Abstract
Malaria is endemic in the Chittagong Hill Districts of southeastern Bangladesh. Previous epidemiological analyses identified the agricultural practice of jhum cultivation as a potential risk factor for malaria infection. We conducted qualitative interviews with jhum cultivators and surveillance workers to describe jhum cultivation and used demographic and malaria surveillance in two study unions from May of 2010 to August of 2012 to better understand the relationship between jhum cultivation and malaria infection. Qualitative interviews revealed that jhum cultivation is conducted on remote, steep hillsides by ethnic tribal groups. Quantitative analyses found that adult jhum cultivators and individuals who live in the same residence had significantly higher incidence rates of symptomatic Plasmodium falciparum infection compared with non-cultivators. These results confirm that jhum cultivation is an independent risk factor for malaria infection and underscore the need for malaria testing and treatment services to reach remote populations in the Chittagong Hill Districts.
Background
In Bangladesh, malaria is endemic in 13 of 64 districts.1 Malaria transmission is hypoendemic and occurs in seasonal epidemics, with the bulk of malaria infections and deaths occurring during the rainy season from May to October.2,3 The intensity of malaria transmission is variable across endemic districts, with the highest estimated malaria incidence in the Chittagong Hill Districts of southeastern Bangladesh.2,4 Malaria infections in Bangladesh are predominantly caused by Plasmodium falciparum; however, as many as 20% of infections are reported to be caused by P. vivax.1,3,5
In 2009, the International Center for Diarrhoeal Disease Research, Bangladesh (icddr,b) and the Johns Hopkins Malaria Research Institute initiated a malaria surveillance system in two demographically defined unions of the malaria-endemic Chittagong Hill Districts. The purpose of this project was to conduct surveillance for symptomatic and asymptomatic malaria infection and understand the epidemiology of malaria in the region.6 Rajbila and Kuhalong unions (subdivisions of Bandarban city subdistrict) were selected for the study area, comprising a total population greater than 20,000 people and 4,500 households.
Epidemiological analyses of symptomatic P. falciparum infection over the first 2 years of the surveillance system identified risk factors for malaria infection in this region.7 One risk factor identified was the practice of jhum cultivation, a traditional agricultural practice using slash-and-burn techniques in the hilly parts of the region.8,9 Jhum cultivation is a form of shifting agriculture practiced by non-Bengali indigenous groups in the Chittagong Hill Districts, of which there are 12 distinct groups.10,11 This practice was associated with the highest malaria incidence among any occupation group and increased the odds of infection by a factor of 1.5 compared with other occupations after controlling for other factors.7
Little is known about how the practice of jhum cultivation relates to malaria transmission in the Chittagong Hill Districts of Bangladesh. Some possible mechanisms for the observed increased malaria incidence in those individuals who practice jhum cultivation compared with other occupations might include increased exposure to mosquitoes because of working in remote areas and at times when mosquitoes are biting, lack of bed net use because of jhum cultivators sleeping away from the home, lack of access to malaria treatment and services because of factors related to jhum cultivation, and that jhum cultivation is associated with other unknown risk factors for malaria infection. Given that jhum cultivation is practiced throughout the Chittagong Hill Districts as well as in Tripura state in India12 and throughout the hilly regions of southeast Asia, where malaria is endemic,13 there may be a large population of cultivators at increased risk for malaria infection.
This study seeks to address the hypothesized relationship between jhum cultivation and malaria infection using both quantitative and qualitative methods. We conducted quantitative analyses based on 28 months of surveillance data from Rajbila and Kuhalong unions, focus group discussions with jhum cultivators, and key informant interviews with icddr,b staff who are knowledgeable about the practice. The objectives of this study are to describe the practice of jhum cultivation and use quantitative methods to confirm that jhum cultivation is an independent risk factor for malaria infection.
Materials and Methods
Study population.
In total, 24,491 individuals in 4,766 households were captured by the demographic surveillance system from October of 2009 to August of 2012. Individuals who migrated out of the study area (N = 409) or died (N = 8) before May of 2010 were removed from the dataset before analysis, leaving a total population of 24,074. This population value inflates the true population at any one point in time, because some of these individuals died (N = 215) during the study interval and in-migration and out-migration (both permanent and seasonal) were common throughout the study period. Both Rajbila and Kuhalong were divided into 12 study clusters for programmatic purposes, comprising approximately 1,000 people per cluster.
Study design.
A population census was conducted from March to April of 2009 to collect global positioning system (GPS) locations of households and count the number of people in the study area. Using a baseline list of households, surveillance workers also collected sociodemographic information at the individual level from October of 2009 to August of 2010. After the initial demographic survey was completed, surveillance workers collected follow-up demographic information approximately every 4 months to ascertain in-migration, out-migration, pregnancies, births, and deaths.6
Passive malaria surveillance was initiated in October of 2009 in Kuhalong and April of 2010 in Rajbila to identify symptomatic malaria cases. Individuals reporting fever to the surveillance workers were tested for malaria at the village level by blood smear microscopy and rapid diagnostic tests and treated with artemether/lumefantrine if either test was positive. Positive individuals were then retested at 2, 7, and 28 days after infection to ensure successful treatment.
A jhum cultivation-specific survey was administered to the study population in May of 2011. The survey included questions to identify those individuals living in jhum cultivation households and those individuals who conducted jhum cultivation in the previous year as well as questions about the initiation and completion of jhum cultivation and bed net use. Most jhum cultivators practice jhum cultivation each year; therefore, this survey was used to identify individuals as jhum cultivators during the entire study period. Survey questionnaires were electronically scanned, reviewed by data entry personnel, and then cleaned and tabulated by the study team to ensure data quality.
Statistical analyses.
We conducted statistical analyses of these existing surveillance data to determine the effect of jhum cultivation on malaria incidence over the study period. Jhum cultivators were identified through either the demographic survey as an occupation or the jhum cultivation-specific survey. Individuals were defined as jhum cohabitants if they did not conduct jhum cultivation and were living in a jhum household, which is a household with at least one jhum cultivator present. Individuals living in non-jhum households and not reporting conducting jhum cultivation were defined as non-jhum cultivators.
Demographic variables of interest were coded into binary and categorical variables for analysis. Binary variables included sex (male or female), bed net use the previous night (yes or no), and study union (Rajbila or Kuhalong). Ethnicities included non-tribal Bengali, Marma, Tanchangya, Tripura, Chakma, Khyang, and other tribal groups (Bawm, Mro, and Rakhaine). Age was divided into infants < 6 months old, children 6–59 months old, children 5–14 years old, and adults ≥ 15 years old. Education level was defined as low (0–2 years), medium (3–5 years), and high (≥ 6 years). Occupations included jhum cultivation, daily labor, agriculture, and other occupations (mostly housewives, students, and unemployed). Because of the nature of the surveys, individuals could report jhum cultivation and another profession, but because of the hypothesized risk associated with jhum cultivation, these individuals were coded as jhum cultivation only.
Malaria infection in these analyses was restricted to symptomatic (identified by passive surveillance) P. falciparum infections identified between May of 2010 and August of 2012. Cases that occurred from May to October were defined as high-transmission season cases, and cases that occurred from November to April were defined as low-transmission season cases. Asymptomatic infections (N = 66) and symptomatic P. vivax infections (N = 24) were not coded as positive cases. Cases in the same individual that occurred within 1 month of each other (31 days) were considered the same case, and one record was removed (N = 1). Because some individuals had multiple infections during the study period (N = 40), their demographic information was included for each time that they had an infection in the dataset. However, because these cases only represented 0.2% of the dataset, we do not believe that these records biased our results in any significant way.
The incidence of symptomatic P. falciparum infection was calculated from May of 2010 to August of 2012. Incidence rates were calculated by dividing the total number of cases by the total person-time at risk (in months) of that group over the study period. Person-time at risk was determined for each individual by subtracting time not at risk to malaria (before birth or after death) from 28 months (the total study period). Incidence rates were presented as the number of cases per 1,000 individuals per month and calculated for both the high- and low-transmission seasons. Statistical significance was determined using Poisson regression. All statistical analyses were performed in Stata (version 12.1; StataCorp LP 2011, College Station, TX).
The timing and demographics of jhum cultivation.
The dates of initiation and completion of jhum cultivation in the year preceding May of 2011 were plotted as box plots to display the median date and interquartile range of jhum initiation and completion. The rainy monsoon season was overlaid to show how the timing of jhum cultivation relates to seasonal rainfall patterns. Demographic variables of interest were tabulated across jhum cultivators, jhum cohabitants, and non-jhum cultivators to compare the demographics of the groups.
The locations of jhum and non-jhum households as well as study clusters were mapped in ArcGIS (version 10.1; ESRI, Redlands, CA). Households were labeled by ethnicity, and study clusters were labeled based on the percentage of the study population that participated in jhum cultivation to show spatial variation in jhum cultivation. Jhum cultivators who were identified through the jhum cultivation-specific survey were asked about bed net use during jhum cultivation. Bed net use was divided into everyday use, use 1–6 days a week, and non-use, and it was tabulated across jhum cultivators.
Malaria risk among jhum cultivators.
The relationship between jhum cultivation and malaria infection was assessed by comparing the incidence of symptomatic P. falciparum infection among jhum cultivators, jhum cohabitants, and non-jhum cultivators. To compare the risk factors for symptomatic P. falciparum infection in jhum cultivators and non-jhum cultivators ≥ 15 years old, incidence rates were calculated for both groups across all sociodemographic categories investigated. Finally, the overall incidence of symptomatic P. falciparum infection for all study participants in each study cluster was plotted against the percentage of the population that participated in jhum cultivation to determine the ecological association between jhum cultivation and malaria incidence at the study cluster level.
The independent relationship between jhum cultivation and malaria infection was determined using multivariate logistic regression modeling. Odds ratios comparing the odds of malaria infection among jhum cultivators and jhum cohabitants with the odds among non-jhum cultivators were adjusted based on other demographic variables. A variable categorizing individuals as living in low, medium, and high malaria incidence areas was included in the model to control for spatial variation in malaria incidence. This variable defined each individual as living in low-incidence clusters (the eight lowest incidence clusters), medium-incidence clusters (the middle eight incidence clusters), and high-incidence clusters (the eight highest incidence clusters). Univariate associations between all covariates of interest and malaria infection were calculated, and a final multivariate logistic regression model was selected using forward and backward selections, where covariates were sequentially selected or removed based on a likelihood ratio test (P < 0.1). The final model was assessed for colinearity using variance inflation factors and goodness of fit using Hosmer–Lemeshow and Pearson's χ2 tests.
Qualitative analyses.
Focus group discussions collected information about the community beliefs, attitudes, and experiences of jhum cultivators as well as their perceptions of health-seeking behavior, malaria infection, and bed net use during jhum cultivation. All focus group discussions occurred at the village level in the local language (Marma). A total of 58 jhum cultivators participated in focus groups discussions. Focus group discussions lasted 45–75 minutes and ranged across many topics, including general information about jhum cultivation, sleeping habits and bed net use during jhum cultivation, perceptions of malaria risk while conducting jhum, and health-seeking behavior when individuals become sick during jhum.
Key informant interviews gathered basic information about the practice of jhum cultivation, including the timing of the practice, the different phases of jhum cultivation, the demographics of jhum cultivators, and where jhum cultivation occurs. Of eight key informant interviews, six interviews were taken from local surveillance workers (three interviews in Marma and three interviews in Bengali), one interview was taken from the medical officer in English, and one interview was taken from the field research manager in English. Interviewees were not reimbursed for their time, and interviews lasted 30–40 minutes. It should be noted that two of the key informants (the medical officer and field research manager) were involved the design of the study, and one informant conducted interviews (three key informant interviews and all focus group discussions); however, these interviews were conducted before any other interviews had occurred to avoid biasing their responses.
All qualitative interviews were recorded using an Olympus Linear PCM Recorder (product LS-7; Olympus America Inc., Center Valley, PA) and subsequently translated into English. Translations were transcribed using Inqscribe (version 2.1; Inquirium LLC 2012, Chicago, IL) and analyzed in NVivo (version 10.0; QSR International Ply. Ltd. 2012, Victoria, Australia) using a codebook to identify specific themes that occurred during the focus groups and interviews.
Ethical considerations.
This study and all study documents were approved by both the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (00004557) and the icddr,b Ethical Review Committee (09021). At the creation of the surveillance system, the icddr,b field research team worked with local administration and leaders to gather permission to work in the study area. The team visited each household and took consent from the head of each household to collect personal information and include the household in malaria surveillance. A copy of the consent form and a completed Family Visit Register was given to each household.
Written or oral consent was taken from key informants and participants in focus groups discussions at the time of the interview. On the day of the interview, the consent form was explained to participants, and consent was taken in the form of a signature or a fingerprint if the participant could not write his or her name. If oral consent was given, a local village leader provided consent to ensure informed consent was properly taken from the participant.
Results
The process of jhum cultivation.
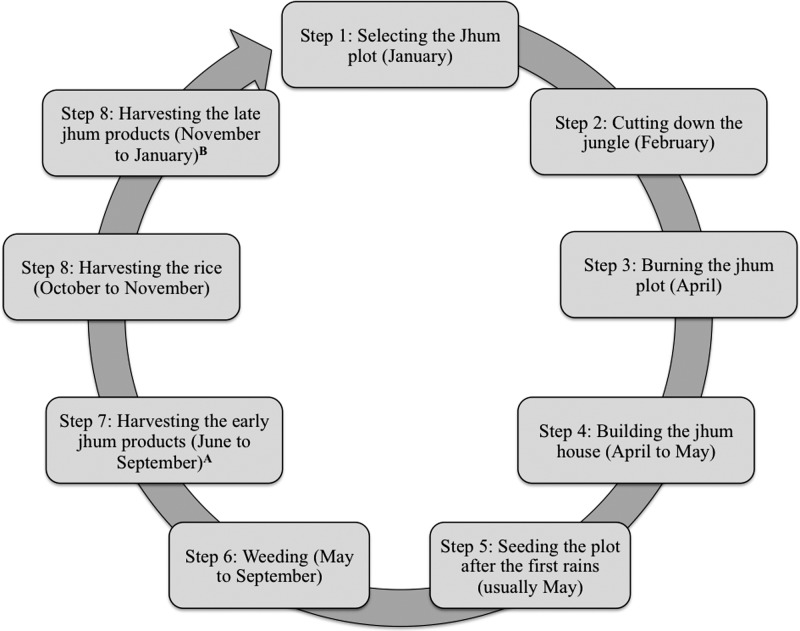
The process of jhum cultivation was investigated through key informant interviews with local surveillance workers and focus group discussions with jhum cultivators. One surveillance worker described jhum cultivation as “…a process of cultivation where a family or group of families go to a significant hilly place and plant varieties of seeds in order to get their crops for livelihoods.” Both jhum cultivators and surveillance workers reported that jhum cultivation occurs strictly on hillsides in the remote areas of the Chittagong Hill Districts. Respondents reported that cultivation is initiated with the clearing and burning of plots from January to March followed by the planting of various types of seeds, including sticky rice, in May or June after the monsoon rains have started (Figure 1). Jhum cultivators reported that they often stay at jhum sites in small houses made of bamboo during the summer months to protect the crops, remove weeds, and collect early crops. The final step is the harvest of the sticky rice and other crops in September or October.
Figure 1.
Annual timing of jhum cultivation. Respondents were asked about the process and timing of jhum cultivation in focus groups discussions with jhum cultivators (N = 58) and key informant interviews with icddr,b staff (N = 8). These results are based on the most common themes provided by respondents. Other than the process of jhum cultivation, respondents also reported that cultivators lived at the jhum cultivation site from July to October. A Indicates maize, cucumbers, chili peppers, bamboo shoots, sour leaf, coriander, cilantro, and turmeric flower. B Indicates turmeric, ginger, til seeds, pumpkin, white pumpkin, chili peppers, coos coos, and cotton.
The quantitative research findings about the timing of jhum cultivation show that, contrary to focus group discussions, where cultivators reported initiating jhum cultivation in January, the middle 50% (interquartile range) of the initiation dates from the jhum-specific survey were between February 11 and April 12, and the median date was April 10 (Figure 2). However, the majority of survey respondents reported completing jhum during November, with a median date of completion of November 17.
Figure 2.
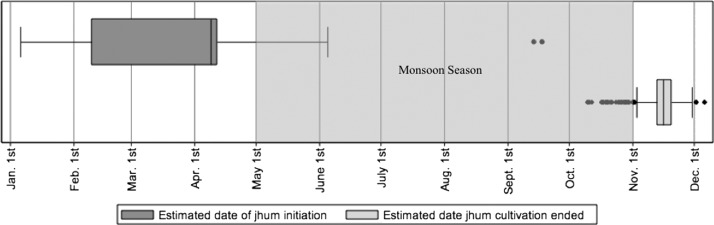
Timing of jhum cultivation and rainfall in Bandarban District from January to December of 2010. A short questionnaire provided to jhum cultivators estimated the dates that individual jhum cultivators initiated and completed jhum cultivation in 2010. Dates of initiation were plotted as box plots, which show the interquartile range of dates (the edges of the box) and the median date (the line through the box). Dates of initiation and completion were reported to the estimated date by the jhum cultivator. The shaded area represents the timing of the rainy monsoon season.
Demographics.
Sociodemographic information was tabulated across jhum cultivators, jhum cohabitants, and non-jhum cultivators to identify sociodemographic differences between the groups (Table 1). Of 24,074 individuals captured by demographic surveillance from May of 2010 to August of 2012, 2,631 (11.3%) individuals reported participating in jhum cultivation. Compared with non-jhum cultivators, these individuals were older (95.9% ≥ 15 years old compared with 61.1%), more likely to be of tribal ethnicity (99.9% compared with 74.5%), less likely to use bed nets (86.7% compared with 90.4%), and more likely to be male (50.8% compared with 48.8%). Jhum cultivators also showed different patterns of education and occupation compared with non-jhum cultivators. Jhum cohabitants were the youngest of the three groups, with 68.0% of jhum cohabitants being younger than 15 years compared with 29.8% for non-jhum cultivators and just 4.1% for jhum cultivators.
Table 1.
Sociodemographic characteristics of jhum cultivators and non-jhum cultivators
| Sociodemographic factors | Total | Non-jhum cultivators | Jhum cultivators | Jhum cohabitants |
|---|---|---|---|---|
| Union | ||||
| Rajbila | 10,789 (44.8) | 8,466 (44.8) | 1,217 (46.2) | 1,106 (43.6) |
| Kuhalong | 13,285 (55.2) | 10,440 (55.2) | 1,414 (53.8) | 1,431 (56.4) |
| Sex | ||||
| Male | 11,791 (49.0) | 9,226 (48.8) | 1,336 (50.8) | 1,229 (48.4) |
| Female | 12,283 (51.0) | 9,680 (51.2) | 1,295 (49.2) | 1,308 (51.6) |
| Age | ||||
| < 6 months | 1,098 (4.6) | 884 (4.7) | 0 (0.0) | 214 (8.4) |
| 6–59 months | 2,683 (11.1) | 2,141 (11.3) | 7 (0.3) | 535 (21.1) |
| 5–14 years | 5,409 (22.5) | 4,331 (22.9) | 101 (3.8) | 977 (38.5) |
| ≥ 15 years | 14,884 (61.8) | 11,550 (61.1) | 2,528 (95.9) | 811 (32.0) |
| Ethnicity | ||||
| Bengali (non-tribal) | 5,019 (20.9) | 5,007 (26.5) | 3 (0.1) | 9 (0.4) |
| Marma | 14,411 (59.9) | 10,660 (56.4) | 1,999 (76.0) | 1,752 (69.1) |
| Tanchangya | 2,142 (8.9) | 1,671 (8.8) | 194 (7.4) | 277 (10.9) |
| Khyang | 1,155 (4.8) | 880 (4.7) | 137 (5.2) | 138 (5.4) |
| Chakma | 824 (3.4) | 458 (2.4) | 185 (7.0) | 181 (7.5) |
| Tripura | 402 (1.7) | 162 (0.9) | 88 (3.3) | 152 (6.0) |
| Other tribal | 108 (0.5) | 55 (0.3) | 25 (1.0) | 28 (1.1) |
| Education, years (age ≥ 15 years) | ||||
| 0–2 | 8,774 (59.0) | 6,423 (55.6) | 1,904 (75.5) | 447 (55.1) |
| 3–5 | 2,731 (18.4) | 2,221 (19.2) | 360 (14.2) | 150 (18.5) |
| 6–8 | 3,379 (22.7) | 2,906 (25.2) | 259 (10.3) | 214 (26.4) |
| Occupation (age ≥ 15 years)* | ||||
| Agricultural | 5,633 (37.9) | 4,489 (38.9) | 936 (37.1) | 208 (25.7) |
| Daily labor | 2,255 (15.2) | 1,968 (17.0) | 204 (8.1) | 83 (10.3) |
| Jhum cultivation only | 999 (6.7) | − | 999 (39.6) | − |
| Other | 5,997 (40.3) | 5,093 (44.1) | 384 (15.2) | 520 (64.1) |
| Bed net use | ||||
| Yes | 19,087 (89.3) | 15,169 (90.4) | 2,239 (86.7) | 1,679 (83.1) |
| No | 2,290 (10.7) | 1,606 (9.6) | 343 (13.3) | 341 (16.9) |
| Total | 24,074 | 18,906 | 2,631 | 2,537 |
Respondents could report jhum cultivation and another occupation, because information about jhum cultivation and occupation status was acquired through two separate surveys.
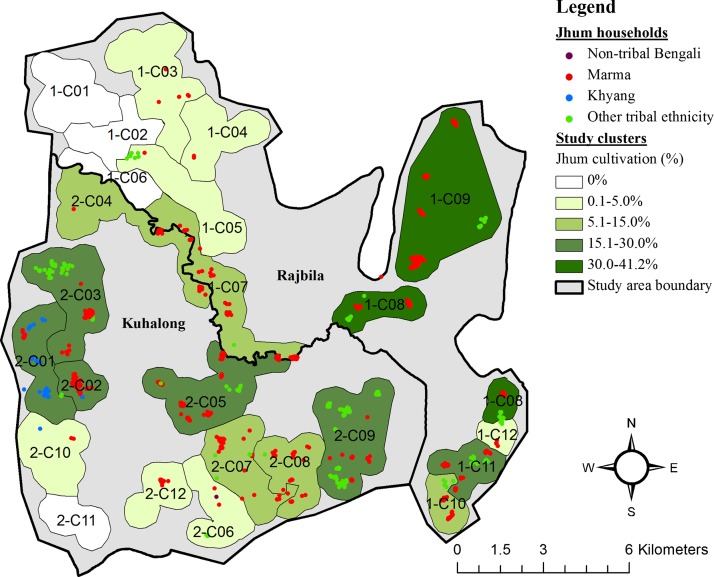
The locations of jhum households, defined as any household containing at least one jhum cultivator, are shown in Figure 3. The map displays the location and ethnicity of each jhum household as well as the percentage of the total population for all study clusters that participated in jhum cultivation during the study period. Among 24 study clusters, < 5% of the population participated in jhum cultivation in 11 clusters, and > 15% of the population conducted jhum in 8 clusters. One cluster in Rajbila union had the highest percentage of the population practicing jhum cultivation at 41.2%. In four clusters, 0% of the population reported conducting jhum cultivation. All but 2 of 1,048 households identified as jhum cultivation households were of tribal ethnicity.
Figure 3.
Locations of jhum households by ethnicity and study clusters labeled by the proportion of inhabitants who participated in jhum cultivation. Surveillance staff collected GPS locations for each household in the study area. Household locations were mapped in ArcGIS and labeled by ethnicity. Programmatic study clusters were also mapped and labeled by the percent of the total population that participated in jhum cultivation.
Malaria infection.
Passive malaria surveillance was conducted from May of 2010 to August of 2012 to detect symptomatic malaria infections. Only P. falciparum malaria cases were considered in this analysis. Jhum cultivators displayed the same temporal pattern of infections as non-jhum cultivators, with the majority of cases occurring in the high-transmission season from May to October, which coincides with the annual monsoon season when rainfall levels are highest. There was no significant difference in the percentage of cases that occurred in the high-transmission season between jhum cultivators (83 of 89 cases [93.3%]; 95% confidence interval = 85.9–97.5%) and non-jhum cultivators (170 of 194 cases [87.6%]; 95% confidence interval = 82.2–91.9%; χ2 test; P = 0.152).
The absolute numbers and incidence rates (per 1,000 per month) of symptomatic P. falciparum infections in the high- and low-transmission seasons for jhum cultivators, jhum cohabitants, and non-jhum cultivators are presented in Table 2. There was a statistically significant difference between the incidence rates of the three groups in both transmission seasons, with the highest rates in jhum cohabitants during both transmission seasons (P < 0.001). Jhum cultivators had an incidence rate of 2.22 infections per month per 1,000 people compared with 0.95 in non-jhum cultivators during the high-transmission season. However, in the low-transmission season, the incidence rates were nearly identical, with 0.19 and 0.17 infections per month per 1,000 people in jhum cultivators and non-jhum cultivators, respectively.
Table 2.
Symptomatic P. falciparum incidence rates among jhum cultivators, jhum cohabitants, and non-jhum cultivators
| N | High-transmission season | Low-transmission season | |||||
|---|---|---|---|---|---|---|---|
| No. of cases | Incidence* | P value | No. of cases | Incidence* | P value | ||
| Jhum cultivators | 2 | 90 | 2.22 | < 0.001 | 6 | 0.19 | < 0.001 |
| Jhum cohabitants | 2 | 94 | 2.41 | 25 | 0.85 | ||
| Non-jhum cultivators | 18 | 280 | 0.95 | 37 | 0.17 | ||
| Total | 24,074 | 464 | 1.23 | 68 | 0.24 | ||
Incidence is reported as the number of Rapid Diagnostic Test (RDT)- or blood smear-positive symptomatic P. falciparum cases per 1,000 per month.
Symptomatic P. falciparum incidence rates were also calculated across different sociodemographic characteristics for both adult jhum cultivators and non-jhum cultivators ≥ 15 years old (Table 3). Adult jhum cultivators had a monthly incidence rate of 1.27 infections per 1,000 individuals compared with 0.55 per 1,000 individuals in non-jhum cultivators. Males had statistically greater rates of infection than females for both jhum cultivators (P = 0.011) and non-jhum cultivators (P < 0.001). For jhum cultivators, there were significantly greater incidence rates in individuals living in Rajbila compared with individuals living in Kuhalong, with monthly incidence rates of 1.88 and 0.75 infections per 1,000 individuals (P < 0.001), respectively. Furthermore, jhum cultivators who reported using a bed net the previous night before the survey had almost one-half of the incidence rate as those jhum cultivators who did not (1.15 versus 2.24 infections per 1,000 individuals per month; P = 0.009). In adult non-jhum cultivators, however, there was no significant difference in the incidence rates observed in the two unions (P = 0.605) and between those individuals using and not using bed nets (P = 0.381).
Table 3.
Monthly symptomatic P. falciparum incidence rates across sociodemographic factors in jhum cultivators and non-jhum cultivators ≥ 15 years old
| Demographic factors | Jhum cultivators | Non-jhum cultivators | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Cases | Incidence* | P value | N | Cases | Incidence* | P value | |
| Union | ||||||||
| Rajbila | 1,169 | 61 | 1.88 | < 0.001 | 5,378 | 85 | 0.57 | 0.605 |
| Kuhalong | 1,354 | 28 | 0.75 | 6,172 | 90 | 0.53 | ||
| Sex | ||||||||
| Male | 1,240 | 58 | 1.61 | 0.011 | 5,627 | 105 | 0.67 | 0.003 |
| Female | 1,194 | 31 | 0.91 | 5,923 | 70 | 0.43 | ||
| Ethnicity | ||||||||
| Bengali (non-tribal) | 3 | 0 | 0 | −† | 2,626 | 30 | 0.41 | < 0.001 |
| Marma | 1,856 | 68 | 1.27 | 6,803 | 107 | 0.56 | ||
| Tanchangya | 180 | 7 | 1.35 | 1,072 | 15 | 0.50 | ||
| Khyang | 127 | 0 | 0 | 487 | 1 | 0.07 | ||
| Chakma | 174 | 5 | 1.03 | 294 | 12 | 1.41 | ||
| Tripura | 87 | 3 | 1.25 | 83 | 5 | 2.03 | ||
| Other tribal | 21 | 6 | 10.22 | 26 | 5 | 5.87 | ||
| Education level, years | ||||||||
| 0–2 | 1,904 | 64 | 1.21 | 0.741 | 6,423 | 101 | 0.57 | 0.094 |
| 3–5 | 360 | 14 | 1.39 | 2,221 | 41 | 0.66 | ||
| ≥ 6 | 259 | 11 | 1.53 | 2,906 | 33 | 0.40 | ||
| Bed net use | ||||||||
| Yes | 2,087 | 69 | 2.24 | 0.009 | 9,464 | 142 | 0.53 | 0.381 |
| No | 302 | 20 | 1.15 | 1,042 | 12 | 0.41 | ||
| Total | 2,523 | 89 | 1.27 | 11,550 | 175 | 0.55 | ||
Incidence is reported as the number of Rapid Diagnostic Test (RDT)- or blood smear-positive symptomatic P. falciparum cases per 1,000 per month.
Could not calculate a χ2 statistic, because there were no cases in the reference group (non-tribal Bengalis).
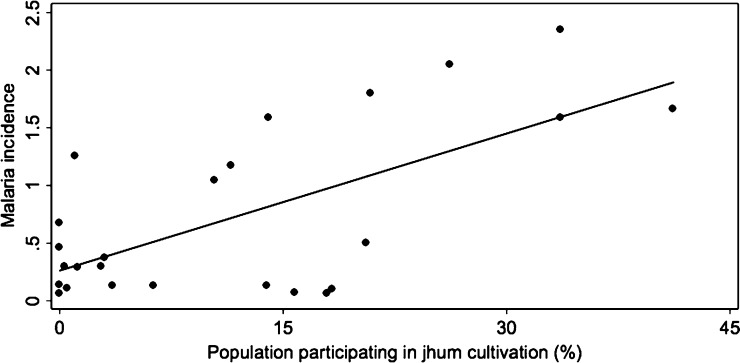
From an ecological perspective, there was an association between the level of jhum cultivation and the overall malaria incidence rate of a cluster (Figure 4). As the proportion of the population within a study cluster that participates in jhum cultivation increases, the incidence rate of symptomatic P. falciparum infection of that cluster increases (coefficient of determination [R2] = 0.417). However, with an R2 of 0.417, not all of the variation in malaria incidence is explained by the proportion of the population conducting jhum cultivation, and therefore, there are other factors that are responsible for spatial variation in malaria incidence.
Figure 4.
Ecological association between jhum cultivation and malaria incidence at the study cluster level. The percent of individuals participating in jhum cultivation and monthly symptomatic P. falciparum incidence were calculated for each study cluster and compared using linear regression analysis from May of 2010 to August of 2012. The percent of the population practicing jhum cultivation and symptomatic P. falciparum incidence were statistically associated (R2 = 0.417; P < 0.001) at the study cluster level.
The odds of malaria infection among jhum cultivators and jhum cohabitants compared with non-jhum cultivators when controlling for other demographic factors were determined using multivariate logistic regression modeling. After selecting a final multivariate model using forward and backward selections, both jhum cultivators (P = 0.001) and jhum cohabitants (P < 0.001) had 1.6-fold higher odds of malaria infection than non-jhum cohabitants after adjusting for age, sex, and the malaria incidence of the geographic area in which they live. Other predictors of malaria infection selected for the final model after controlling for spatial variation in malaria incidence were age, because children 5–14 years old had significantly higher odds of infection than individuals ≥ 15 years old (odds ratio = 1.8; P < 0.001), and sex, because men had marginally higher odds of infection than women (odds ratio = 1.2; P = 0.095).
The qualitative results addressing possible mechanisms of malaria infection during jhum cultivation are summarized in Table 5. In focus group discussions, jhum cultivators consistently reported that fever was common among jhum cultivators or during jhum, although it should be noted that participants often did not always differentiate between fever and malaria. Fever or malaria was listed as one of the major ailments during jhum in all five focus groups. Key informants, however, were much more mixed in their views of malaria infection during jhum cultivation. Of eight key informants interviewed, four informants specifically listed malaria as being more common during jhum cultivation, and three informants did not think that there was any connection.
Table 5.
Summary of qualitative findings on malaria infection, treatment, and prevention
| Hypothesized mechanism | Results |
|---|---|
| (1) Lack of bed net use during jhum cultivation | Jhum cultivators consistently reported bringing and using bed nets while living at jhum sites in both focus groups and the jhum-specific survey |
| Jhum cultivators reported that bed nets were important to protect from the bite of mosquitoes and other insects | |
| Key informants were doubtful that jhum cultivators used bed nets during cultivation | |
| (2) Increased exposure to mosquito vectors and malaria parasites during jhum cultivation | Both key informants and cultivators reported that mosquitoes are not only abundant but also, that they bite during the day at jhum sites |
| Jhum cultivation occurs during the rainy season, when mosquitoes are common and malaria infections are most prevalent | |
| Jhum cultivators tend to cluster at jhum sites, because cultivators take turns assisting one another with different phases of cultivation | |
| (3) Lack of access to malaria treatment services | Both key informants and cultivators reported that jhum cultivators often do not initially seek care when they become sick |
| Jhum cultivators reported seeking care; however, they often used other local treatments (such as hot chilies or drinking tea) so that they can stay at jhum sites and work | |
| (4) Malaria infection is associated with some other factor that is also associated with jhum cultivation | Although jhum cultivators reported that they believed that jhum cultivators have higher rates of malaria infection, key informants were mixed in their views of the relationship between jhum cultivation and malaria infection |
Common perceptions expressed by cultivators during discussions about the possible mechanism of jhum cultivation and malaria infection include exposure to mosquitoes while working jhum and walking to and from the jhum site, clustering of jhum cultivators, and a combination of the two factors. Both jhum cultivators and key informants reported that jhum cultivators live at the jhum site during much of jhum cultivation, which occurs during the rainy season, when cultivators have to be present to plant the seeds, remove weeds, harvest the crops, and protect the ripening crops from animals. Jhum cultivators noted that they often gather to help one another with different phases of jhum cultivation, leading to clustering of cultivators at jhum sites for days or weeks. Furthermore, both key informants and cultivators in focus group discussions reported that mosquitoes were more common at jhum sites. Mosquitoes were specifically mentioned as “common” and a “great problem,” and two key informants reported that, at jhum sites, mosquitoes are biting even during the daytime.
Jhum cultivators surveyed in the jhum-specific survey overwhelming reported high bed net use, with 94.9% of those individuals surveyed reporting using bed nets every night during jhum and 99.7% of respondents reporting using bed nets at least one time per week during jhum. These results are consistent with qualitative results about bed net use during jhum cultivation. Respondents in all focus groups reported owning and using bed nets during jhum. When asked about bed net use during jhum, one respondent replied: “Yes, we take the mosquito nets when we go to jhum and the mosquito nets are helpful to protect against … the bite of insects and mosquitoes.” Respondents in all five focus groups specifically listed mosquitoes as a reason to use bed nets. Among key informants, however, there was some disagreement as to whether jhum cultivators used bed nets. When asked if jhum cultivators used a bed net during jhum, four of eight key informants (50%) expressed some doubt.
Finally, both jhum cultivators and key informants reported that jhum cultivators did not have the same access to malaria services as other groups. Because jhum cultivation occurs in remote areas and cultivators often have to stay at sites to protect ripening crops from wild animals, jhum cultivators who have a fever often stay at sites until the illness becomes serious. Although many cultivators reported that, after someone becomes seriously sick, he or she returns to villages for treatment, others reported that some individuals use local remedies, such as hot chilies or teas, to treat fever.
Discussion
This study described the practice of jhum cultivation and confirmed that it is an independent risk factor for P. falciparum infection in the Chittagong Hill Districts of Bangladesh. Jhum cultivators had higher incidence rates of P. falciparum infection compared with non-jhum cultivators over 28 months of prospective malaria surveillance. Furthermore, this relationship was only statistically significant during the high-transmission season when jhum cultivation occurs, providing additional evidence that jhum cultivation is causally related to malaria infection. Finally, jhum cultivators had significantly greater odds of infection than non-cultivators after controlling for other predictors of infection in a multivariate logistic regression model. These results were consistent with qualitative interviews. Jhum cultivators in all five focus group discussions and four of seven key informants reported that malaria is more common during jhum cultivation, and both jhum cultivators and key informants reported that mosquitoes were more common at jhum sites and that jhum cultivators often do not immediately seek care when they become sick.
This study also suggests that jhum cultivation may facilitate malaria transmission to other individuals not involved with the practice. Jhum cohabitants or those individuals living with jhum cultivators but not participating in cultivation had the highest incidence rates of P. falciparum infection during both the high- and low-transmission seasons. Furthermore, multivariate logistic regression revealed that both jhum cultivators as well as jhum cohabitants had a 1.6-fold higher odds of infection compared non-jhum cultivators, controlling for age, sex, and spatial variation in malaria incidence. These results indicate that jhum cultivation not only affects individual risk to malaria infection but may also play a role in community transmission.
These results are in agreement with previous studies that show that agricultural practices may affect an individual's risk of malaria infection. Agricultural practices have been previously implicated as an occupational risk factor for malaria infection in other settings, including palm oil plantations in Papua New Guinea, irrigated rice paddies in Ethiopia and Cote d'Ivoire, and forest settings in Vietnam.14–17 In one study, agricultural irrigation was associated with greater abundance of anopheline vectors in rural Tanzania.18 In contrast, one study in Peru found that agricultural practices were statistically associated with lower malaria incidence rates.19 These studies show that a specific agricultural practice may impact malaria transmission, depending on the ecology of local anopheline vectors and the nature of the agricultural practice.
We hypothesized several mechanisms to explain this observed association between jhum cultivation and malaria incidence. Jhum cultivation may increase the time of exposure to mosquitoes because of the abundance of mosquitoes in remote areas where jhum cultivation occurs, lack of bed net use at jhum sites during the high-transmission season when many jhum cultivators live at jhum sites, lack of access to treatment when jhum cultivators are conducting jhum cultivation, or some unknown risk factor that is related to the practice.
Qualitative research results were consistent with the hypothesized mechanism that the abundance of mosquitoes at jhum sites leads to higher rates of infection in jhum cultivators. Both jhum cultivators and surveillance workers reported that mosquitoes are very common at jhum sites. This finding is consistent with entomology studies that have been conducted in the region,20,21 which show that two species of forest mosquitoes, Anopheles baimaii (An. dirus D) and An. minimus, are found in this region. Although both studies found that these mosquitoes are relatively uncommon compared with other species, mosquitoes were captured in the village setting and may not be representative of more remote regions where jhum cultivation takes place. Furthermore, several studies in Asia have observed increases in malaria vectors after deforestation, conceivably because it allows surface water accumulation at cleared sites.22 Although these studies looked at large-scale general deforestation, the same mechanism may apply at jhum sites after the removal of the jungle.
The results of this study do not support that lack of bed net use at jhum sites contributed to malaria incidence in jhum cultivators. Quantitative surveys revealed that 99.7% of jhum cultivators reported using a bed net (94.9% every night) during jhum. These results were confirmed by focus groups with jhum cultivators, who reported using bed nets during jhum and identified the need to protect against mosquitoes and malaria using bed nets. These results are also consistent with other reports on bed net use in the Chittagong Hill Districts, which have reported high-bed net use in the area.23,24
Another possible mechanism that was consistent with the results of this study is that lack of access to care may contribute to higher infection rates in jhum cultivators. Qualitative results found that jhum cultivators delayed treatment until the symptoms became severe because of the distance of jhum sites to the village and perceived need to stay at jhum sites and continue cultivation. Many cultivators also reported using herbal treatments, such as tea or chili peppers, instead of seeking medical treatment. These results are consistent with others studies of treatment-seeking behavior conducted in southeast Asia. Ahmed and others24 found that allopathic treatment is the most common form (> 90%) of malaria treatment in Bangladesh. Furthermore, studies in Myanmar and on the Thai–Cambodian border found that malaria treatment was often delayed in remote areas.25,26 Because jhum cultivators often work in large groups at various phases of cultivation, lack of prompt malaria treatment may facilitate local malaria outbreaks that can later be spread when jhum cultivators return to their villages.
There are a number of limitations of this study. Because of the nature of the demographic surveys in this study, jhum cultivators and non-jhum cultivators may have been misclassified. Jhum cultivators were identified during either the initial demographic survey or the jhum-specific survey in May of 2011. Therefore, only at two specific moments could individuals report conducting jhum, which may oversimplify the dynamics of occupations in this area. However, qualitative results revealed that most jhum cultivators conduct jhum cultivation every year; therefore, we do not believe that a meaningful percentage of respondents was misclassified.
Based on the qualitative research results that jhum cultivators only seek treatment when the illness becomes severe, one limitation of this study is that it may underestimate the incidence of malaria in jhum cultivators. If jhum cultivators do not seek treatment when they have a mild case of malaria, that case will not be captured by the surveillance system and counted in the study. Therefore, this study may have, in fact, underestimated the strength of the association between jhum cultivation and malaria infection.
Finally, this study could not assess the overall burden of malaria infection in the population, because it did not account for asymptomatic infections, which are believed to make up the burden of malaria infections in this region. Additional work is needed to assess the effect of jhum cultivation on asymptomatic infection.
Previous work in this area identified demographic, temporal, and spatial clusters of P. falciparum infection in this region.7 This study confirms that jhum cultivation is an independent risk factor for malaria infection in this region and may also play a role in community transmission by increasing the risk to those individuals living with jhum cultivators. The National Malaria Control Program in Bangladesh has been successful in reducing malaria incidence in the Chittagong Hill Districts by distributing insecticide-treated bed nets and conducting testing and treatment, but this study shows that there may be other populations that require more targeted approaches to further reduce infections. Expanding services to remote areas where cultivators work will be essential to decrease infection rates in these clusters and may be important to prevent transmission to high-risk groups, such as children living with jhum cultivators.
Table 4.
Unadjusted and adjusted odds of malaria infection of non-jhum cultivators, jhum cultivators, and jhum cohabitants
| Demographic factor | Unadjusted (N = 24,074) | Adjusted (N = 21,377) | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Occupation | ||||
| Non-jhum cultivator | 1.0 | 1.0 | ||
| Jhum cultivator | 2.2 (1.8–2.8) | < 0.001 | 1.6 (1.2–2.0) | 0.001 |
| Jhum cohabitant | 2.9 (2.3–3.6) | < 0.001 | 1.6 (1.2–2.0) | < 0.001 |
| Union | ||||
| Rajbila | 1.7 (1.4–2.0) | < 0.001 | −* | |
| Kuhalong | 1.0 | |||
| Sex | ||||
| Male | 1.2 (1.0–1.5) | 0.013 | 1.2 (1.0–1.4) | 0.075 |
| Female | 1.0 | 1.0 | ||
| Age | ||||
| < 6 months | −† | −† | ||
| 6–59 months | 1.3 (1.0–1.7) | 0.097 | 1.2 (0.8–1.6) | 0.390 |
| 5–14 years | 1.0 (1.7–2.4) | < 0.001 | 1.9 (1.6–2.4) | < 0.001 |
| ≥ 15 years | 1.0 | 1.0 | ||
| Ethnicity | ||||
| Bengali | 1.0 | −* | ||
| Tribal | 2.6 (2.0–3.5) | < 0.001 | ||
| Bed net use | ||||
| Yes | 0.7 (0.5–0.9) | 0.003 | −* | |
| No | 1.0 | |||
| Education level, years | ||||
| 0–2 | 1.0 | −* | ||
| 3–5 | 0.9 (0.7–1.1) | 0.391 | ||
| ≥ 6 | 0.6 (0.4–0.8) | < 0.001 | ||
| Geography (study cluster) | ||||
| Low incidence | 1.0 | 1.0 | ||
| Medium incidence | 4.2 (2.8–6.3) | < 0.001 | 3.7 (2.4–5.6) | < 0.001 |
| High incidence | 15.6 (10.7–22.6) | < 0.001 | 13.1 (8.9–19.3) | < 0.001 |
| Goodness-of-fit tests | χ2 (df) | |||
| Hosmer–Lemeshow | 7.4 (8) | 0.496 | ||
| Pearson's | 45.8 (43) | 0.355 | ||
CI = confidence interval; df = degree of freedom; OR = odds ratio.
Not selected into the final model using forward and backward selections.
Could not be modeled because of the lack of variation in the outcome (no positives cases in this group).
ACKNOWLEDGMENTS
We acknowledge the surveillance staff at the icddr,b field office in Bandarban for helping to arrange this study and participating in key informant interviews. We thank the local population for their participation in the study and their enthusiasm, especially those cultivators that participated in focus group discussions. Finally, we acknowledge the contribution of Intekhab Hossain, an intern at the icddr,b who helped with qualitative interviews and translations. We also thank Pablo Peñataro Yori for providing extensive scientific review of this manuscript.
Footnotes
Financial support: This study was funded by Johns Hopkins Malaria Research Institute at the Johns Hopkins Bloomberg School of Public Health Grant 00679. The icddr,b also gratefully acknowledges the following donors, which provide unrestricted support to research efforts: the Australian Agency for International Development (AusAID), the Government of the People's Republic of Bangladesh, the Canadian International Development Agency (CIDA), the Swedish International Development Cooperation Agency (SIDA), and the Department for International Development, United Kingdom (DFID). We thank the Johns Hopkins Center for Global Health and John Snow, Inc., which provided travel funding for a student investigator.
Authors' addresses: Sean R. Galagan, Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, and International Training and Education Center for Health (I-TECH), Department of Global Health, University of Washington, Seattle, WA, E-mail: sgalagan@uw.edu. Chai Shwai Prue, Jacob Khyang, Wasif Ali Khan, Sabeena Ahmed, Mohammad Shafiul Alam, M. Zahirul Haq, Jasmin Akter, and Peter Kim Streatfield, Centre for Population, Urbanization and Climate Change, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mails: dr_prue@icddrb.org, jacob@icddrb.org, wakhan@icddrb.org, sabeena@icddrb.org, shafiul@icddrb.org, mzhaq@icddrb.org, jakter@icddrb.org, and pkstreatfield@icddrb.org. Malathi Ram, Douglas E. Norris, Myaing Myaing Nyunt, Timothy Shields, David J. Sullivan, and David A. Sack, Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mails: mram@jhsph.edu, dnorris@jhsph.edu, mnyunt@jhsph.edu, tshields@jhsph.edu, dsulliva@jhsph.edu, and dsack@jhsph.edu. Gregory Glass, Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, and Global Biological Threat Reduction Program, Southern Research Institute, Birmingham, AL, E-mail: ggurrigl@jhsph.edu.
References
- 1.Ministry of Health and Family Welfare, Government of Bangladesh, World Health Organization, Country Office for Bangladesh . Dhaka, Bangladesh: Ministry of Health and Family Welfare, Government of Bangladesh; 2009. Annual Report: July 2008 to May 2009. [Google Scholar]
- 2.Reid H, Haque U, Clements ACA, Tatem AJ, Vallely A, Ahmed SM, Islam A, Haque R. Mapping malaria risk in Bangladesh using Bayesian geostatistical models. Am J Trop Med Hyg. 2010;83:861–867. doi: 10.4269/ajtmh.2010.10-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude RJ, Hasan MU, Hossain MA, Sayeed AA, Paul SK, Maude RR, Vaid N, Ghose A, Amin R, Samad R, Yunus EB, Rahman MR, Bengali AM, Hoque MG, Day NP, White NJ, White LJ, Dondorp AM, Faiz MA. Temporal trends in severe malaria in Chittagong, Bangladesh. Malar J. 2012;11:323. doi: 10.1186/1475-2875-11-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque U, Ahmed SM, Hossain S, Huda M, Hossain A, Alam MS, Mondal D, Khan WA, Khalequzzaman M, Haque R. Malaria prevalence in endemic districts of Bangladesh. PLoS ONE. 2009;4:e6737. doi: 10.1371/journal.pone.0006737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, Khanum H, Sullivan DJ, Jr, Haque R. Real-time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malar J. 2011;10:175. doi: 10.1186/1475-2875-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan WA, Sack DA, Ahmed S, Prue CS, Alam MS, Haque R, Khyang J, Ram M, Akter J, Nyunt MM, Norris D, Glass G, Shields T, Haq MZ, Cravioto A, Sullivan DJ., Jr Mapping hypoendemic, seasonal malaria in rural Bandarban, Bangladesh: a prospective surveillance. Malar J. 2011;10:124. doi: 10.1186/1475-2875-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, Galagan S, Scobie H, Khyang J, Prue CS, Khan WA, Ram M, Alam MS, Haq MZ, Akter J, Glass G, Nyunt MM, Shields T, Sullivan D, Sack DA. Malaria hotspots drive hypoendemic transmission in the Chittagong Hill Tracts of Bangladesh. PLoS One. 2013;8:e69713. doi: 10.1371/journal.pone.0069713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borggaard OK, Gafur A, Petersen L. Sustainability appraisal of shifting cultivation in the Chittagong Hill Tracts of Bangladesh. Ambio. 2003;32:118–123. doi: 10.1579/0044-7447-32.2.118. [DOI] [PubMed] [Google Scholar]
- 9.Gafur A, Jensen JR, Borggaard OK, Petersen L. Runoff and losses of soil and nutrients from small watersheds under shifting cultivation (Jhum) in the Chittagong Hill Tracts of Bangladesh. J Hydrol (Amst) 2003;274:30–46. [Google Scholar]
- 10.Human Development Research Center (HRDC) Socio-Economic Baseline Survey of the Chittagong Hill Tracts. Dhaka, Bangladesh: United Nations Development Programme, Bangladesh.; 2009. [Google Scholar]
- 11.Ahmed SM. Differing health and health-seeking behaviour: ethnic minorities of the Chittagong Hill Tracts, Bangladesh. Asia Pac J Public Health. 2001;13:100–108. doi: 10.1177/101053950101300208. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta M. Jhumias of Tripura. Econ Polit Wkly. 1986;21:1955–1960. [Google Scholar]
- 13.Rasul G, Thapa GB. Shifting cultivation in the mountains of South and Southeast Asia: regional patterns and factors influencing the change. Land Degrad Dev. 2003;14:495–508. [Google Scholar]
- 14.Pluess B, Mueller I, Levi D, King G, Smith TA, Lengeler C. Malaria—a major health problem within an oil palm plantation around Popondetta, Papua New Guinea. Malar J. 2009;8:56. doi: 10.1186/1475-2875-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erhart A, Thang ND, Van Ky P, Tinh TT, Van Overmeir C, Speybroeck N, Obsomer V, Hung LX, Thuan LK, Coosemans M. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kibret S, Alemu Y, Boelee E, Tekie H, Alemu D, Petros B. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop Med Int Health. 2010;15:41–50. doi: 10.1111/j.1365-3156.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- 17.Koudou BG, Tano Y, Keiser J, Vounatsou P, Girardin O, Klero K, Koné M, N'Goran EK, Cissé G, Tanner M, Utzinger J. Effect of agricultural activities on prevalence rates, and clinical and presumptive malaria episodes in central Côte d'Ivoire. Acta Trop. 2009;111:268–274. doi: 10.1016/j.actatropica.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Ijumba JN, Mosha FW, Lindsay SW. Malaria transmission risk variations derived from different agricultural practices in an irrigated area of northern Tanzania. Med Vet Entomol. 2002;16:28–38. doi: 10.1046/j.0269-283x.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- 19.Guthmann JP, Hall AJ, Jaffar S, Palacios A, Lines J, Llanos-Cuentas A. Environmental risk factors for clinical malaria: a case-control study in the Grau region of Peru. Trans R Soc Trop Med Hyg. 2001;95:577–583. doi: 10.1016/s0035-9203(01)90084-7. [DOI] [PubMed] [Google Scholar]
- 20.Alam MS, Khan MG, Chaudhury N, Deloer S, Nazib F, Bangali AM, Haque R. Prevalence of anopheline species and their Plasmodium infection status in epidemic-prone border areas of Bangladesh. Malar J. 2010;9:15. doi: 10.1186/1475-2875-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam MS, Chakma S, Khan WA, Glass GE, Mohon AN, Elahi R, Norris LC, Podder MP, Ahmed S, Haque R, Sack DA, Sullivan DJ, Jr, Norris DE. Diversity of anopheline species and their Plasmodium infection status in rural Bandarban, Bangladesh. Parasit Vectors. 2012;5:150. doi: 10.1186/1756-3305-5-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- 23.Ahmed SM, Hossain S, Kabir MM, Roy S. Free distribution of insecticidal bed nets improves possession and preferential use by households and is equitable: findings from two cross-sectional surveys in thirteen malaria endemic districts of Bangladesh. Malar J. 2011;10:357. doi: 10.1186/1475-2875-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed SM, Haque R, Haque U, Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J. 2009;8:173. doi: 10.1186/1475-2875-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wangroongsarb P, Satimai W, Khamsiriwatchara A, Thwing J, Eliades JM, Kaewkungwal J, Delacollette C. Respondent-driven sampling on the Thailand-Cambodia border. II. Knowledge, perception, practice and treatment-seeking behaviour of migrants in malaria endemic zones. Malar J. 2011;10:117. doi: 10.1186/1475-2875-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J-W, Xu Q-Z, Liu H, Zeng Y-R. Malaria treatment-seeking behaviour and related factors of Wa ethnic minority in Myanmar: a cross-sectional study. Malar J. 2012;11:417. doi: 10.1186/1475-2875-11-417. [DOI] [PMC free article] [PubMed] [Google Scholar]