Abstract
Currently, the body louse is the only recognized vector of Bartonella quintana, an organism that causes trench fever. In this work, we investigated the prevalence of this bacterium in human lice in different African countries. We tested 616 head lice and 424 body lice from nine African countries using real-time polymerase chain reaction targeting intergenic spacer region 2 and specific B. quintana genes. Overall, B. quintana DNA was found in 54% and 2% of body and head lice, respectively. Our results also show that there are more body lice positive for B. quintana in poor countries, which was determined by the gross domestic product, than in wealthy areas (228/403 versus 0/21, P < 0.001). A similar finding was obtained for head lice (8/226 versus 2/390, P = 0.007). Our findings suggest that head lice in Africa may be infected by B. quintana when patients live in poor economic conditions and are also exposed to body lice.
Introduction
Sucking lice (Anoplura) are hematophagous wingless insects that can infest birds and mammals.1 Among these hosts, humans constitute the preferred host for only two species: Pediculus humanus and Pthirus pubis (pubic lice). P. humanus includes two morphotypes: P. humanus morphotype capitis (head lice) and P. humanus morphotype humanus (body lice).2 Each louse has a specific ecotype; head lice live and lay eggs in the hair and are prevalent in all countries and all levels of society, whereas body lice live in clothing and multiply when cold, promiscuity, and lack of hygiene are present.3 Louse coloration was described at the beginning of 20th century. The variability in louse color on a single host may be affected by not only the color of the skin but also, the color of the hair and clothing.4,5
Many genetic studies have been conducted on human lice—first based on 18S ribosomal RNA6,7 and then, the mitochondrial genes (cytochrome oxidase subunit 1 [Cox1] and cytochrome b [Cytb]). These studies allowed scientists to classify lice into three different clades: Clade A, the most common clade found worldwide and comprised of both head and body lice; Clade B, composed of only head lice and found in Central and North America, Europe, and Australia8; and Clade C, including black head lice found in Nepal,9 Ethiopia,10 and Senegal.11
The body louse is linked to poverty. Its transmission occurs in crowded environments, such as homeless shelters, refugee camps, and jails, especially when hygienic standards are lacking.12 The body louse is the main vector of three pathogenic bacteria: Bartonella quintana, the agent of trench fever; Borrelia recurrentis, the agent of louse-borne relapsing fever; and Rickettsia prowazekii, the agent of epidemic typhus.13,14 B. quintana is a Gram-negative bacterium that causes trench fever, bacillary angiomatosis, endocarditis, chronic bacteremia, and chronic lymphadenopathy.15 It has typically been transmitted by body lice, but recently, DNA of B. quintana has also been found in head lice collected from homeless individuals in Nepal,9 the United States,16 France,17 Senegal,11 and Ethiopia.10,18
The detection of B. quintana in African lice remains limited to only a small number of countries. Therefore, the objectives of this study were to investigate the presence of B. quintana in head and body lice in different areas of African countries suffering from poverty, social instability, or war and identify the relationship between louse phenotypes and genotypes.
Materials and Methods
Ethics statement.
Lice from African countries were obtained from the private frozen collection of our laboratory (The Unité de Rcherche sur les Maladies Infectieuses et Tropicales Emergentes [URMITE]/World Health Organization [WHO] Collaborative Research Center). The lice in that collection were required for various epidemiological and entomological studies or to perform diagnoses abroad, and they were sent to our laboratory as a WHO reference facility. Body lice were collected from clothing and head lice were removed from hair with the verbal consent of the infested individuals. Written consent was not obtainable in the majority of cases, because most of the subjects were illiterate. However, in most instances, the investigator, local authorities/Institutional Review Boards (IRBs), and/or village/family chief approved and were present when collection was performed (with individual consent).
Sampling/country.
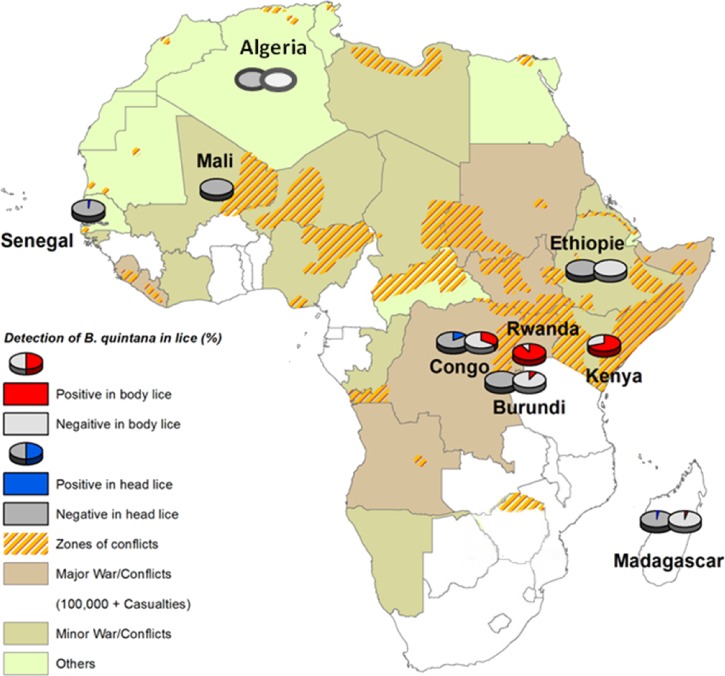
Human lice samples were collected from patients in various regions of Africa (Figure 1). Nine countries were investigated. In total, 1,040 lice were collected: 616 head lice and 424 body lice. Exactly 381 head lice were collected in Senegal (2011), 75 head lice and 22 body lice were collected in Madagascar (2009, 2011, and 2012), 14 head and body lice were collected in Ethiopia (2011), 92 head lice were collected in Mali (2010), 37 body lice were collected in Kenya (1999), 166 body lice were collected in Rwanda (2011), 10 head and body lice were collected in Burundi (2008), 35 head lice and 154 body lice were collected in Congo (2010), and 9 head lice and 21 body lice were collected in Algeria (2000). In our laboratory, each sample was photographed on dorsal and ventral sides with a camera (Olympus DP71, Rungis, France). Lice were preserved in 70% alcohol, rinsed two times with sterile distilled water for 2 minutes, and dried with filter paper. Samples were then cut in half on the longitudinal plane, and one-half was stored at −20°C for subsequent analysis.
Figure 1.
Map of Africa showing the prevalence of B. quintana in head and body lice collected in the various study areas during 1999–2012.
Distribution of regions.
We classified these countries into two regions: poor (with the gross domestic product [GDP] < $1,844 per capita: Madagascar, Ethiopia, Mali, Kenya, Rwanda, Burundi, and Congo) and wealthy (with GDP ≥ $1,844 per capita: Algeria and Senegal) areas. GDP being an economic indicator of the wealth produced annually in a particular country for our classification, we estimated the average per capita GDP of all regions.19 The average GDP corresponding to the total of GDP per capita from nine countries divided by nine ($16,600/9 = $1,844) was then compared with the infection rate of B. quintana in lice.
DNA preparation and detection of B. quintana in lice.
After incubation at 56°C in a dry bath overnight, the lice were extracted on an automaton EZ1 device using the QIAamp Tissue Kit (Qiagen, Hilden, Germany) according the manufacturer's recommendations. The DNA was used as a template in a real-time polymerase chain reaction (PCR) assay targeting a portion of the Bartonella 16S–23S intergenic spacer (ITS) region and a specific B. quintana gene (yopP) that encodes a hypothetical intracellular effector.17 Each real-time PCR assay was performed using a CFX96 TM REAL-Time System C1000 Thermal Cycler (Bio-Rad Laboratories, Foster City, CA). For each PCR assay, two negative and positive controls were used. The identification of B. quintana was confirmed by amplification of both ITS2 and yopP genes.
Genotypic status of lice.
All samples that tested positive for B. quintana DNA were analyzed by multiplex real-time PCR that targeted a portion of the Phum PHUM540560 gene. This assay allowed for the discrimination of body lice from head lice, which has described previously.20 As a positive control, we used a head louse and body louse with genotypic statuses that were known (VIC-positive for the head louse and FAM-positive for the body louse). Monoplex and multiplex real-time PCRs were performed in a CFX96 Thermal Cycler (Bio-Rad Laboratories, Foster City, CA).
Standard PCR and sequencing.
Lice genotype was identified by the analysis of partial (347-bp) mitochondrial Cytb DNA. The primers used for the Cytb gene were CytbF1 (5′-GAGCGACTGTAATTACTAATC-3′) and CytbR1 (5′-GGACCCGGATAATTTTGTTG-3′). Overall, 10 lice belonging to each region were tested with a standard PCR assay targeting the Cytb gene as described previously.8 Each amplification reaction was performed on a PTC-200 thermocycler machine (MJ Research, Waltham, MA). The PCR reaction contained 9.9 μL water, 4 μL buffer 5× Fusion HF Buffer, 0.4 μL 10 mM deoxynucleoside triphosphates (dNTPs), 1.25 μL each primer, 0.2 μL Phusion polymerase (Finnzymes, Thermo Scientific, Vantaa, Finland), and 3 μL DNA template to obtain a total volume of 20 μL. The cycling conditions were 98°C for 30 seconds, 35 cycles of 98°C for 5 seconds, 56°C for 30 seconds, and 72°C for 15 seconds, and a final extension time of 5 minutes at 72°C. The success of the PCR amplification was then detected by the migration of the PCR product on 2% agarose gel (Electrophoresis grade; Invitrogen, Carlsbad, CA) prepared with 0.5% Tris Borate ethylenediaminetetraacetic (EDTA) (TBE; Euromodex, Lake Placid, NY) and charged with a solution of 0.5% ethidium bromide (Invitrogen, Carlsbad, CA). Purification of PCR-amplified products was performed using distilled RNase-DNase free water on NucleoFast 96 PCR plates (Macherey-Nagel EURL, Hoerdt, France). Sequencing of positive samples was performed using the ABI Prism Big Dye Terminator Cycle Sequencing Kit, version 1.1 (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
Data analysis.
Microsoft Excel was used for data management. Descriptive statistics, such as percentages and means, were computed to summarize the proportions of infestations with lice. Statistical analysis was performed with Epi Info 6 (www.cdc.gov/epiinfo/Epi6/EI6dnjp.htm), and a P value of < 0.05 was considered significant.
Phylogenetic analysis.
Each DNA sequence was aligned using multisequence alignment software (CLUSTALX, version 2.0.11). The ChromasPro program (Technelysium PTY, Australia) was used to analyze, assemble, and correct sequences. The sequence similarities were determined using MEGA 5, and phylogenetic trees were obtained using the maximum likelihood (ML) method with 100 bootstrap replicates.21 We compared our sequences with other sequences that are present in GenBank.
Results
Morphological analysis.
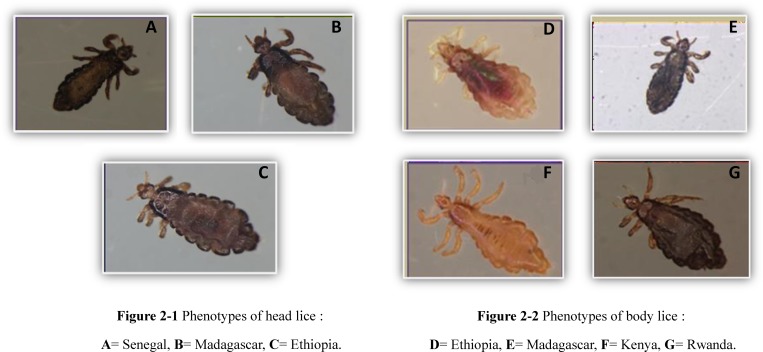
Phenotypical examination of the lice showed that all head lice from Senegal, Madagascar, and Ethiopia have a black color (Figure 2A –C). The color of all body lice in Madagascar and Rwanda is black; in Kenya, all body lice are brown, whereas in Ethiopia, all body lice are gray (Figure 2D–G). During our study, the phenotypes of lice from Congo, Burundi, Mali, and Algeria were not investigated, because they had been destroyed previously for other studies.
Figure 2.
Phenotypes of head and body lice collected from African countries during 1999–2012 (Head lice: A, B and C; Body lice: D, E, F and G).
Phylogenetic analysis.
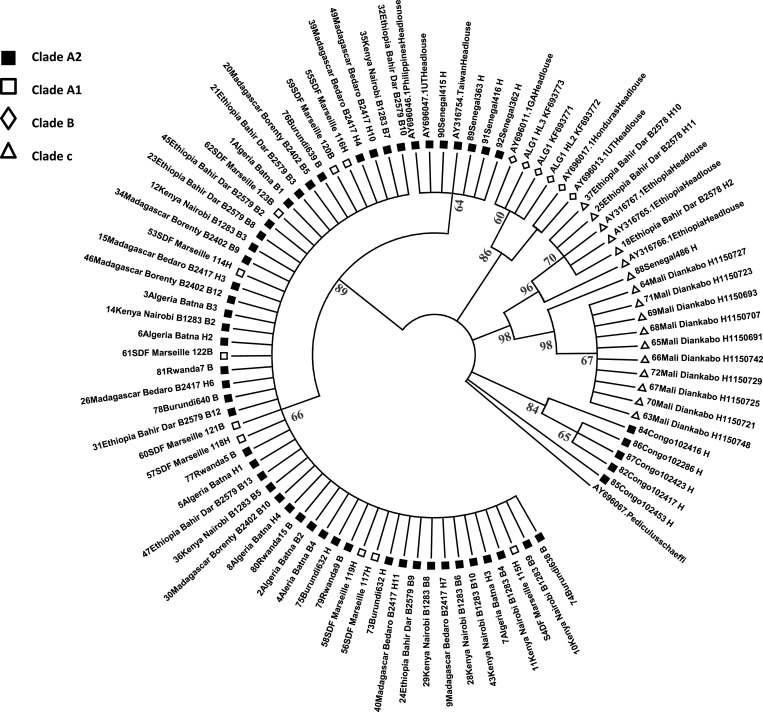
On the basis of phylogenetic study of lice Clade A based on ITSs (ultispacer typing method [MST])22 and through the analysis of Cytb in this work (Figure 3 ), our results show that head lice from Algeria, Madagascar, Burundi, and Senegal belong to Clade A2. Clade C was found in head lice from Ethiopia, Mali, and Senegal. Clade B was found in head lice from Algeria. Based on Cytb analysis, all head and body lice that are positive for B. quintana DNA belong to Clade A2 (Table 1).
Figure 3.
Phylogenetic tree of lice based on the Cytb (using the ML method with 100 bootstrap replicates).
Table 1.
Distribution of B. quintana DNA in head lice (N = 616) and body lice (N = 424) collected from African countries during 1999–2012
| Country/area | Lice analyzed | Positive B. quintana DNA (%) | Cytb | |||
|---|---|---|---|---|---|---|
| Head lice | Body lice | Head lice | Body lice | Head lice | Body lice | |
| Senegal | ||||||
| Yeumbeul | 101 | 0 | 0 | − | Clade A2 | − |
| Malika | 69 | 0 | 0 | − | Clade A2 | − |
| Dielmo village | 71 | 0 | 2 (2.8) | − | Clade A2 | − |
| Ndiop village | 77 | 0 | 0 (0) | − | Clade C | − |
| Keur Massar | 63 | 0 | 0 | − | Clade A2 | − |
| Total | 381 | 0 | 2 (0.52) | |||
| Madagascar | ||||||
| Borenty village | 0 | 9 | − | 1 (11.1) | − | Clade A2 |
| Tsiroanomandidy | 21 | 13 | 0 | 0 | Clade A2 | Clade A2 |
| Anjozorobe | 39 | 0 | 2 (5.1) | − | Clade A2 | − |
| Bedaro village | 15 | 0 | 0 | − | Clade A2 | − |
| Total | 75 | 22 | 2 (2.66) | 1 (4.54) | ||
| Ethiopia | ||||||
| Gondar | 14 | 14 | 0 | 0 | Clade C | Clade A2 |
| Mali | ||||||
| Diankabou | 92 | 0 | 0 | − | Clade C | − |
| Kenya | ||||||
| Nairobi | 0 | 37 | − | 27 (72.9) | − | Clade A2 |
| Rwanda | ||||||
| Kigali | 0 | 166 | − | 149 (89.7) | − | Clade A2 |
| Burundi | ||||||
| Bujumbura | 10 | 10 | 0 | 1 (10) | Clade A2 | Clade A |
| Congo | ||||||
| RDC | 35 | 154 | 6 (17.1) | 50 (32.5) | Clade A2 | Clade A2 |
| Algeria | ||||||
| Batna | 9 | 21 | 0 | 0 | Clades A and B | Clade A2 |
| Total | 616 | 424 | 10 (1.6) | 228 (53.7) | ||
Molecular detection of B. quintana in lice.
The DNA of B. quintana was detected in 10 of 616 (1.6%) head lice (the mean cycle thresholds [ct] value ± SD: 30.64 ± 6.34). Specifically, B. quintana DNA was found in 2 of 71 (2.8%) head lice from Senegal (Dielmo village), 2 of 39 (5.1%) head lice from Madagascar (Anjozorobe), and 6 of 35 (17.1%) head lice from Congo.23 No B. quintana DNA was detected in Senegal (Yeumbeul, Malika, Ndiop village, and Keur Massar), Madagascar (Tsiroanomandidy and Bedaro village), Ethiopia, Mali, Burundi, or Algeria (Table 1).
The DNA of B. quintana was detected in 228 of 424 (54%) body lice (the mean ct value ± SD: 33.19 ± 3.79). Among 228 B. Quintana-positive body lice, 149 of 166 (89.7%) body lice are from Rwanda, 27 of 37 (72.9%) body lice are from Kenya, 50 of 154 (32.5%) body lice are from the Democratic Republic of Congo,23 1 (11.1%) body louse is from Madagascar, and 1 (10%) body louse is from Burundi. No B. quintana DNA was detected in the Madagascar area of Tsiroanomandidy, Ethiopia, or Algeria. There are significantly more body lice with B. quintana DNA than head lice (54% versus 1.6%, P < 0.001) (Table 1).
Genotypic status of positive lice to B. quintana DNA.
Among the African Clade A lice positive for B. quintana DNA, all head lice had the head louse genotype, and all body lice had the body louse genotype. No signal was detected in the negative controls.
Relationship between B. quintana in body or head lice and socioeconomic level.
We compared our results with the life conditions of the regions tested and found that the body lice that were B. quintana-positive were more likely to be found in poor countries than wealthy countries (228/403 versus 0/21, P < 0.001). The same finding was made for head lice (8/226 versus 2/390, P = 0.007).
We correlated the GDP to the level of B. quintana infection. Our results indicate that the higher the GDP in a region, the less prevalent that B. quintana DNA was and vice versa (correlation r = −0.178).
Discussion
This study of 1,040 human lice of the genus Pediculus collected from nine African countries has enabled us to better specify some characteristics of human lice. The phenotypic study allowed us to confirm several previously described observations, including the black phenotype of head lice in Senegal and Ethiopia.10,11,24 For the first time, we have established the phenotype of lice collected in Madagascar and Kenya. Body lice from Madagascar and Rwanda are black; body lice collected in Kenya are brown. The head lice from Madagascar had the same black color as body lice collected in this country (Figure 2). In addition, it seems that the color of lice is more complex than indicated in the first findings provided in 1926 by Ewing,25 and as suggested by a recent study,10 lice color may be independent of the host's skin color.
On the basis of phylogenetic study of lice Clade A, based on ITSs (MST)22 and through the analysis of Cytb in this work (Figure 3), we confirmed that head lice in Senegal are Clades A2 and C11 and that head lice in Ethiopia are Clade C.10 In addition, we have found that head lice in Madagascar, Burundi, Congo, and Algeria belong to Clade A2 and that head lice in Mali are Clade C (Figure 3). The body lice of Algeria, Ethiopia, Madagascar, Kenya, Burundi, and Rwanda belong to Clade A2; it is the main clade in sub-Saharan Africa.26 Therefore, the geographical distribution of lice seems to be complex and independent of the phenotype.
B. quintana is a re-emerging pathogen that is responsible for a range of clinical manifestations in humans.27 It has long been established that trench fever can be transmitted by the body louse.28 However, the role played by the head louse as a reservoir or vector of B. quintana remains unclear.29 In total, B. quintana was found in 54% and 2% of body and head lice collected in Africa, respectively. Here, we find that there are more B. quintana in body lice than head lice. This finding could probably be explained through the role played by the body louse in transmission of diseases. With the new method of multiplex real-time PCR assay with the Phum_PHUM540560 gene, all head lice were genotyped as head lice by the signal emitted by the VIC-labeled probe specific to head lice, and all body lice were genotyped as body lice by the signal emitted by the FAM-labeled probe specific to body lice. In our study, the infection rate was higher in Rwanda (149/166 versus 6/262, P < 0.001) (Table 2) and lower in Burundi (1/10 versus 158/346, P = 0.025) than the rate reported in 2002 by Fournier and others.30 These differences could be explained by the living conditions in jail in Rwanda and probably, the sample size in this study in Burundi. We have confirmed the presence of B. quintana in body lice in Rwanda and Burundi.30 B. quintana has also been detected in body lice from homeless people in Zimbabwe (16.7%, 2/12).30 This bacterium has not been found in body lice in Tunisia.30 We detected B. quintana for the first time in body lice collected in Madagascar (4.54%, 1/22) and Kenya (72.9%, 27/37). The high rate of B. quintana in body lice in some African countries (Kenya, Rwanda, and Congo) may reflect the low socioeconomic level of the study population. We tested this hypothesis by comparing the average GDPs of nine different countries with the level of B. quintana infection. We found that the higher the GDP increases, the lower the level of B. quintana decreases and vice versa (correlation r = −0.178). For example, for countries with high GDP, this hypothesis could be justified through the previous work by Fournier and others30 in 2002, which reported 0% B. quintana in head lice in Portugal, China, Thailand, Australia, Algeria, and France.30 This percentage of infection with B. quintana remains stable (0%) in body lice in Australia, Algeria, and Peru. In France, Bouvresse and others31 also found the same result (0%) in head lice. Roux and Raoult32 reported a rate of 1.4% in body lice in Peru. For countries with low GDP, this hypothesis could be confirmed by the work of Piarroux and others23 in 2010, which reported 32.5% B. quintna in body lice in Congo, and the work of Fournier and others,30 which found a higher prevalence of 93.9% B. quintana in body lice in Burundi (a refugee camp in 2001). Elsewhere, we compared the infection rate in B. quintana body lice in people in Africa with the infection rate in B. quintana body lice in homeless people in Marseille, France,30,32,33 and the result was statistically significant (228/424 versus 77/560, P < 0.001) (Table 2). In this study, we show a relationship between B. quintana presence in body lice and socioeconomic level, and we found that the body lice that were B. quintana-positive were more likely to be found in poor countries (GDP < $1,844 per capita) than wealthy countries (GDP > $1,844 per capita; 228/403 versus 0/21, P < 0.001).
Table 2.
Prevalence of infections of B. quintana in body lice (this study and the literature)
| Country/source | Year of collection | Percent (no. B. quintana/no. tested) | Source |
|---|---|---|---|
| Algeria | |||
| Schoolchildren | 2000 | 0 (0/21) | This study |
| Homeless in Batna | 2001 | 0 (0/33) | 30 |
| Madagascar | |||
| Local population | 2009/2011/2012 | 4.5 (1/22) | This study |
| Ethiopia | |||
| Bahir Dar | 2011 | 0 (0/14) | This study |
| Poor regions (Jimma) | 2010 | 3 (1/33) | 29 |
| Poor regions (Jimma) | 2010 | 18 (76/424) | 10 |
| Kenya | |||
| Local population | 1999 | 72.9 (27/37) | This study |
| Rwanda | |||
| Jail | 2011 | 89.7 (149/166) | This study |
| Jail | 2001 | 2.3 (6/262) | 30 |
| Burundi | |||
| Refugee camp | 2008 | 10 (1/10) | This study |
| During typhus outbreak | 1997 | 0 (0/10) | 32 |
| Refugee camp | 1997 | 9.5 (6/63) | 32 |
| After outbreak in camp | 1998 | 14.3 (13/91) | 32 |
| During typhus outbreak | 1997 | 0 (0/10) | 30 |
| Refugee camp | 1997 | 9.5 (6/63) | 30 |
| After typhus outbreak | 1998 | 14.3 (13/91) | 30 |
| Refugee camp | 1998 | 21 (8/38) | 30 |
| Refugee camp | 2000 | 90 (100/111) | 30 |
| Refugee camp | 2001 | 93.9 (31/33) | 30 |
| Congo | |||
| Local population | 2010 | 32.5 (50/154) | This study |
| Refugee camp | 1998 | 0 (0/7) | 32 |
| Refugee camp | 1998 | 0 (0/7) | 30 |
| Local population | 2010 | 32.5 (50/154) | 23 |
| France | |||
| SDF (homeless) | 1997 | 20 (3/15) | 34 |
| SDF (homeless) | 1998 | 4 (3/75) | 32 |
| SDF (homeless) | 2000 | 26 (42/161) | 33 |
| SDF (homeless) | 1998–2001 | 9.8 (32/324) | 30 |
| Tunisia | |||
| Homeless in Sousse | 2000 | 0 (0/3) | 30 |
| Zimbabwe | |||
| Homeless in Harare | 1998 | 16.7 (2/12) | 30–32 |
| The Netherlands | |||
| Homeless in Utrecht | 2001 | 36 (9/25) | 30 |
| United States | |||
| SDF (homeless) | 2007–2008 | 33.3 (11/33) | 16 |
| Nepal | |||
| Street children and children in slums | 2002 | 20 (4/20) | 9 |
| Russia | |||
| Homeless in Moscow | 1998 | 12.3 (33/268) | 32 |
| Australia | |||
| Homeless | 2001 | 0 (0/2) | 30 |
| Peru | |||
| Andean rural population | NA | 1.4 (1/73) | 32 |
| Andean rural population | NA | 0 (0/10) | 30 |
NA = not available.
Head lice can also be infected with B. quintana. In our study, we detected the DNA of B. quintana in 1.6% (10/616) of head lice collected: in Senegal, 0.52% (2/381); in Madagascar for the first time, 2.66% (2/75); in the Congo, 17.1% (6/35). B. quintana was detected in 7% (19/274) of head lice collected in Dakar, Senegal11 and 9.2% (6/65) of head lice pools and 7% (19/271) of head lice on persons living in the poorest areas of Jimma, Ethiopia.10,29 No B. quintana DNA was detected in head lice from schoolchildren in Marseille, France,30,31 contrary to the work by Angelakis and others,10 which have reported this result in the homeless population. In Russia, Portugal, Algeria, Burundi, China, Thailand, and Australia, no B. quintana was found in schoolchildren30 (Table 3). In this study, we also found the head lice that were B. quintana-positive were more likely to be found in poor countries (GDP < $1,844 per capita) than wealthy countries (GDP > $1,844 per capita; 8/226 versus 2/390, P < 0.007). Head lice are prevalent around the world and in all levels of society. Thus, head lice may be infected with B. quintana when the host is coinfected with body lice in a precarious environment in which proper hygiene is lacking.
Table 3.
Prevalence of infections of B. quintana in head lice (this study and the literature)
| Country/source | Year of collection | Percent (no. B. quintana/no. tested) | Source |
|---|---|---|---|
| Algeria | |||
| Schoolchildren | 2000 | 0 (0/9) | This study |
| Schoolchildren | NA | 0 (0/18) | 30 |
| Burundi | |||
| Schoolchildren | 2008 | 0 (0/10) | This study |
| Schoolchildren | NA | 0 (0/20) | 30 |
| Senegal | |||
| Rural community | 2011 | 0.52 (2/381) | This study |
| Rural community | NA | 6.9 (19/274) | 11 |
| Ethiopia | |||
| Poor region | 2011 | 0 (0/14) | This study |
| Poor regions (Jimma) | 2010 | 7 (19/271) | 10 |
| Poor regions (Jimma) | 2010 | 9.2 (6/65) | 29 |
| Congo | |||
| Local population | 2010 | 17.1 (6/35) | This study |
| Local population | 2010 | 17.1 (6/35) | 23 |
| Madagascar | |||
| Local population | 2010–2011 | 2.6 (2/75) | This study |
| Mali | |||
| Schoolchildren | 2010 | 0 (0/92) | This study |
| Portugal | |||
| Schoolchildren | NA | 0 (0/20) | 30 |
| China | |||
| Schoolchildren | NA | 0 (0/23) | 30 |
| Thailand | |||
| Schoolchildren | NA | 0 (0/29) | 30 |
| Australia | |||
| Schoolchildren | NA | 0 (0/3) | 30 |
| United States | |||
| SDF (homeless) | 2007–2008 | 25 (3/12) | 16 |
| Nepal | |||
| Street children and children in slums | 2002 | 9.5 (2/21) | 9 |
| Russia | |||
| Schoolchildren | NA | 0 (0/10) | 30 |
| France | |||
| Schoolchildren | NA | 0 (0/20) | 30 |
| Schoolchildren | 2008–2009 | 0 (0/288) | 31 |
| SDF (homeless) | 2008 | 100 (3/3) | 17 |
NA = not available.
In conclusion, it has been estimated that trench fever affected several million people, especially in Russia and on the Eastern, Central, and Western European fronts during World War II.12,15 Our study on the prevalence of B. quintana in lice from various geographical and socioecological situations associated with clinical information will help to evaluate the role of head lice in the transmission of this disease.
ACKNOWLEDGMENTS
The authors thank Mr. Jean-Michel Berenger (entomologist) and the molecular biology team of URMITE Marseille for their technical support.
Footnotes
Authors' addresses: Abdoul Karim Sangaré, Amina Boutellis, Rezak Drali, Cristina Socolovschi, Georges Diatta, and Didier Raoult, URMITE, UM63, 7278 CNRS, IRD 198, Inserm 1095, University of Aix, Marseille, France, and IRD, Campus Commun UCAD-IRD of Hann, Dakar, Senegal, E-mails: sangareak@icermali.org, amina.boutell@yahoo.fr, rezakdrali@hotmail.com, cr_socolovschi@yahoo.com, georges.diatta@ird.fr, and didier.raoult@gmail.com. Stephen C. Barker, Parasitology Section, School of Chemistry and Molecular Biosciences (SCMB), University of Queensland, Brisbane, Queensland, Australia, E-mail: s.barker@uq.edu.au. Christophe Rogier and Marie-Marie Olive, Pasteur Institute of Madagascar, Ambohitrakely, Madagascar, E-mails: crogier@pasteur.mg and mmolive@pasteur.mg. Ogobara K. Doumbo, University of Bamako, MRTC/DEAP/FMPOS-UMI3189, Bamako, Mali, E-mail: okd@icermali.org.
References
- 1.Barker SC. Phylogeny and classification, origins, and evolution of host associations of lice. Int J Parasitol. 1994;24:1285–1291. doi: 10.1016/0020-7519(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 2.Light JE, Toups MA, Reed DL. What's in a name: the taxonomic status of human head and body lice. Mol Phylogenet Evol. 2008;47:1203–1216. doi: 10.1016/j.ympev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332–337. doi: 10.1111/j.1469-0691.2012.03778.x. [DOI] [PubMed] [Google Scholar]
- 4.Nuttall GHF. The biology of Pediculus humanus. Parasitology. 1917;10:80–185. [Google Scholar]
- 5.Busvine JR. Blackwell Publishing Ltd; 1946. On the pigmentation of the body louse pediculus humanus L. In Proceedings of the Royal Entomological Society of London. Series A, General Entomology (Vol. 21, No. 10--12, pp. 98--103) [Google Scholar]
- 6.Yong Z, Fournier PE, Rydkina E, Raoult D. The geographical segregation of human lice preceded that of Pediculus humanus capitis and Pediculus humanus humanus. C R Biol. 2003;326:565–574. doi: 10.1016/s1631-0691(03)00153-7. [DOI] [PubMed] [Google Scholar]
- 7.Leo NP, Barker SC. Unravelling the evolution of the head lice and body lice of humans. Parasitol Res. 2005;98:44–47. doi: 10.1007/s00436-005-0013-y. [DOI] [PubMed] [Google Scholar]
- 8.Raoult D, Reed DL, Dittmar K, Kirchman JJ, Rolain JM, Guillen S, Light JE. Molecular identification of lice from pre-Columbian mummies. J Infect Dis. 2008;197:535–543. doi: 10.1086/526520. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Poudel SK, Isawa H, Hayashi T, Seki N, Tomita T, Sawabe K, Kobayashi M. First molecular evidence of Bartonella quintana in Pediculus humanus capitis (Phthiraptera: Pediculidae), collected from Nepalese children. J Med Entomol. 2006;43:110–112. doi: 10.1093/jmedent/43.1.110. [DOI] [PubMed] [Google Scholar]
- 10.Angelakis E, Diatta G, Abdissa A, Trape JF, Mediannikov O, Richet H, Raoult D. Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg Infect Dis. 2011;17:2357–2359. doi: 10.3201/eid1712.110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutellis A, Veracx A, Angelakis E, Diatta G, Mediannikov O, Trape JF, Raoult D. Bartonella quintana in head lice from Senegal. Vector Borne Zoonotic Dis. 2012;12:564–567. doi: 10.1089/vbz.2011.0845. [DOI] [PubMed] [Google Scholar]
- 12.Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357–374. doi: 10.1146/annurev-ento-120709-144739. [DOI] [PubMed] [Google Scholar]
- 13.Cutler SJ. Relapsing fever–a forgotten disease revealed. J Appl Microbiol. 2010;108:1115–1122. doi: 10.1111/j.1365-2672.2009.04598.x. [DOI] [PubMed] [Google Scholar]
- 14.Raoult D, Ndihokubwayo JB, Tissot-Dupont H, Roux V, Faugere B, Abegbinni R, Birtles RJ. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352:353–358. doi: 10.1016/s0140-6736(97)12433-3. [DOI] [PubMed] [Google Scholar]
- 15.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla DL, Kabeya H, Henn J, Kramer VL, Kosoy MY. Bartonella quintana in body lice and head lice from homeless persons, San Francisco, California, USA. Emerg Infect Dis. 2009;15:912–915. doi: 10.3201/eid1506.090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelakis E, Rolain JM, Raoult D, Brouqui P. Bartonella quintana in head louse nits. FEMS Immunol Med Microbiol. 2011;62:244–246. doi: 10.1111/j.1574-695X.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 18.Boutellis A, Mediannikov O, Bilcha KD, Ali J, Campelo D, Barker SC, Raoult D. Borrelia recurrentis in head lice, Ethiopia. Emerg Infect Dis. 2013;19:796–798. doi: 10.3201/eid1905.121480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CIA Factbook . 2012. The World Factbook. Central Intelligence Agency.https://www.cia.gov/library/publications/the-world-factbook/index.html Available at. [Google Scholar]
- 20.Drali R, Boutellis A, Raoult D, Rolain JM, Brouqui P. Distinguishing body lice from head lice by multiplex real-time PCR analysis of the Phum_PHUM540560 gene. PLoS ONE. 2013;8:e58088. doi: 10.1371/journal.pone.0058088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Ortiz G, Fournier PE, Gimenez G, Reed DL, Pittendrigh B, Raoult D. Genotyping of human lice suggests multiple emergencies of body lice from local head louse populations. PLoS Negl Trop Dis. 2010;4:e641. doi: 10.1371/journal.pntd.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piarroux R, Abedi AA, Shako JC, Kebela B, Karhemere S, Diatta G, Davoust B, Raoult D, Drancourt M. Plague epidemics and lice, Democratic Republic of the Congo. Emerg Infect Dis. 2013;19:505–506. doi: 10.3201/eid1903.121542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veracx A, Boutellis A, Merhej V, Diatta G, Raoult D. Evidence for an African cluster of human head and body lice with variable colors and interbreeding of lice between continents. PLoS ONE. 2012;7:e37804. doi: 10.1371/journal.pone.0037804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Ewing HE. A revision of the American lice of the genus Pediculus, together with aconsideration of the significance of their geographical and host distribution. Proc US Natl Museum. 1926;68:1–30. [Google Scholar]
- 26.Boutellis A, Drali R, Rivera MA, Mumcuoglu KY, Raoult D. Evidence of sympatry of clade A and clade B head lice in a pre-Columbian Chilean mummy from Camarones. PLoS ONE. 2013;8:e76818. doi: 10.1371/journal.pone.0076818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217–223. doi: 10.3201/eid1202.050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacot A. On the probable identity of Rickettsia pediculi with Rickettsia quintana. BMJ. 1921;1:156–157. doi: 10.1136/bmj.1.3135.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler S, Abdissa A, Adamu H, Tolosa T, Gashaw A. Bartonella quintana in Ethiopian lice. Comp Immunol Microbiol Infect Dis. 2012;35:17–21. doi: 10.1016/j.cimid.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Fournier PE, Ndihokubwayo JB, Guidran J, Kelly PJ, Raoult D. Human pathogens in body and head lice. Emerg Infect Dis. 2002;8:1515–1518. doi: 10.3201/eid0812.020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouvresse S, Socolovshi C, Berdjane Z, Durand R, Izri A, Raoult D, Chosidow O, Brouqui P. No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp Immunol Microbiol Infect Dis. 2011;34:475–477. doi: 10.1016/j.cimid.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Roux V, Raoult D. Body lice as tools for diagnosis and surveillance of reemerging diseases. J Clin Microbiol. 1999;37:596–599. doi: 10.1128/jcm.37.3.596-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La SB, Fournier PE, Brouqui P, Raoult D. Detection and culture of Bartonella quintana, Serratia marcescens, and Acinetobacter spp. from decontaminated human body lice. J Clin Microbiol. 2001;39:1707–1709. doi: 10.1128/JCM.39.5.1707-1709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouqui P, La Scola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]