Abstract
Human sparganosis is one of the neglected diseases but important food-borne parasitic zoonoses. The disease is caused by larvae (spargana) of diphyllobothriidean tapeworm. Here, we describe nine cases of human sparganosis, caused by Spirometra erinaceieuropaei in a hospital in Thailand during 2001–2012. Clinical characteristics, treatment, and outcome of cases were revealed. Diagnosis and identification of causative parasite species was made by histopathological investigations followed by a polymerase chain reaction-based molecular method using formalin-fixed paraffin embedded tissues. The DNA samples were extracted from tissues and a partial fragment of cytochrome c oxidase subunit 1 (cox1) gene was amplified for the detection of parasitic DNA. Infection could be prevented by increasing activities on health communication by responsible public health agencies.
Introduction
Humans are known to serve as the accidental host for several cestodes. Human sparganosis is one of the zoonotic diseases caused by plerocercoid type larvae called spargana of diphyllobothriidean tapeworm.1–3 The disease can be classified into two types, non-proliferative sparganosis caused by an infection with canine and feline tapeworm,4 genus Spirometra, and proliferative sparganosis caused by an infection with Sparganum proliferum.5 Human non-proliferative sparganosis is endemic mainly in East and Southeast Asia, especially in China, Japan, Korea, Taiwan, and Thailand.6–10 Genus Spirometra uses freshwater cycloploid copepods as the first intermediate host and a cluster of amphibians, reptiles, birds, and mammals as the second intermediate hosts/paratenic hosts.5,11 Infections with the different species show no evident differences in clinical presentation, except for the disease caused by S. proliferum, which is a serious migratory form.5,11 Human infection occurs by consuming raw or undercooked meat of the second intermediate hosts or paratenic hosts, including frogs and snakes. Infection also can occur by drinking water polluted with infected cycloploid copepods, or by the use of frog meat as a poultices.5,11 In humans, spargana invade the brain, eyes, central nervous system (CNS), breast, and subcutaneous tissues, causing the disease in local tissue damage, blindness, paralysis, and even death, and are a major threat to human health.6–10
In Thailand, there were 55 cases of sparganosis (53 non-proliferative and 2 proliferative) in the period of 1943–2011.10,12 This report describes nine cases of human sparganosis, caused by Spirometra erinaceieuropaei in a hospital in Thailand during 2001–2012. Diagnosis and identification of causative parasite species was made by histopathological investigations followed by molecular confirmation using formalin-fixed paraffin embedded (FFPE) tissues.
Materials and Methods
Patients and the specimens.
Between March 2001 and January 2012, nine patients were diagnosed with human sparganosis by clinical characteristics and pathological findings (Tables 1 and 2) at Siriraj Hospital, Mahidol University, Thailand. All patients underwent surgery. Based on a retrospective review of medical records, clinical characteristics such as age, symptoms, duration and location of symptoms, past diet history, radiographic findings, and treatment were obtained. The definite diagnosis of sparganosis was made from the histological findings. Hematoxylin and eosin stained sections from parafilm blocks reveal parasites surrounded with tegument and several calcareous bodies were found in the parasitic stroma. This study protocol was approved by the Siriraj Institutional Review Board Certificate of Approval (COA no. Si 189/2012). Informed consent was obtained from all human adult participants and from parents or legal guardians of minors.
Table 1.
Clinical characteristics of 9 cases of sparganosis from year 2001 to 2012*
| No. | Year | Age | Sex | Locations | Symptoms | Duration | Organ |
|---|---|---|---|---|---|---|---|
| 1 | 2001 | 51 | F | C | Firm mass at right lower eye lid | 2 mo | Lymph node of right lower eye lid |
| 2 | 2004 | 74 | F | C | Firm mass at suprapubic area | N/A | Abdominal wall at pubic region |
| 3 | 2004 | 26 | F | NE | Firm mass at left scapular area | 5 yr | Soft tissue of left scapular area |
| 4 | 2005 | 45 | F | S | Firm mass at right lower quadrant area of abdomen | 6 mo | Abdominal wall at right lower quadrant area of abdomen |
| 5 | 2007 | 26 | F | C | Firm mass at left arm | 2 mo | Soft tissue of left arm |
| 6 | 2008 | 26 | M | C | Complete ptosis and lateral rectus muscle palsy of left eye. Hearing loss of right ear. Hemiparesis of left leg. | 2 yr | Right cerebellopontine angle and left interpeduncular region. Serpiginous-masslike lesion at L1–L5 region. |
| 7 | 2008 | 51 | M | C | Firm mass at left chest wall. | 2 mo | Soft tissue of left chest wall. |
| 8 | 2011 | 31 | M | N | Left leg pain and numbness at left buttock | 6 mo | Edematous conus medullaris lesion with an irregular tubular lesion in the caudal sac from L4–S1. |
| 9 | 2012 | 46 | F | C | Low back pain, paraparesis with sensory impairment. | 2 mo | Intradural extramedullary serpiginous-cystic like lesion at C2–C5, C7, T1, T5–T10, L3–L5, S1. |
F = female; M = male; N = north; NE = northeast; C = central; S = south of Thailand; N/A = data not available.
Table 2.
Pathological finding, definite diagnosis, treatment, and outcome of nine cases of sparganosis from 2001 to 2012
| Case no. | Gross finding | Histological finding | Diagnosis | Treatment | Recovery |
|---|---|---|---|---|---|
| 1. [THA-Se1] KF539833 | An irregular piece of rubbery well-circumscribed mass measuring 1.8 × 1 × 0.8 cm, the cut surface shows grayish white and light brown non-homogenous tissue. | Acute necrotizing lymphadenitis with calcified parasite, probable Sparganum spp. like. | Necrotizing lymphadenitis of right lower eye lid (Cutaneous sparganosis). | Excision | Completed |
| 2. [THA-Se2] KF539834 | An irregular piece of rubbery light brown tissue measuring 4.5 × 1 × 0.5 cm. The cut surface shows brown non-homogenous tissue. | Probable Sparganum spp. liked cyst. | Subcutaneous mass of abdominal wall at suprapubic area (Cutaneous sparganosis). | Excision | Completed |
| 3. [THA-Se3] KF539835 | An irregular piece of rubbery light brown tissue measuring 4.5 × 4 × 2 cm. The cut surface shows a 1.5 × 1.5 × 0.7 cm yellow material containing cyst in muscular tissue. | Intramuscular wall of abscess with infected Sparganum spp. like. | Intramuscular mass at left scapular area (Cutaneous sparganosis). | Excision | Completed |
| 4. [THA-Se4] KF539836 | Irregular pieces of soft fibrofatty tissue, varying from 1.5, 2.2, and 2.5 cm. The cut surface shows fatty yellow homogenous tissue. | Parasitic infection, morphology identical to Sparganum spp. like. | Subcutaneous mass of abdominal wall at right lower quadrant area (Cutaneous sparganosis). | Excision | Completed |
| 5. [THA-Se5] KF539837 | Irregular pieces of soft light brown tissue, measuring 0.4 × 0.5 × 0.4 cm and 0.9 × 1.2 × 0.5 cm. | Parasitic infestation morphologically compatible with Sparganum spp. like. | Subcutaneous mass of left arm (Cutaneous sparganosis). | Excision | Completed |
| 6. [THA-Se6] KF539838 | It consists of multiple pieces of small irregular soft light brown and grey white tissue, measuring 1.2 × 1.1 × 0.3 cm. | Compatible with parasite infection compatible with Sparganum spp. like. | Sparganosis of brain (right cerebellopontine angle and left interpeduncular region) and spinal cord (L1–L5) (Cerebral and spinal sparganosis). | Laminoplasty at L1–L5 and duraplasty with tumor removal, Right median pressure ventriculoperitoneal shunt. | Partial |
| 7. [THA-Se7] KF539839 | It consists of 3 pieces of irregular soft light brown and grey white tissue, measuring from 1.2–3 cm in greatest dimension. | Parasitic infestation associated with necrotic tracts in the subcutaneous tissue, morphologically compatible with Sparganum spp. like. | Subcutaneous mass of left chest wall (Cutaneous sparganosis). | Excision | Completed |
| 8. [THA-Se8] KF539840 | It consists of a piece of irregular soft grey white tissue, 0.3 × 0.3 × 0.2 cm. | Parasitic infestation, cestode. morphologically suggestive of with Sparganum spp. like. | Sparganosis of spinal cord (conus medullaris, L4–S1) (Spinal sparganosis/ involved conus medullaris). | Laminotomy at L1–L4 with laminectomy at L5–S1 and partial cystic mass removal. | Partial |
| 9. [THA-Se9] KF539841 | Multiple pieces of light brown, soft tissue measure 1 × 1 × 0.7 cm. | Specimen consists of membranous tissue fragments. They are foreign tissue and composed of three distinctive layers. Consistent with Sparganum spp. cyst like. | Sparganosis of spinal cord (C2–C5,C7,T1,T5–T10, L3–L5, S1) (spinal sparganosis). | Laminectomy at T9–T11, L1–L2 with multiple debridement, corticosteroid, praziquantel and ivermectin therapy. | No |
DNA extraction, polymerase chain reaction (PCR), DNA sequencing, and sequence analysis.
For molecular identification of the causative parasite species, the parasites in the sections were confirmed by hematoxylin-eosin stain before preparing DNA. The DNA was extracted from 10 μm unstained serial sections (cut from the plerocercoid in the tissue of FFPE) attached to glass slides using a DEXPAT kit (TaKaRa Bio Inc., Shiga, Japan) as reported previously.13,14 The resulting supernatants were used as the DNA template for PCR. Amplification of a partial mitochondrial cytochrome c oxidase subunit 1 (cox1) gene by PCR was performed as shown in Table 3. A fragment of cox1 gene was amplified using the primers Se658-F and Se1124-R, which were designed from the cox1 gene of S. erinaceieuropaei (GenBank accession no. AB369250). The PCR was carried out using a GeneAmp PCR System 9700 (Applied Biosystems, Singapore). Amplified product was run on a 1% agarose gel and a 467 base pair (bp) fragment was cut and sequenced using the Applied Biosystems 3730 × I DNA Analyzer and ABI big dye Version 3.1 (Foster City, CA). The partial cox1 gene sequences of individual S. erinaceieuropaei from FFPE specimens of each patient were deposited in the GenBank Database with the accession nos. of KF539833–KF539841.
Table 3.
The primers and polymerase chain reaction conditions
| Primers | PCR master mixed (25 μL total volume) | PCR steps |
|---|---|---|
| Forward: Se658-F 5′-TTTGATCCTTTGGGTGGTGG-3′ | 2.5 μL of 10× FastStart High Fidelity Reaction buffer with 18 mM MgCl2 (Roche, Mannheim, Germany), 200 μM of dNTP, 0.2 μM of primer (Invitrogen, Carlsbad, CA), 0.625 units of FastStart High Fidelity Enzyme Blend (Roche). | Denatured 94°C 5 mins, 35 cycles at 95°C 30 sec, 59°C for 30 sec, 72°C 45 sec, a final extension at 72°C for 10 min. |
| Reverse: Se1124-R 5′-ACCACAAACCACGTGTCATG-3′ |
They were analyzed using BLAST-N search (National Center for Biotechnology Information, Bethesda, MD). Published cox1 sequences from S. erinaceieuropaei were aligned with our new sequences (alignment length 426 bp when trimmed to the length of the shortest sequence) using ClustalW15 and a maximum likelihood tree constructed using MEGA 5.2.16 The best-fit substitution model was determined in MEGA to be the Hasegawa-Kishino-Yano (HKY) model with uniform rates among sites, but assuming a proportion (0.65) of invariant sites. Sequences from Spirometra mansonoides (GenBank no. AF096239) were used as an out group.
Results
According to this study (N = 9) there have been 10 cases of sparganosis in Siriraj Hospital since 2001 (including Boonyasiri and others).12 Details of clinical characteristics of all patients were shown in Table 1. Subcutaneous sparganosis was six cases followed by CNS involvement (three cases). The CNS infections were all in spinal cord with one patient having both brain and spinal lesions. The mean age was 41.8 ± 16.2 years (range 26–74 years). There were six females and three males, a ratio of 2:1. Risk behaviors were not recorded in most cases. Only one patient had a history of eating uncooked snake meat (case no. 6, THA-Se6). Patients are mostly from the central part of the country (67%). The median duration before diagnosis was 4 months. Pathological findings, definite diagnosis, treatment, and outcome of cases were described in Table 2 and Figure 1. All patients underwent surgical excision or debridement for treatment. All of the cutaneous sparganosis patients were completely cured after excision and no recurrence after ∼1 year of a followed period.
Figure 1.
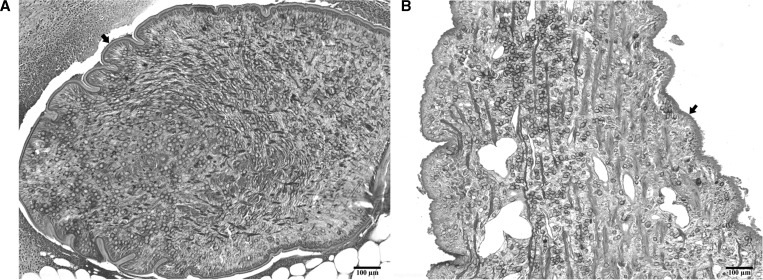
Representative plerocercoids detected in the paraffin-embedded sections used for molecular identification. (A) A plerocercoid (arrow) isolated from the subcutaneous nodule of the patient 4 (THA-Se4); (B) a plerocercoid (arrow) detected in the spinal cord from the patient 8 (THA-Se8). The sections were stained with hematoxylin-eosin. Scale bar = 100 μm.
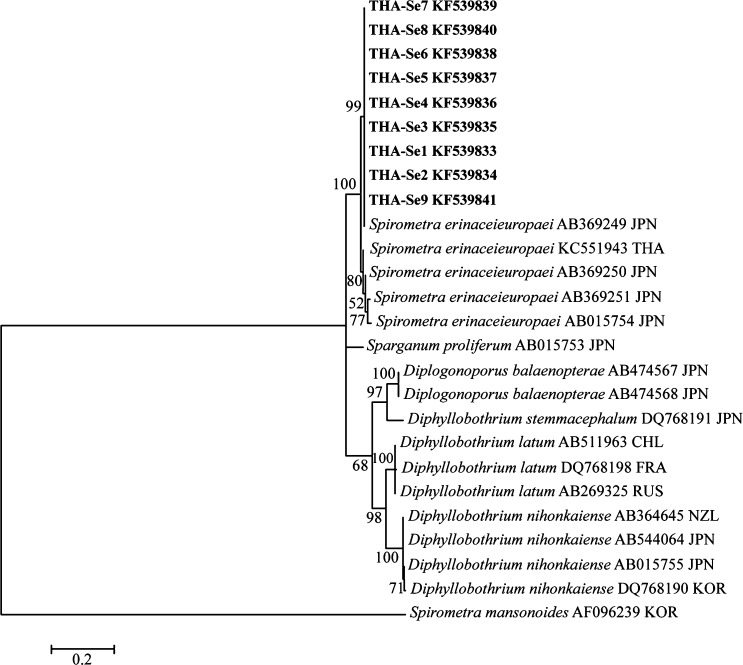
The partial cox1 sequence of parasite DNA recovered from nine patients, which were deposited in the GenBank database under the accession nos. KF539833–KF539841 (Table 2), was almost identical (97–99%) with those of S. erinaceieuropaei from various geographical localities Figure 2. From these results and the previous report, the parasites obtained from nine patients were identified as S. erinaceieuropaei.
Figure 2.
Maximum Likelihood tree based on partial cytochrome c oxidase subunit 1 (cox1) gene sequences. Sequences of Spirometra spp. and other tapeworm obtained from GenBank are indicated with accession number and country code (ISO 3166-1 alpha-3 codes). Spirometra erinaceieuropaei sequences of this study are presented in bold (KF539833-KF539841). The sequences were deposited in GenBank numbers as shown in Table 2. Numbers at the nodes indicate bootstrap P values (1,000 replicates).
Discussion
In Thailand, there were 55 sparganosis cases (53 non-proliferative and 2 proliferative).10,12,13 As was pointed out in their comprehensive review,10 previously Tesjaroen17 reviewed 34 cases of sparganosis in Thailand including 13 cases from Siriraj Hospital, the same hospital as this study. Because the overall sparganosis cases in Thailand is just over 60 cases including nine cases reported here, about one-third of the cases have been diagnosed and treated in the Siriraj Hospital, Mahidol University, Bangkok, Thailand. When Anantaphruti and others10 reviewed sparganosis cases in Thailand, 18 of 52 cases were ocular sparganosis. However, the majority of them was recorded before 1990 and has become rare since 1991. In this study, six cases were subcutaneous sparganosis and three cases were CNS involvement, but we did not come across ocular cases. The chronological changes of the clinical features of sparganosis in Thailand indicate the reduced access to the traditional medicine and persistence of eating habits.
Previous study of sparganosis in Thailand, only three cases were identified as infection with S. erinaceieuropaei plerocercoids by PCR-based molecular identification of the worms preserved as FFPE tissues.12,13 In this study, we added nine cases (one case was reported previously)12 of human sparganosis in Thailand with molecular identification of the causative parasite species as S. erinaceieuropaei. In this study, we used partial mitochondrial cox1 gene as a marker and found slight variation among the samples. Such an intraspecies variation is comparable with the intraspecies variation of cox1 gene of S. erinaceieuropaei previously reported.18 Although S. erinaceieuropaei plerocercoids is a causative agent of nonproliferative sparganosis in Asia/Oceania, S. mansonoides is proven as the causative agent of sparganosis in the Americas.4 This report is the biggest sparganosis case series in Thailand.
Present results together with the previous review10 clearly show that sparganosis remains an important food-borne zoonosis and infection can occur anywhere in Thailand. The infection is possibly caused by drinking water collected directly from ponds19 or eating uncooked meat, including frogs and snakes.10 In fact, in this study, case no. 6 (THA-Se6) had a history of eating raw snake meat. From the results described here, sparganosis is one of the neglected but important food-borne parasitic zoonoses in Thailand. Infection could be avoided by expanding focus on health communication regarding safe food and water by responsible agencies.
ACKNOWLEDGMENTS
We wish to acknowledge the support of the Khon Kaen University Publication Clinic, Research and Technology Transfer Affairs, Khon Kaen University, for their assistance.
Footnotes
Financial support: This study was supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004 to Pewpan M. Intapan and Wanchai Maleewong. This research was also funded by grants from the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Thailand through the Health Cluster (SHeP-GMS) and the Faculty of Medicine, Khon Kaen University.
Authors' addresses: Adhiratha Boonyasiri, Division of Clinical Epidemiology, Department of Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, E-mail: adhiratha.bon@mahidol.ac.th. Pornsuk Cheunsuchon, Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, E-mail: pcheunsuchon@gmail.com. Yupin Suputtamongkol, Division of Infectious Diseases and Tropical Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, E-mail: ysupattamongkol@gmail.com. Hiroshi Yamasaki, Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: hyamasak@nih.go.jp. Oranuch Sanpool, Wanchai Maleewong, and Pewpan M. Intapan, Department of Parasitology, Faculty of Medicine and Research and Diagnostic Center for Emerging Infectious Diseases, Khon Kaen University, Khon Kaen, Thailand, E-mails: sanpoolor@yahoo.com, pewpan@kku.ac.th, and wanch_ma@kku.ac.th.
References
- 1.Waeschenbach A, Webster BL, Bray RA, Littlewood DT. Added resolution among ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with complete small and large subunit nuclear ribosomal RNA genes. Mol Phylogenet Evol. 2007;45:311–325. doi: 10.1016/j.ympev.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Kuchta R, Scholz T, Bray RA. Revision of the order Bothriocephalidea Kuchta, Scholz, Brabec and Bray, 2008 (Eucestoda) with amended generic diagnoses and keys to families and genera. Syst Parasitol. 2008;71:81–136. doi: 10.1007/s11230-008-9153-7. [DOI] [PubMed] [Google Scholar]
- 3.Bray RA, Jones A, Andersen KI. Order Pseudophyllidea Carus, 1863. In: Khalil LF, Jones A, Bray RA, editors. Key to the Cestode Parasites of Vertebrates. Wallingford: CAB International; 1994. pp. 205–247. [Google Scholar]
- 4.Bowman DD, Hendrix CM, Lindsay DS, Barr SC. Feline Clinical Parasitology. Hoboken, NJ: Wiley-Blackwell; 2002. [Google Scholar]
- 5.Miyazaki I. Spirometriasis. In: Miyazaki I, editor. An Illustrated Book of Helminthic Zoonoses. Tokyo: International Medical Foundation of Japan; 1991. pp. 207–214. [Google Scholar]
- 6.Qiu MH, Qiu MD. Human plerocercoidosis and sparganosis: II. A historical review on pathology, clinics, epidemiology and control. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi (Chin J Parasitol Parasit Dis) 2009;27:251–260. [in Chinese with English abstract] [PubMed] [Google Scholar]
- 7.Cho SY, Bae JH, Seo BS. Some aspects of human sparganosis in Korea. Korean J Parasitol. 1975;13:60–77. doi: 10.3347/kjp.1975.13.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa M, Ouji Y, Nishiofuku M, Ishizaka S, Nawa Y. Sparganosis cases reported in Japan in the recent decades, 2000–2009. Clin Parasitol. 2010;21:33–36. [in Japanese] [Google Scholar]
- 9.Chung CC, Tsai BJ, Lin TY, Kuo HM. Cutaneous sparganosis: a case report. Dermatol Sinica (Taiwan) 2000;18:204–210. [English with Chinese abstract] [Google Scholar]
- 10.Anantaphruti MT, Nawa Y, Vanvanitchai Y. Human sparganosis in Thailand: an overview. Acta Trop. 2011;118:171–176. doi: 10.1016/j.actatropica.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Beaver PC, Jung RC, Cupp EW. Pseudophyllidean tapeworms. In: Beaver PC, Jung RC, Cupp EW, editors. Clinical Parasitology. Ninth edition. Philadelphia, PA: Lea & Febiger; 1984. pp. 494–504. [Google Scholar]
- 12.Boonyasiri A, Cheunsuchon P, Srirabheebhat P, Intapan PM, Yamasaki H, Maleewong W. Sparganosis presenting as cauda equine syndrome with molecular identification of a causative parasite species. Korean J Parasitol. 2013;51:739–742. doi: 10.3347/kjp.2013.51.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koonmee S, Intapan PM, Yamasaki H, Sugiyama H, Muto M, Kuramochi T, Kularbkeaw J, Kanpittaya J, Maleewong W, Nawa Y. Molecular identification of a causative parasite species using formalin-fixed paraffin embedded (FFPE) tissues of a complicated human pulmonary sparganosis case without decisive clinical diagnosis. Parasitol Int. 2011;60:460–464. doi: 10.1016/j.parint.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki H, Nakaya K, Nakao M, Sako Y, Ito A. Significance of molecular diagnosis using histopathological specimens in cestode zoonoses. Trop Med Health. 2007;35:307–321. [Google Scholar]
- 15.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesjaroen S. Sparganosis in Thais. Siriraj Hosp Gaz. 1991;43:743–749. [Google Scholar]
- 18.Okamoto M, Iseto C, Shibahara T, Sato MO, Wandra T, Craig PS, Ito A. Intraspecific variation of Spirometra erinaceieuropaei and phylogenetic relationship between Spirometra and Diphyllobothrium inferred from mitochondrial CO1 gene sequences. Parasitol Int. 2007;56:235–238. doi: 10.1016/j.parint.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Wiwanitkit V. A review of human sparganosis in Thailand. Int J Infect Dis. 2005;9:312–316. doi: 10.1016/j.ijid.2004.08.003. [DOI] [PubMed] [Google Scholar]