such as diarrhea, abdominal pain, and vomiting, were noted among these children. According to the parents, 50% (50 of 100) of the participants had one or more of the previously mentioned symptoms: 39% (39 of 100) of the participants were suffering diarrhea, 39% (39 of 100) had abdominal pain, and 23% (23 of 100) experienced vomiting close to the time of the survey conduction. Intestinal pathogens were detected in 61% (61 of 100) of the participating children, G. lamblia in 32% (32 of 100), A. lumbricoides in 14% (14 of 100), EAEC in 12.6% (11 of 87), E. coli in 12% (12 of 100), Iodamoeba büstchlii in 8% (8 of 100), Entamoeba histolytica/Entamoeba dispar complex in 4% (4 of 100), and Ancylostoma duodenale/Necator americanus, Cryptosporidium spp., Cyclospora cayetanensis, Endolimax nana, and Hymenolepis nana in 1% (1 of 100) each.
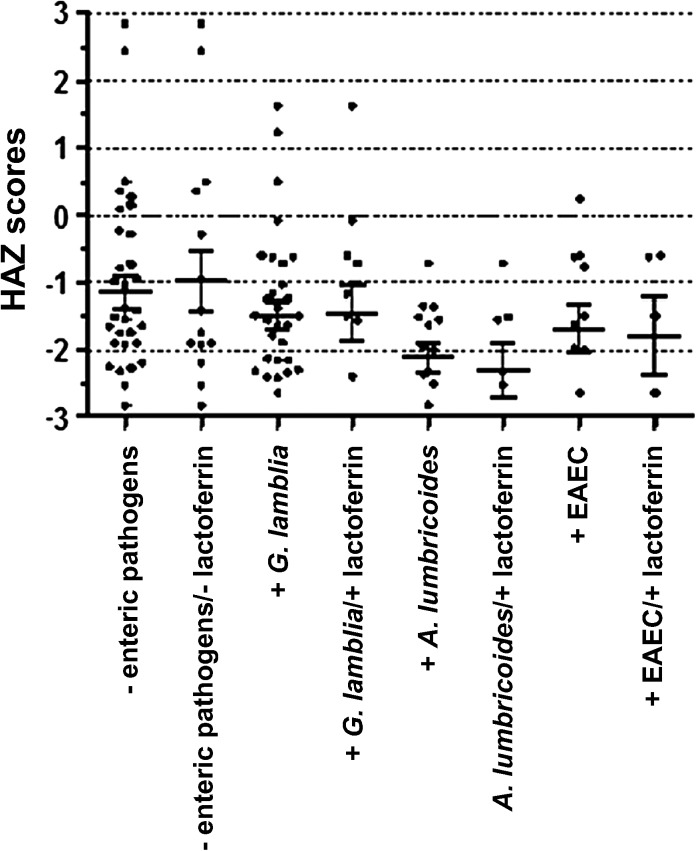
After analyzing the anthropometric measurements of the participants, their HAZ were grouped according to the presence or absence of fecal lactoferrin, as a marker of intestinal inflammation, and the most common enteric pathogens. The following information summarizes the number of children (in parentheses) in each of the eight resulted groups: children with no enteric pathogens (39); children with no enteric pathogens and no fecal lactoferrin (15); children with G. lamblia (32); children with G. lamblia and fecal lactoferrin (12); children with A. lumbricoides (14); children with A. lumbricoides and fecal lactoferrin (8); children with enteroaggregative E. coli (11). Study participants with no enteric pathogens and no fecal lactoferrin had the highest HAZ mean of −0.97, closely followed by those children who presented no enteric pathogens with a HAZ mean of −1.14 (Figure 2). The rest of the data sets had lower means of HAZ, −1.49, −1.67, and −2.11 for G. lamblia, EAEC, and A. lumbricoides infections, respectively. Except for the HAZ mean of children infected with G. lamblia, which showed an increase of 0.03 when the presence of fecal lactoferrin was taken into account, the study participants with A. lumbricoides and EAEC infections showed a decrease of 0.19 and 0.13, respectively, in their HAZ means when the presence of intestinal inflammation was included as a factor. Overall, the HAZ means of the children with any of the three most common enteric infections and of those with fecal lactoferrin, but no intestinal pathogens, were all more than one standard deviation below the international reference value. Of note, even the study participants with no enteric pathogens and no fecal lactoferrin possessed a HAZ mean ∼1 standard deviation below the mean of the reference population.
Figure 2.
Height-for-age z-scores (HAZ) of study participants according to presence or absence of enteric pathogens and fecal lactoferrin, as a marker of intestinal inflammation.
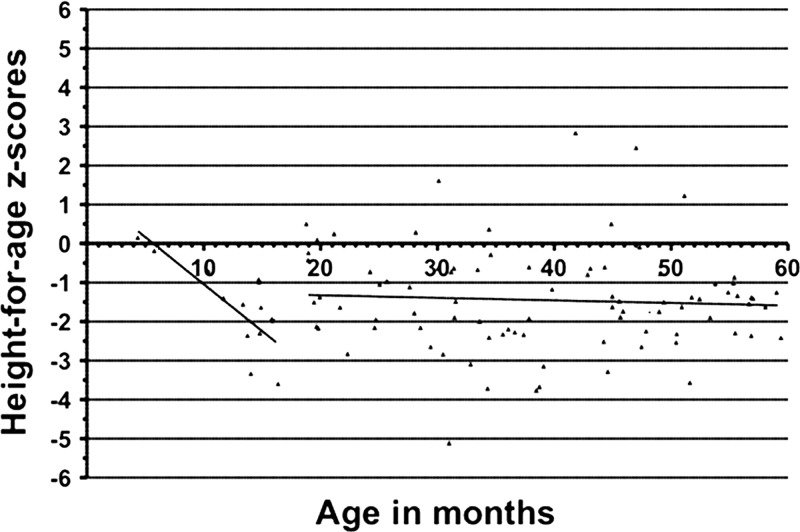
As shown in Figure 3, the HAZ of the study participants > 12 months of age experienced a decline (HAZ mean of −1.449) from the reference population. As a group, the children in the study 1 to 5 years of age did not show recovery from the previously mentioned decline in terms of their HAZ, suggesting that growth impairment may occur in early childhood and may persist over the first 5 years of life.
Figure 3.
Height-for-age z-scores (HAZ) of all study participants according to age in months. Out of 100 subjects, two were excluded from this analysis because anthropometric data were not collected from them.
Discussion
According to the Panamanian Ministry of Health, 76,417 new cases (21,779.5 of 100,000 children) of diarrheal illness occurred in children < 5 years of age in the Republic of Panama in 2007, and 76,032 new cases (22,009 of 100,000 children) ensued in the same population of young children in 2008.22 Over a 16-month period in 2008 and 2009, a study on diarrhea, intestinal protozoan infection, and stunting in 373 preschool children of the comarca Ngäbe-Buglé, an impoverished, rural indigenous area within the Besiko District in western Panama, identified chronic protozoan infections (primarily G. lamblia) as a risk factor for low height-for-age.23 Thus, it highlighted the link between chronic intestinal infection and stunting in a region in which 91% of the inhabitants live in extreme poverty.23 Our investigation assessed the prevalence of gastrointestinal infections with parasites and EAEC in children < 5 years of age in four rural communities within Cañazas County of Veraguas Province in central Panama. Given that the incidence of poverty in Panama's rural non-indigenous areas comprises ∼50.7%, in contrast to 17.7% in urban areas, and Veraguas possesses a poverty level of 52%, the study participants were also among the poorest inhabitants of Panama.24 Overall, 61% (61 of 100) of the children in the study presented with at least one enteric pathogen, mainly G. lamblia (32 of 100), A. lumbricoides (14 of 100), and EAEC (11 of 87), at the time of stool collection. Similarly, the investigation in the comarca Ngäbe-Buglé detected Giardia spp. in 18–34% of the collected fecal samples.23 Our survey by direct observation and formally obtained data from the participants' parents indicated that the environmental hygienic conditions were poor in the study population. Houses with unchlorinated water supply and deficiencies in physical infrastructure, such as earth floor and inappropriate traditional latrines, were commonly registered. This information, which is suggestive of a widespread lack of clean water and sanitation in these communities, may explain the high prevalence of enteric infections in the study participants.
Furthermore, this research study intended to determine whether these young children presented arrested linear growth in association with enteric infections and/or intestinal inflammation. As mentioned previously, study participants with no enteric pathogens and no fecal lactoferrin, a marker of intestinal inflammation, had the highest HAZ mean of −0.97, closely followed by those children who presented no enteric pathogens with a HAZ mean of −1.14. The means of the HAZ of the children infected with G. lamblia, EAEC, and A. lumbricoides were −1.49, −1.67, and −2.11, respectively. It is important to mention that, to our knowledge, this investigation was the first report of EAEC detection in Panama, confirming the presence of this pathogen in this region of the country. In addition, the presence of intestinal inflammation also appeared to play a role in decreasing these young subjects' linear growth. The study participants with A. lumbricoides and EAEC infections in the presence of fecal lactoferrin showed another decrease of 0.19 and 0.13, respectively, in their HAZ means. The groups of children with the most frequently detected gastrointestinal pathogens and fecal lactoferrin tended to have lower means of HAZ than those study participants without enteric infections and without intestinal inflammation. Of note, even the study participants with no enteric pathogens and no fecal lactoferrin possessed a HAZ mean ∼1 standard deviation below the mean of the reference population. This observation could be related to the fact that the study population lived in remote rural communities with high rates of poverty, which probably contributed to exposure to water and/or food contaminated with gastrointestinal pathogens and poor access to good diets and proper healthcare.
Because moderate stunting or chronic malnutrition is defined as an HAZ between 2 and 3 standard deviations below the international reference mean value, the previous findings could be indicative of a mild degree of stunting or chronic malnutrition within the study population. Given the high rate of gastrointestinal infections in these children, it is probable that many of the participants, regardless of whether they presented enteric pathogens at the time of the study, had been infected in the past. Overall, these infections may have contributed to the low HAZ means reported. A cross-sectional analysis of the participants' anthropometric measurements by age revealed a decline of more than 1 standard deviation below the international reference mean and might denote mild stunting or chronic malnutrition in these children. These growth shortfalls may have been caused by a combination of factors, including an insufficient diet, the weaning process, and the introduction of water and/or food contaminated with gastrointestinal pathogens. It is important to note that the nutritional stage during this early period of life has been considered crucial for the linear growth.25 A closely related situation has been described in Tanzania.26 To improve the healthy growth of children, these authors recommend the promotion of exclusive breastfeeding during the first months of life and appropriate feeding and hygiene practices.26
Regarding the investigation's limitations, the study population may have been too small to provide accurate representation of each subset of children according to a particular gastrointestinal pathogen and the presence or absence of fecal lactoferrin. Another limitation comprised the use of only a single stool sample for detecting enteric pathogens, which was probably less accurate than repeated stool examinations. Furthermore, the study's enteric panel did not include viral or bacterial targets, which have been isolated frequently from children in other low-income settings associated with diarrhea or stunting.27 In addition, as a cross-sectional study, this investigation did not provide a longitudinal assessment to track the children's linear growth and detect temporal patterns of enteric infections with a wider spectrum of bacteria and parasites. To truly describe growth faltering in relation to pathogens and lactoferrin measurements, a longitudinal study design would be necessary. Future studies in this area could test for other biomarkers of intestinal dysfunction, such as fecal alpha-1 antitrypsin or lactulose absorption, and its consequences, which include stunted growth in children.28 Anemia may also be used as an indicator of stunting.29,30 Nevertheless, it is urgent to call on health authorities to reinforce programs for the diagnosis, management, and prevention of child malnutrition and intestinal infections in this region.
ACKNOWLEDGMENTS
We are grateful to Vivian E. Thomson for her logistical assistance.
Footnotes
Financial support: This work received financial support from the Secretaria Nacional de Ciencia, Tecnologia e Innovación (SENACYT, Panamá) grant No. FID08-048, the Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES, Panama), and the University of Virginia's Panama Initiative Student Scholar Program and the Center for Global Health-University Scholar Award Program.
Disclosure: Azael Saldaña and Jose E. Calzada are members of Sistema Nacional de Investigación (SNI), SENACYT-Panama.
Authors' addresses: Elena Jiménez Gutiérrez, Richard L. Guerrant, Jones B. Lima Neto, and Relana C. Pinkerton, Center for Global Health, Division of Infectious Diseases and International Health, University of Virginia, Charlottesville, VA, E-mails: ej5de@virginia.edu, RLG9A@hscmail.mcc.virginia.edu, Jb5vk@virginia.edu, and rlg9a@virginia.edu. Vanessa Pineda, Jose E. Calzada, and Azael Saldaña, Departamento de Parasitología, Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES), Panama City, Panama, E-mails: vpineda@gorgas.gob.pa, jcalzada@gorgas.gob.pa, and asaldana@gorgas.gob.pa.
Reprint requests: Azael Saldaña, Departamento de Parasitología, Instituto Conmemorativo Gorgas de Estudios de la Salud, Apartado Postal No.0816-02593, Panama City, Republic of Panama, Tel: 507-227-4111, Fax: 507-225-4366, E-mail: asaldana@gorgas.gob.pa.
References
- 1.Koopman JS, Fajardo L, Bertrand W. Food, sanitation, and the socioeconomic determinants of child growth in Colombia. Am J Public Health. 1981;71:31–37. doi: 10.2105/ajph.71.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland MG, Cole TJ, Whitehead RG. A quantitative study into the role of infection in determining nutritional status in Gambian village children. Br J Nutr. 1977;37:441–450. doi: 10.1079/bjn19770047. [DOI] [PubMed] [Google Scholar]
- 3.Cole TJ, Parkin JM. Infection and its effect on the growth of young children: a comparison of The Gambia and Uganda. Trans R Soc Trop Med Hyg. 1977;71:196–198. doi: 10.1016/0035-9203(77)90005-0. [DOI] [PubMed] [Google Scholar]
- 4.Mata LJ, Kromal RA, Urrutia JJ, Garcia B. Effect of infection on food intake and the nutritional state: perspectives as viewed from the village. Am J Clin Nutr. 1977;30:1215–1227. doi: 10.1093/ajcn/30.8.1215. [DOI] [PubMed] [Google Scholar]
- 5.Martorell R, Habicht JP, Yarbrough C, Lechtig A, Klein RE, Western KA. Acute morbidity and physical growth in rural Guatemalan children. Am J Dis Child. 1975;129:1296–1301. doi: 10.1001/archpedi.1975.02120480022007. [DOI] [PubMed] [Google Scholar]
- 6.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 7.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrheal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 8.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 9.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 10.Nataro JP, Steiner T, Guerrant RL. Enteroaggregative Escherichia coli. Emerg Infect Dis. 1998;4:251–261. doi: 10.3201/eid0402.980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–18. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 12.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis. 2010;202:506–514. doi: 10.1086/654894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 14.Gadewar S, Fasano A. Current concepts in the evaluation, diagnosis and management of acute infectious diarrhea. Curr Opin Pharmacol. 2005;5:559–565. doi: 10.1016/j.coph.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 16.Huicho L, Campos M, Rivera J, Guerrant RL. Fecal screening tests in the approach to acute infectious diarrhea: a scientific overview. Pediatr Infect Dis J. 1996;15:486–494. doi: 10.1097/00006454-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Garcia LS. Diagnostic Medical Parasitology. Fifth edition. Washington, DC: ASM Press; 2007. [Google Scholar]
- 18.Monteiro BT, Campos LC, Sircili MP, Franzolin MR, Bevilacqua LF, Nataro JP, Elias WP. The dispersin-encoding gene (aap) is not restricted to enteroaggregative Escherichia coli. Diagnost Micro Infect Dis. 2009;65:81–84. doi: 10.1016/j.diagmicrobio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins C, Chart H, Willshaw GA, Cheasty T, Smith HR. Genotyping of enteroaggregative Escherichia coli and identification of target genes for the detection of both typical and atypical strains. Diagn Microbiol Infect Dis. 2006;55:13–19. doi: 10.1016/j.diagmicrobio.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman S, Ramakrishna BS, Kang G, Rajan DP, Mathan VI. Fecal lactoferrin as a predictor of positive fecal culture in south Indian children with acute diarrhea. Ann Trop Paediatr. 2003;23:9–13. doi: 10.1179/000349803125002805. [DOI] [PubMed] [Google Scholar]
- 21.Huicho L, Garaycochea V, Uchima N, Zerpa R, Guerrant RL. Fecal lactoferrin, fecal leukocytes and occult blood in the diagnostic approach to childhood invasive diarrhea. Pediatr Infect Dis J. 1997;16:644–647. doi: 10.1097/00006454-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Benítez L, Tejada I, Ruiloba AM. Boletín Estadístico de Salud 2008. República de Panamá; Ministerio de Salud: 2009. p. 58. [Google Scholar]
- 23.Halpenny CM, Koski KG, Valdés VE, Scott ME. Prediction of child health by household density and asset-based indices in impoverished indigenous villages in rural Panamá. Am J Trop Med Hyg. 2012;86:280–291. doi: 10.4269/ajtmh.2012.11-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estado nutricional de niños y niñas menores de cinco años. Republica de Panamá. Encuesta de Niveles de Vida . Contraloría General de la República. Instituto Nacional de Estadística y Censo; 2008. p. 2. [Google Scholar]
- 25.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 26.Muhimbula HS, Issa-Zacharia A. Persistent child malnutrition in Tanzania: risks associated with traditional complementary foods. Afr J Food Sci. 2010;4:679–692. [Google Scholar]
- 27.Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, Mølbak K, Rasmussen ZA, Sack RB, Valentiner-Branth P, Checkley W. Childhood malnutrition and infection network. Diarrhea in early childhood: short-term association with weight and long-term association with length. Am J Epidemiol. 2013;178:1129–1138. doi: 10.1093/aje/kwt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhagen LM, Incani RN, Franco CR, Ugarte A, Cadenas Y, Sierra Ruiz CI, Hermans PW, Hoek D, Campos Ponce M, de Waard JH, Pinelli E. High malnutrition rate in Venezuelan Yanomami compared to Warao Ameridians and Creoles: significant associations with intestinal parasites and anemia. PLoS ONE. 2013;8:e77581. doi: 10.1371/journal.pone.0077581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoya MA, Ngnie-Teta I, Seraphin MN, Mamadoultaibou A, Boldon E, Saint-Fleur JE, Koo L, Bernard S. Prevalence and risk factors of anemia among children 6–59 months old in Haiti. Anemia. 2013 doi: 10.1155/2013/502968. [DOI] [PMC free article] [PubMed] [Google Scholar]