Abstract
Zanzibar has transitioned from malaria control to the pre-elimination phase, and the continued need for intermittent preventive treatment during pregnancy (IPTp) has been questioned. We conducted a prospective observational study to estimate placental malaria positivity rate among women who did not receive IPTp with sulfadoxine-pyrimethamine. A convenience sample of pregnant women was enrolled from six clinics on the day of delivery from August of 2011 to September of 2012. Dried placental blood spot specimens were analyzed by polymerase chain reaction (PCR); 9 of 1,349 specimens (0.7%; precision estimate = 0.2–1.1%) were PCR-positive for Plasmodium falciparum. Placental infection was detected on both Pemba (N = 3) and Unguja (N = 6). Placental malaria positivity in Zanzibar was low, even in the absence of IPTp. It may be reasonable for the Ministry of Health to consider discontinuing IPTp, intensifying surveillance efforts, and promoting insecticide-treated nets and effective case management of malaria in pregnancy.
Introduction
The devastating consequences of malaria in pregnancy (MIP) on maternal and newborn health are well-documented. Pregnant women in areas of both stable and unstable malaria transmission are at increased risk for peripheral and placental Plasmodium falciparum infection, with symptomatic infections being more common in areas of unstable transmission.1–4 The adverse maternal outcomes of MIP in both transmission settings include severe anemia and mortality, with higher case fatality rates in lower-transmission settings.3,5,6 In addition, MIP is associated with low birth weight (LBW; < 2,500 g),7 an important risk factors for infant mortality.2,8,9
In areas of stable malaria transmission, the World Health Organization (WHO) promotes a three-pronged strategy to prevent adverse consequences of MIP, including (1) intermittent preventive treatment during pregnancy (IPTp) using sulfadoxine-pyrimethamine (SP), (2) use of insecticide-treated bed nets (ITNs), and (3) effective case management through prompt detection and treatment of malaria and anemia in pregnancy.10–12 Zanzibar adopted this three-pronged approach in 2004 when malaria transmission was high and stable.13 Since 2006, Zanzibar has scaled up long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), improved case detection with malaria rapid diagnostic tests (mRDTs), and introduced artemisinin-based combination therapy (ACT). A marked decline in malaria prevalence in Zanzibar over the past decade (from 50% in 2003 to 0.5% in 2012 among children under 5 years of age) has shifted Zanzibar to the malaria pre-elimination phase.13,14 In response, Zanzibar is now revising its MIP control strategy. One question is whether to continue IPTp. This study aimed to estimate the rate of placental malaria infection at delivery among Zanzibari pregnant women who had not received IPTp to help determine whether IPTp is still warranted in Zanzibar.
Methods and Materials
Study area and sites.
Zanzibar is comprised of two main islands approximately 40 km from mainland Tanzania: Unguja (2012 population = 896,721; 1,666 km2) and Pemba (2012 population = 406,848; 998 km2).15,16 This study was conducted at a purposive sample of 6 of 38 health facilities in Zanzibar where routine deliveries occur (3 facilities in Unguja [Mnazi Mmoja Hospital, Mwembeladu Hospital, and Kivunge Health Center] and 3 facilities in Pemba [Chake Chake Hospital, Wete Hospital, and Micheweni Health Center]) from August of 2011 to September of 2012. These facilities were engaged in an ongoing program supported by the US Agency for International Development (USAID) designed to improve maternal and newborn health services. Although these six hospitals covered approximately 82% of hospital deliveries on both islands, 50% of women in Zanzibar deliver at home.17 The peak rainfall period occurs from March to June, and the peak malaria transmission period is from April to July.18
Eligibility and enrollment.
Residents of Unguja or Pemba delivering at a study facility were considered eligible if their antenatal care (ANC) card indicated they had received no doses of IPTp SP. Women were enrolled 24 hours/day every day of the week by the clinic staff. Consecutive sampling was used to enroll all eligible women during the 12-month study period. However, because of the added burden on the clinic staff, no listing was maintained of women who were eligible but not enrolled. The 12-month enrollment ensured that both low- and high-transmission seasons were represented.
Procedures.
Women who delivered at the six facilities were screened by examining their ANC card, which contains a designated space for recording doses of IPTp SP received. No additional questions were asked, because eligible clients were typically in active labor. Eligible women who agreed to participate were read an informed consent statement by trained maternity nurses or nurse midwives, and their verbal consent was obtained and recorded. No additional assent options were offered to minors. A brief form, including age of mother, gravidity, hemoglobin or hematocrit and week of gestation at which the measurement was taken, whether it was a singleton or multiple birth, outcome of the birth, and birth weight of the baby, was filled out by the health worker with information extracted from each consenting mother's ANC card. The form was placed into the client's file and transferred to the delivery room with the client.
Collection of placental samples.
After management of the birth, a trained maternity nurse or nurse midwife at each facility obtained a placental dried blood spot (DBS) specimen from the maternal side of the placenta. A single deep incision was made, and five drops of blood were drawn up with a pipette and dotted onto Whatman 903 Protein Saver Card filter paper labeled only with the woman's study identification (ID) number.19,20 DBS specimens were labeled, packed with desiccant, and transferred to the University of California, San Francisco for polymerase chain reaction (PCR) to detect infection with Plasmodium (all species). Although exact timing was not monitored, collection of each specimen generally occurred within 30 minutes of delivery. After collection of the specimen, the placenta was disposed of per the facility's routine procedures. Multiple births were noted on the client's study form. In the case of twins with one placenta, one ID was issued. In the case of twins with two placentas, each placenta was assigned a unique ID number and tested. Corresponding placental ID numbers were noted on the client form.
Laboratory analysis.
DNA from each DBS was extracted using the QIAamp DNA Microkit following the manufacturer's instructions (Qiagen, Germantown, MD). Plasmodium DNA was amplified by 18S ribosomal DNA PCR, with the species-specific nested round confirming falciparum species.21 The presence of PCR product was evaluated using agarose gel electrophoresis stained with ethidium bromide. Testing of control samples extracted from DBSs with known parasite densities confirmed a limit of detection of < 10 parasite/μL.
Sample size.
The required sample size was calculated to be 1,825 assuming a parasitemia prevalence of 5% with a 95% confidence interval with precision of ±1% point. The sample size was calculated assuming independence of observations, because intracluster correlations could not be assessed, but the expected prevalence was liberally estimated to help account for unknown variables. Initial results indicated prevalence well under the liberal prevalence estimate used in the sample size assumptions, indicating that fewer samples were needed to attain the necessary power for the study, but data collection continued to allow for coverage throughout both the rainy and dry seasons.
Data processing and analysis.
Client forms were entered into an Access database and date stamped, and hard copies were filed chronologically along with the consent forms. Data on PCR status were provided in an Excel spreadsheet, and the ID numbers were matched between the two files. Cleaned data were exported into SPSS, version 20 and Stata, version 11 for analysis. Although we attempted to enroll all eligible women, given the total number of deliveries and the expected proportion of women who did not receive IPTp (25%),14 it is likely that some of the eligible women were not enrolled. Given that the participants in this study were a non-probability sample of all eligible women, the 95% confidence interval given for the proportion of women with placental parasitemia who did not receive IPTp is considered a precision interval rather than a confidence interval.
Ethics/institutional review.
Ethical approval was provided by the Zanzibar Research Council, Johns Hopkins School of Public Health Institutional Review Board, and the US Centers for Disease Control and Prevention, Atlanta.
Results
Patient characteristics.
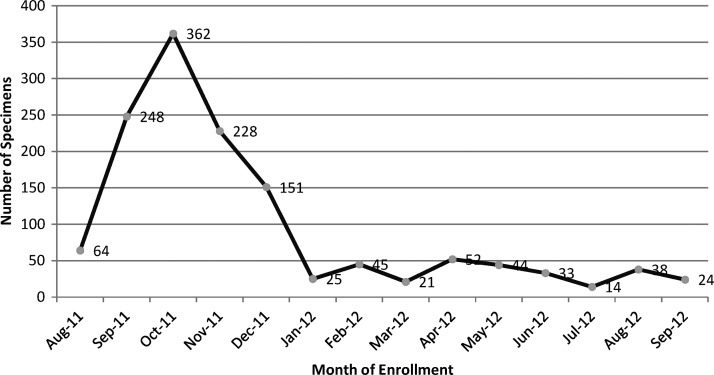
In total, 1,411 pregnant women were enrolled at the time of delivery. Data and specimens from 74 enrollees were not analyzed because of mislabeling, duplicate IDs, missing client cards, or loss. After exclusion of these 74 women, our analysis included 1,339 women from Pemba (N = 694) and Unguja (N = 645). The average age of enrolled women was 27 years (range = 15–48 years), and 433 (32%) women were primigravidae (Table 1). The proportion of primigravidae varied by facility (from 14% of the enrolled women at Mwembeladu to 42% of the enrolled women at Mnazi Mmoja). The enrollees included both singleton (N = 1,326) and twin (N = 13) pregnancies. Birth outcomes experienced by these women included 1,308 (97%) live births, 22 (2%) stillbirths, and 73 (5%) LBW deliveries (Table 1). The majority of enrolments (67%) occurred in the first 4 months of the study (Figure 1), likely because of SP stockouts preceding and during this period. Overall, 6% of all deliveries at the six facilities were enrolled (ranging from 4% to 15%). Although only 23% of all deliveries at Zanzibar health facilities occurred on Pemba in 2011, deliveries from Pemba comprised 52% of the total enrolment in this study.
Table 1.
Characteristics of pregnant women enrolled at delivery (N = 1,339) on Pemba and Unguja islands of Zanzibar in 2011–2012
| Island/health facility | Annual deliveries (2011)* | Enrolled women with specimen analyzed, n | Mean age (range), years | Primigravid, n (%) | Twin pregnancies, n (%) |
|---|---|---|---|---|---|
| Unguja | |||||
| Mnazi Mmoja | 10,338 | 375 | 27 (15–46) | 160 (42) | 5 (1) |
| Mwembeladu | 5,665 | 205 | 28 (17–46) | 29 (14) | 2 (1) |
| Kivunge | 1,420 | 65 | 25 (16–42) | 25 (37) | 2 (3) |
| Pemba | |||||
| Chake Chake | 2,838 | 415 | 27 (15–48) | 132 (32) | 3 (1) |
| Wete | 1,607 | 189 | 27 (16–48) | 50 (26) | 1 (1) |
| Micheweni | 645 | 90 | 25 (17–42) | 37 (41) | 0 (0) |
| Total | 22,563 | 1,339 | 27 (15–48) | 433 (32) | 13 (1) |
These figures represent deliveries during the 2011 calendar year.
Figure 1.
Placental specimens collected by month in Pemba and Unguja, Zanzibar.
Characteristics of placental parasitemia cases.
In total, 1,349 placental specimens were processed and analyzed for presence of malaria infection by PCR (Table 2). Nine (0.7%; precision estimate = 0.2–1.1%) were positive for P. falciparum, with no other Plasmodium species detected.
Table 2.
Distribution of malaria-positive placental specimens (N = 9) among study participants in 2011–2012
| Characteristic | Positive, n (%) | Total, n | P value |
|---|---|---|---|
| Season | 0.63 | ||
| September to December | 6 (1) | 1,014 | |
| January to April | 2 (1) | 143 | |
| May to August | 1 (1) | 192 | |
| Facility | 0.004 | ||
| Unguja | |||
| Mnazi Mmoja | 1 (< 1) | 380 | |
| Mwembeladu | 4 (2) | 207 | |
| Kivunge | 1 (1) | 67 | |
| Pemba | |||
| Chake Chake | 0 (0) | 416 | |
| Wete | 0 (0) | 189 | |
| Micheweni | 3 (3) | 90 | |
| Gravidity | 1.0 | ||
| Primigravidae | 3 (1) | 433 | |
| Multigravidae | 6 (1) | 916 | |
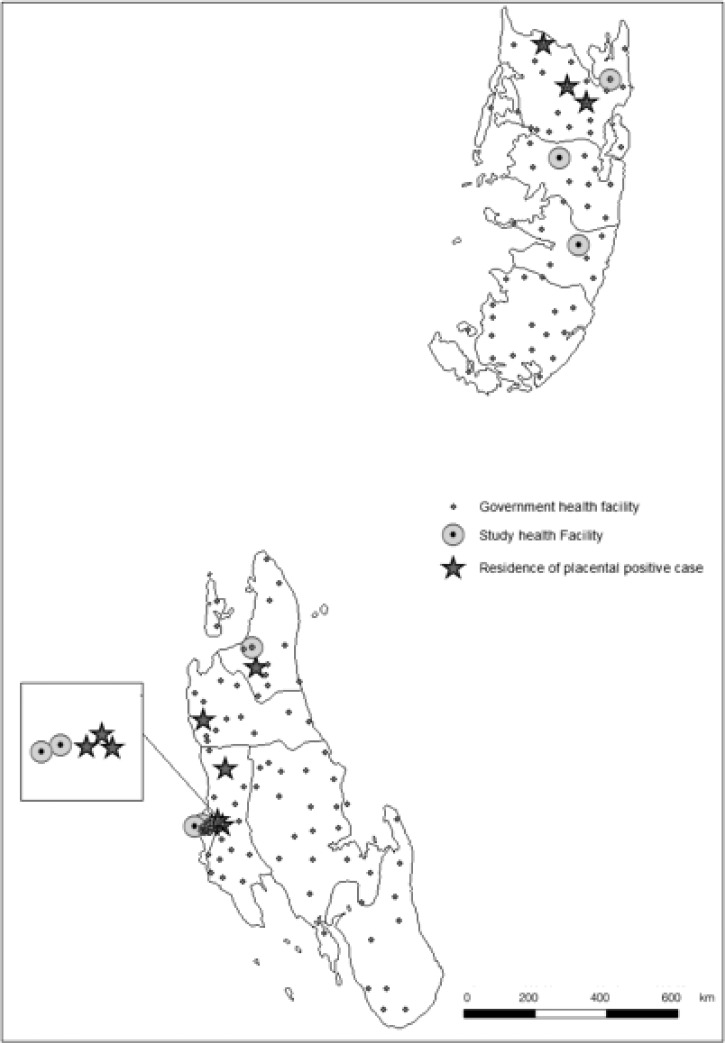
Placental infection was detected during 6 of 12 months of enrolment, with no seasonal variation. Unguja and Pemba accounted for six (66%) and three (33%) of the positive placental specimens, respectively (Figure 2). Detection of cases in the low-transmission season (September to December) was likely the result of increased study enrolment eligibility during these months as a result of SP stockouts (Figure 1). Seven (78%) positives originated from two of six enrolment facilities: Mwembeladu Hospital and Micheweni Health Center on Unguja and Pemba, respectively. In Pemba, placental infection (N = 3) was only detected at Micheweni, but at least one placental infection was detected at each of three Unguja facilities.
Figure 2.
Placental malaria infection cases in Pemba and Unguja, Zanzibar.
Birth outcomes among nine placental infections included one stillborn infant of LBW (< 2.5 kg). None of the remaining women with placental parasitemia delivered an LBW infant. Three women with placental parasitemia had hemoglobin tests; two women were anemic (hemoglobin < 11 g/dL) (Table 3). The proportion of anemic women at time of delivery was similar in women with and without placental malaria. Three positive specimens came from primigravidae women, proportionate to the percent of primigravidae sampled in the study.
Table 3.
Characteristics of malaria-positive placental specimens (N = 9) among study participants in 2011–2012
| Case number | Island | Health facility | Month of delivery | Parity | Birth weight (kg) |
|---|---|---|---|---|---|
| 1 | Pemba | Micheweni | September 2011 | 1 | 2.5 |
| 2 | Unguja | Mwembeladu | October 2011 | 6 | 3.8 |
| 3 | Unguja | Mwembeladu | October 2011 | 3 | 2.8 |
| 4 | Unguja | Mnazi Mmoja | November 2011 | 5 | 4.2 |
| 5 | Unguja | Mwembeladu | December 2011 | 2 | 3.1 |
| 6 | Unguja | Kivunge | December 2011 | 1 | 3.5 |
| 7 | Pemba | Micheweni | April 2012 | 1 | 2.5 |
| 8* | Pemba | Micheweni | April 2012 | 3 | 1.8 |
| 9 | Unguja | Mwembeladu | May 2012 | 4 | 2.5 |
Macerated stillbirth.
Discussion
The prevalence of placental malaria at the time of delivery in Zanzibar was very low among women in this study who did not receive IPTp SP, with an infection rate of 0.7% (precision estimate = 0.2–1.1%) by PCR. Data from screening women for peripheral parasitemia at first ANC visit with mRDTs at government health facilities shows a similar low prevalence of 0.2% among 19,724 women in 2011 and 27,186 women in 2012,22,23 confirming the low risk of MIP in Zanzibar. These data triangulate well with Zanzibar's Malaria Early Epidemic Detection System (MEEDS), which collects weekly data from all government facility outpatient departments. MEEDS reported a malaria positivity rate of 1.2% and 0.9% among all febrile or otherwise symptomatic outpatients tested during 2011 and 2012, respectively (Zanzibar Malaria Control Program, unpublished data).
The low prevalence of malaria overall and particularly, the low prevalence documented in pregnant women who deliver in health facilities raises multiple questions about the use of continuing IPTp SP as part of ANC services in Zanzibar. In areas of moderate to high malaria transmission, the WHO recommends IPTp in addition to ITNs and effective case management. In African countries that have substantially reduced malaria transmission after successful scale up of multiple control efforts, the WHO continues to recommend provision of IPTp.12,24 Despite this recommendation, Rwanda discontinued their national IPTp program in 2008 because of increased prevalence of SP resistance combined with declining malaria prevalence.25 The WHO's Malaria Policy Advisory Committee recently considered an Evidence Review Group (ERG) recommendation to discontinue IPTp SP when malaria transmission has been very low (falciparum malaria population prevalence in children under 15 years of age below 5%) for at least 3 years. The ERG's recommendation was based on findings from two unpublished analyses that suggested that, as transmission falls to very low levels, the fraction of malaria-attributable LBW prevented by IPTp SP continues to decline.26 The ERG emphasized that discontinuation of IPTp would require simultaneous maintenance and strengthening of vector control, prompt diagnosis and effective treatment, and surveillance systems to monitor malaria transmission. As of late 2013, the WHO had not changed its recommendation against discontinuation of IPTp, because the threshold value where IPTp SP confers no benefit remains undefined.
The potential for malaria resurgence in Zanzibar remains, given historical observations, population movement, and proximity to mainland Tanzania.27 It is reassuring that, since 2007, the population prevalence of malaria in cross-sectional household surveys in Zanzibar has remained below 1% and that, since 2008, MEEDS data indicate positivity rates well below 5% among febrile outpatients. Tanzania mainland has also reduced malaria prevalence among children 6–59 months of age (from 18% in 2007 to 10% in 2012).14 However, transmission in the nearby coastal regions Pwani and Tanga (10% and 6% prevalence, respectively, in 2012) remains substantially higher than in Zanzibar. Zanzibar's proximity to the mainland must be taken into consideration in any discussion to discontinue IPTp, particularly considering complexities and costs associated with restarting the IPTp SP program after discontinuation in case that is warranted.
The seasonal increases in malaria transmission and focal hot spots in Zanzibar must also be considered. After the rainy season each year, confirmed cases of malaria rise during May to July. Weekly malaria positivity rate has exceeded 5% (threshold for pre-elimination) one time per year in both 2010 and 2012 (maximum weekly positivity of 10% among patients ≥ 5 years of age).28 Thus, although the overall annual malaria prevalence and outpatient positivity rates remain extraordinarily low, periodic localized increases in malaria transmission do occur. As a strategy for prevention of MIP, however, it is unlikely that administering SP to all Zanzibari pregnant women throughout the year would significantly modulate risk during these brief periods of increased malaria transmission.
Because of Zanzibar's proximity to the mainland, approaches to control of MIP must include a strong component of surveillance and maintenance of high ITN use as well as ensure access to prompt diagnosis and effective treatment. The MEEDS surveillance system does not adequately monitor MIP for two reasons: (1) the entry point to MEEDS is limited to outpatient departments (OPDs), whereas symptomatic ANC clients may report to ANC clinics, and (2) the system does not capture pregnancy status of adults who present to OPDs. Enhanced surveillance of MIP could be achieved through inclusion of ANC malaria test results into MEEDS and modification of MEEDS to allow reporting of outpatient test results stratified by pregnancy status.
Among the alternatives to IPTp SP are two screening and treatment approaches: (1) intermittent screening and treatment of MIP (ISTp), in which every woman is tested at every ANC visit, and (2) single screen and treatment (SSTp), in which all pregnant women are tested at the first ANC visit and only symptomatic women are screened thereafter. Data comparing ISTp with IPTp from a single trial in Ghana show similar reductions in maternal morbidity, peripheral parasitemia, and LBW with ISTp (using SP or amodiaquine-artesunate) compared with IPTp SP.29 Additional studies comparing the effectiveness of IPTp SP with ISTp with artemether-lumefantrine are underway in East Africa, and results are expected in 2015 (ClinicalTrials.gov Identifier NCT01084213). SSTp is currently the national policy in Indonesia, where an ongoing study is comparing SSTp and ISTp using dihydroartemisinin-piperaquine for treatment. However, these data are not available to address the current decision-making process for a revised MIP strategy in Zanzibar. Another complication is that neither ISTp nor SSTp is currently endorsed by the WHO.
Both IPTp and widespread screening in ANC have cost implications, including commodity, logistics, training, supervision, and monitoring costs. Screen and treat strategies are likely to be more costly than IPTp SP given the higher cost of the RDTs compared with SP. In a setting with virtually no cases of malaria, these costs must be weighed against the potential risks and benefits to mothers and newborns, including the potential for serious adverse drug reactions related to SP use (estimated at 11.6–25.0 events per 100,000 exposures with a mean cost per episode of US$24.15 [range = US$0–226.04]).30 Given the cost implications of screen and treat programs, a setting such as Zanzibar, with a prevalence persistently below 1%, may reasonably consider discontinuing IPTp SP without implementing a screen and treat program. Such a move would necessitate maintaining and improving the other pillars of malaria prevention, including use of ITNs, effective case management, and additional enhancement of the existing surveillance system.
ITNs remain a critical part of the overall malaria control strategy supported by the Roll Back Malaria Partnership. ITNs not only provide protection to pregnant women but also reduce all-cause mortality among infants and young children by up to 20%.31 High ITN use at the community level has also achieved malaria transmission reductions.32 Provision of ITNs to pregnant women is one avenue to maintain continuous high coverage. Furthermore, in a large randomized controlled trial in a low-transmission setting in Uganda, ITNs and IPTp provided equivalent benefit to pregnant women.33 This finding suggests that, where ITN distribution forms part of the basis for general malaria control, it may be reasonable to discontinue IPTp and continue high ITN coverage as one strategy to avert MIP.
Limitations.
This study is limited to the cross-sectional prevalence of placental parasitemia among a consecutive sample of women delivering in health facilities in Zanzibar, but more than one-half of the women in Zanzibar deliver at home. The representativeness of our study sample may be questioned, because it is not unreasonable to presume that women delivering at home are less likely to receive IPTp and thus, more likely to be at risk for malaria infection. However, the high level of concordance among the results of our study, MEEDS, and findings from ANC screening provides reassurance that these results are valid. Receipt or non-receipt of IPTp SP was determined solely by reviewing the client ANC card at delivery, which means that the quality of information for enrolment eligibility was limited by the recording practices of ANC providers. In most cases where women had not received IPTp SP, it was indicated rather than just an absence of a marking on the card. Because SP is not widely available outside of ANC (in 2011, the Zanzibar Food and Drug Board banned the sale of SP and artemisinin monotherapies from private drug shops), the proportion of women who could have received SP outside of ANC (and thus, been misclassified as not receiving SP) is negligible. National survey data confirm that the proportion of women reporting receipt of any antimalarial in pregnancy (85%) is virtually identical to the proportion reporting having received SP from the ANC (84%).14 Because of SP stockouts, most women were enrolled in low-transmission months (September to December), potentially resulting in lower prevalence of placental malaria. Although we attempted consecutive enrollment of all eligible women, it is likely that it did not occur. Because no record was maintained of women who were not enrolled, to minimize the study-associated workload among busy labor/delivery ward staff, the exact number of eligible women remains uncertain. However, failure to enroll eligible women was likely related to the workload at the time that the woman presented, which is unlikely to be related to the outcome of interest. This limitation did not likely compromise the generalizability of our results to all pregnant women who did not receive IPTp delivering at these facilities. Because the facilities were selected purposively from the facilities supported by a USAID-funded program designed to improve maternal and newborn health services, the generalizability of the findings to all pregnant women who deliver at health facilities in Zanzibar cannot be assured. The use of histopathology, rather than PCR alone, would have allowed us a better estimation of the prevalence of malaria throughout a longer proportion of the pregnancy; however, it was beyond the scope of this study. Finally, because this study did not enroll women who did receive IPTp SP, no information was collected on the current protective efficacy of IPTp SP in Zanzibar.
Conclusion.
As malaria transmission in Zanzibar reaches the pre-elimination phase, MIP policy and strategies must be suitably adjusted. This study found a negligible level of malaria among women who had not received IPTp SP. Given the extremely low levels of malaria in both the general population and pregnant women, discontinuing IPTp SP is one reasonable option for Zanzibar to consider. A shift away from IPTp SP as part of Zanzibar's MIP control strategy should only be considered if increased commitment is made to ensure uninterrupted, high-quality access to malaria diagnosis and treatment during pregnancy combined with enhanced measures to drive current transmission levels down closer to zero. We further recommend enhanced surveillance of MIP through reinforcement of the existing surveillance system to ensure that cases among pregnant women are reported from both ANC and outpatient settings in a timely manner. Efforts should continue to ensure high ownership and use of ITNs, particularly among women of reproductive age. Findings from ongoing studies in higher-transmission areas on the cost effectiveness of integrating screen and treat programs into ANC services may not be directly relevant in Zanzibar, where prevalence of malaria is much lower. The Zanzibar Ministry of Health should consider an internal review of costs and findings from surveillance to inform on whether the cost of screening every pregnant woman in ANC is a sound public health investment.
ACKNOWLEDGMENTS
The technical input of Mr. Abdullah Suleiman Ali (Programme Manager, Zanzibar Malaria Elimination Programme (ZMEP)) and Ráz Stevenson (US Agency for International Development) is gratefully acknowledged. We also acknowledge the dedication of the 65 nurses and nurse midwives who collected placental specimens in these six health facilities on top of their normal workload for the period of 1 year. The map was created by Mohamed Haji Ali of ZMEP. In memory of Ms. Stella Mziray, who entered all of the data for this study and unexpectedly passed away in June of 2013.
Disclaimer: The contents are the responsibility of the authors and do not necessarily reflect the views of the US President's Malaria Initiative, US Agency for International Development, or US Centers for Disease Control and Prevention. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Financial support: This work was supported by the US President's Malaria Initiative, US Agency for International Development (USAID) under the terms of Cooperative Agreement No. 621-A-00-08-00023-00, Mothers and Infants, Safe Healthy Alive Program. This study was made possible by the support of the American people through USAID.
Authors' addresses: Marya Plotkin, Natalie Hendler, and Asma Ramadhan Khamis, Jhpiego Tanzania, Dar es Salaam, Tanzania, E-mails: Marya.Plotkin@jhpiego.org, Natalie.Hendler@jhpiego.org, and AsmaRamadan.Khamis@jhpiego.org. Khadija Said and Mwinyi I. Msellem, Zanzibar Malaria Control Programme, Zanzibar Ministry of Health, Tanzania, E-mails: khadijasd@yahoo.com and mmwinyi@hotmail.com. Rachel P. Chase, Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: rchase@jhu.edu. Elaine Roman, Jhpiego Baltimore, Baltimore, MD, E-mail: Elaine.Roman@jhpiego.org. Chonge Kitojo, Centers for Disease Control and Prevention, Dar es Salaam, Tanzania, E-mail: Kitojoc@tz.cdc.gov. Alanna C. Schwartz, Department of Medicine, University of California, San Francisco, CA, E-mail: alanna.schwartz@ucsf.edu. Julie Gutman and Peter D. McElroy, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jgutman@cdc.gov and pgm9@cdc.gov.
References
- 1.Brabin B, Romagosa C, Abdelgalil S, Menéndez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D'Alessandro U, Nosten F, Fischer PR, Ordi J. The sick placenta—the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaira in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64((Suppl)):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 3.Nosten F, Ter Kuile F, Maelankirri I, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg. 1991;85:424–429. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 4.Newman R, Hailemariam A, Jimma D. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopica during a nonepidemic year. J Infect Dis. 2003;187:1765–1772. doi: 10.1086/374878. [DOI] [PubMed] [Google Scholar]
- 5.Brabin B, Verhoeff F. The contribution of malaria. In: Maclean AB, editor. Maternal Morbidity and Mortality. London, United Kingdom: Royal College of Obstetricians and Gynaecologists; 2002. pp. 65–78. [Google Scholar]
- 6.Brabin B, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131((Suppl 2)):604–615. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 7.De Beaudrap P, Turyakira E, White L, Nabasumba C, Tumwebaze B, Muehlenbachs A, Guérin P, Boum Y, McGready R, Piola P. Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment. Malar J. 2013;12:139. doi: 10.1186/1475-2875-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyatt H, Snow RW. Impact of malaria during pregnancy on low birth weigh in Sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760–769. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Geertruyden J, Thomas F, Erhart A, D'Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71((Suppl)):35–40. [PubMed] [Google Scholar]
- 10.World Health Organisation . Updated WHO Policy Recomendations: Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva: World Health Organization; 2012. [Google Scholar]
- 11.World Health Organisation . A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region: Brazzaville: WHO Regional Office for Africa. Geneva: World Health Organization; 2004. [Google Scholar]
- 12.World Health Organization . WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine. 2013. http://www.who.int/malaria/publications/atoz/Policy_brief_IPTp-SP_implementation_11april2013.pdf.pdf Available at. Accessed June 1, 2013. [Google Scholar]
- 13.Aregawi M, Ali SA, Al-mafazy A, Molteni F, Katikiti S, Warsame M, Njau R, Komatsu R, Korenromp E, Hosseini M, Low-Beer D, Bjokman A, D'Alessandro U, Coosemans M, Otten M. Reduction in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malar J. 2011;10:46. doi: 10.1186/1475-2875-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Bureau of Statistics . HIVAIDS and Malaria Indicator Survey 2011/12. Dar es Salaam, Tanzania: National Bureau of Statistics; 2012. [Google Scholar]
- 15.National Bureau of Statistics and Ministry of Finance . Tanzania in Figures 2010. Dar es Salaam, Tanzania: National Bureau of Statistics; 2010. [Google Scholar]
- 16.National Bureau of Statistics and Ministry of Finance Dar es Salaam; Office of Chief Goverment Statistician President Office Finance Economy and Development Planning Zanzibar . Population and Housing Census. Dar es Salaam, Tanzania: National Bureau of Statistics; 2012. [Google Scholar]
- 17.National Bureau of Statistics (NBS) Tanzania and ICF Macro . 2010 Tanzania Demographic and Health Survey. Dar es Salaam, Tanzania: National Bureau of Statistics and ICF Macro; 2011. [Google Scholar]
- 18.Zanzibar Malaria Control Program and Ministry of Health . Malaria Epidemic Early Detection System Biannual Report Year - End 2011. Zanzibar: Zanzibar Ministry of Health; 2011. [Google Scholar]
- 19.Othoro C, Moore JM, Wannemuehler K, Nahlen BL, Otieno J, Slutsker L, Lal AA, Shi YP. Evaluation of various methods of maternal placenta blood collection for immunology studies. Clin Vaccine Immunol. 2006;13:568–574. doi: 10.1128/CVI.13.5.568-574.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman P, Wanzira H, Tumwine G, Arinaitwe E, Waldman S, Achan J, Havlir D, Rosenthal PJ, Dorsey G, Clark TD, Cohan D. Placental malaria among HIV- infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snounow G, Viryakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosairio VE, Thaithong S, Brown KN. High sensitivity of detection of human parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 22.Zanzibar Malaria Control Program and Ministry of Health . ZMCP Annual Report. 2012. [Google Scholar]
- 23.Zanzibar Malaria Control Program and Ministry of Health . ZMCP Annual Report. 2011. [Google Scholar]
- 24.WHO Malaria Policy Advisory Committee and Secretariat Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar J. 2012;11:424. doi: 10.1186/1475-2875-11-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institut National de la Statistique du Rwanda (INSR) and ICF Macro . Enquête intermédiaire sur les indicateurs démographiques et de santé Rwanda 2007–2008 Ministere de la Sante (MINISANTE) 2009. [Google Scholar]
- 26.WHO Evidence Review Group on Intermittent Preventive Treatment (IPT) of Malaria in Pregnancy . Draft Recommendations on Intermittent Preventive Treatment in Pregnancy (IPTp) 2013. http://www.who.int/malaria/mpac/mpac_sep13_erg_ipt_malaria_pregnancy_report.pdf Available at. Accessed October 4, 2013. [Google Scholar]
- 27.Smith DL, Cohen JM, Moonen B, Tatem AJ, Sabot OJ, Ali A, Mugheiry SM. Infectious disease. Solving the Sisyphean problem of malaria in Zanzibar. Science. 2011;332:1384–1385. doi: 10.1126/science.1201398. [DOI] [PubMed] [Google Scholar]
- 28.Al-mafazy A, McElroy P, Msellem M, Molteni F, Ramsan M, Kakuko J, Murugasampillay S, Njau R, Jiddawi M, Ali AS. Atlanta, GA: 2012. Examination of surveillance data milestones as Zanzibar transitions to the malaria pre-elimination phase, 2008–2012. Proceedings of the 61st American Society of Tropical Medicine and Hygiene Annual Meeting, November 11–15, 2012. [Google Scholar]
- 29.Tagbor H, Jane B, Mitchel A, Brian G, Daniel C. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS ONE. 2010;5:e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njau J, Kabanywanyi A, Goodman C, Macarthur JR, Kapella BK, Gimnig JE, Kahigwa E, Bloland PB, Abdulla SM, Kachur SP. Adverse drug events resulting from use of drugs with sulphonamide-containing anti-malarials and artemisinin-based ingredients: findings on incidence and household costs from three districts with routine demographic surveillance systems in rural Tanzania. Malar J. 2013;12:236. doi: 10.1186/1475-2875-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Hawley W, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68((Suppl)):121–127. [PubMed] [Google Scholar]
- 33.Ndyomugyenyi R, Clarke SE, Hutchison CL, Hansen KS, Magnussen P. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually-randomised placebo-controlled trial using intermittent preventive treatment and insecticide-treated nets in the Kabale Highlands, southwestern Uganda. Trans R Soc Trop Med Hyg. 2011;105:607–616. doi: 10.1016/j.trstmh.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]