Abstract
Quality by design (QbD) concept is a paradigm for the improvement of botanical injection quality control. In this work, water precipitation process for the manufacturing of Xueshuantong injection, a botanical injection made from Notoginseng Radix et Rhizoma, was optimized using a design space approach as a sample. Saponin recovery and total saponin purity (TSP) in supernatant were identified as the critical quality attributes (CQAs) of water precipitation using a risk assessment for all the processes of Xueshuantong injection. An Ishikawa diagram and experiments of fractional factorial design were applied to determine critical process parameters (CPPs). Dry matter content of concentrated extract (DMCC), amount of water added (AWA), and stirring speed (SS) were identified as CPPs. Box-Behnken designed experiments were carried out to develop models between CPPs and process CQAs. Determination coefficients were higher than 0.86 for all the models. High TSP in supernatant can be obtained when DMCC is low and SS is high. Saponin recoveries decreased as DMCC increased. Incomplete collection of supernatant was the main reason for the loss of saponins. Design space was calculated using a Monte-Carlo simulation method with acceptable probability of 0.90. Recommended normal operation region are located in DMCC of 0.38–0.41 g/g, AWA of 3.7–4.9 g/g, and SS of 280–350 rpm, with a probability more than 0.919 to attain CQA criteria. Verification experiment results showed that operating DMCC, SS, and AWA within design space can attain CQA criteria with high probability.
Introduction
Quality by Design (QbD) is a systematic approach based on knowledge management and risk management [1]–[3]. It has become a paradigm for pharmaceutical industry to establish analytical methods [4]–[6], develop new manufacturing processes [7]–[12], and optimize existing manufacturing processes [13]. Manufacturing process optimization under the framework of QbD will benefit the target patient population, the pharmaceutical industry, and the regulation agency. Implementation of QbD concept usually contains several steps, such as critical quality attribute (CQA) definition, risk assessment, critical process parameter (CPP) determination, design space development, control strategy design, and continual improvement in drug lifecycle [13]–[15].
To keep batch-to-batch consistency, design space development is very important in the implementation of QbD concept. Parameter variations within design space are not considered to affect product quality. Quantitative models between process parameters and process CQAs should be established to develop design space. CPPs can be determined using risk assessment or design of experiments. Plackett–Burman design or fractional factorial design are usually applied to select CPPs [16]. In order to obtain design space, response surface methodology, such as central composite design [17] or Box-Behnken design [18], is widely applied. Recently, Rozet et al. suggested that design space is “a multivariate domain of input factors ensuring that critically chosen responses are included within predefined limits with an acceptable level of probability” [16]. The Monte-Carlo method is adopted by several researchers to calculate the probability in the development of analytical methods [19], [20].
Botanical drugs have gained increasing popularity in recent years. The compositions of most botanical drugs are very complicated. There is usually more than one distinct active ingredient in a botanical drug. Accordingly, the quality control of botanical drugs is difficult. Recently, Zhang et al. applied the QbD concept to develop botanical drug manufacturing processes [15].
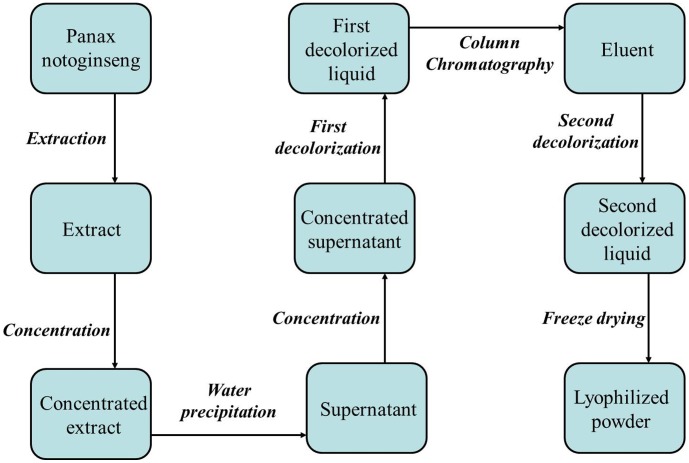
In this work, water precipitation process in the manufacturing of Xueshuantong powder was optimized in the framework of QbD concept as a sample. Xueshuantong powder was applied to treat retinal vein occlusion and cerebrovascular disease in China [21]. It is made from Notoginseng Radix et Rhizoma (Sanqi) through a series of unit operations, including extraction with a mixed ethanol-water solution, concentration, water precipitation, decolorization, column chromatography, and freeze drying, as shown in Figure 1 [22]. The active pharmaceutical ingredients (APIs) of Xueshuantong powder are saponins, including Notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rd, and ginsenoside Re.
Figure 1. The manufacturing processes of Xueshuantong powder.
The mixed solvent of ethanol and water is widely applied to extract active ingredients from medicinal herbs [23]–[26]. Water precipitation is a precipitation process usually used in the treatment of plant extracts extracted using ethanol solution. It can be realized by simply adding water into the concentrated extracts of plants. The purpose of this process is to remove impurities with weak polarity. In the production of Xueshuantong injection, the loss of saponins are observed in water precipitation. Therefore water precipitation process affects both drug efficacy and drug safety. However, there are very few publications on water precipitation.
In this work, water precipitation was optimized using a design space approach. Process CQAs of water precipitation were obtained through risk assessment. Process parameters are also discussed in risk assessment. Fractional factorial designed experiments were used to determine CPPs of water precipitation. Design space was calculated based on the Box-Behnken designed experimental results with a Monte-Carlo simulation method. Finally, design space was verified.
Experimental
Materials and Chemicals
Sanqi was collected from Wenshan of Yunnan Province (China) and purchased from Zhejiang Chinese Medical University Medical Pieces Co. Ltd. (Hangzhou, China). No specific permissions were required for the described field studies. The locations are neither privately owned nor protected by the Chinese government. No endangered or protected species were sampled.
Standard substances of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd were purchased from Shanghai Winherb Pharmaceutical Technology Development. Co., Ltd. (Shanghai, China). Acetonitrile (HPLC grade) and methanol (HPLC grade) were obtained from Merck (Darmstadt, Germany). The formic acid (HPLC grade) was purchased from Tedia (Darmstadt, Germany). Deionized water was produced using a Milli-Q academic water purification system (Milford, MA, USA).
Procedures
Sanqi was crushed and sieved. After that, a mixed solvent of 60% ethanol and 40% water (v/v) was used to extract Sanqi with water bath (W501, Shanghai Shen Sheng Biotechnology Co., Ltd) operated at 95°C for three times. The obtained extracts were mixed and concentrated. The dry matter content of concentrated extract (DMCC) was 509.1 mg/g. Concentrated extracts with lower DMCC were obtained by dilution with water.
Water was added to concentrated extract in a conical flask under stirring with a roller pump (BT300-2J, Baoding Longer Precision Pump Co., Ltd.) at a desired flowrate. After adding water, the stirring was kept for 10 min. The flask then was refrigerated in a low-temperature thermostat bath (THD-1008W, Ningbo Tianheng Instrument Factory) for the desired time at the desired temperature. Finally, the supernatant was collected and weighed. Saponin contents and dry matter content of the supernatant then were determined.
Experimental design
Six parameters were investigated, including DMCC, amount of water added (AWA), stirring speed (SS), refrigeration temperature, refrigeration time, and the flowrate of water addition. The coded and uncoded values of parameters are listed in Table 1. Fractional factorial designed experiments were applied to select CPPs, as seen in Table 2. Box-Behnken designed experiments were applied to obtain the quantitative models between CQAs and CPPs, as seen in Table 3. After the development of design space, verification experiments with conditions listed in Table 4 were carried out. Verification experiments were repeated three times.
Table 1. Coded and uncoded values for the factors.
| Parameters | Symbols | Coded values | |
| −1 | 1 | ||
| DMCC (g/g) | A | 0.34 | 0.55 |
| AWA (g/g) | B | 2.5 | 5.5 |
| Stirring speed (rpm) | C | 200 | 400 |
| Refrigeration temperature (°C) | D | 5.0 | 30.0 |
| Refrigeration time (h) | E | 12 | 48 |
| Flowrate (ml/min) | F | 3.0 | 7.0 |
Table 2. Fractional factorial designed experiments and results.
| Run | A | B | C | D | E | F | Recovery of each saponin in supernatant (%) | Total saponin purity in supernatant (%) | ||||
| Notoginsenoside R1 | Ginsenoside Rg1 | Ginsenoside Re | Ginsenoside Rb1 | Ginsenoside Rd | ||||||||
| 1 | 0.55 | 5.5 | 400 | 5.0 | 12 | 3.0 | 92.6 | 91.3 | 92.8 | 94.3 | 96.0 | 39.2 |
| 2 | 0.34 | 2.5 | 400 | 5.0 | 48 | 3.0 | 89.1 | 88.5 | 85.6 | 93.1 | 98.6 | 39.8 |
| 3 | 0.34 | 5.5 | 400 | 30.0 | 48 | 3.0 | 94.0 | 93.6 | 90.4 | 97.7 | 103.2 | 42.1 |
| 4 | 0.55 | 5.5 | 200 | 30.0 | 12 | 7.0 | 90.5 | 91.3 | 90.7 | 94.6 | 100.9 | 38.5 |
| 5 | 0.34 | 5.5 | 400 | 5.0 | 12 | 7.0. | 93.3 | 93.2 | 88.4 | 96.8 | 99.2 | 40.6 |
| 6 | 0.55 | 2.5 | 400 | 30.0 | 12 | 3.0 | 84.6 | 84.0 | 85.0 | 90.9 | 104.8 | 42.1 |
| 7 | 0.55 | 5.5 | 400 | 30.0 | 48 | 7.0 | 92.3 | 91.9 | 90.2 | 95.6 | 101.2 | 43.8 |
| 8 | 0.34 | 5.5 | 200 | 5.0 | 48 | 7.0 | 92.8 | 93.3 | 89.7 | 82.7 | 78.6 | 37.6 |
| 9 | 0.34 | 2.5 | 400 | 30.0 | 12 | 7.0 | 89.8 | 90.0 | 85.2 | 95.2 | 100.6 | 42.3 |
| 10 | 0.55 | 2.5 | 400 | 5.0 | 48 | 7.0 | 84.8 | 84.1 | 84.5 | 91.3 | 99.9 | 39.3 |
| 11 | 0.55 | 2.5 | 200 | 30.0 | 48 | 3.0 | 84.7 | 84.1 | 85.1 | 89.6 | 96.9 | 38.3 |
| 12 | 0.55 | 5.5 | 200 | 5.0 | 48 | 3.0 | 90.8 | 90.7 | 90.4 | 92.9 | 97.9 | 38.3 |
| 13 | 0.55 | 2.5 | 200 | 5.0 | 12 | 7.0 | 85.5 | 85.0 | 84.6 | 91.0 | 99.7 | 39.6 |
| 14 | 0.34 | 2.5 | 200 | 30.0 | 48 | 7.0 | 88.3 | 88.6 | 83.7 | 94.1 | 101.8 | 38.0 |
| 15 | 0.34 | 5.5 | 200 | 30.0 | 12 | 3.0 | 94.2 | 94.3 | 89.5 | 98.7 | 106.2 | 41.4 |
| 16 | 0.34 | 2.5 | 200 | 5.0 | 12 | 3.0 | 88.9 | 88.6 | 84.2 | 93.5 | 101.8 | 41.0 |
Table 3. Box-Behnken designed experiments and results.
| Run | A | B | C | Recovery of each saponin in supernatant/(%) | Total saponin purity in supernatant (%) | ||||
| Notoginsenoside R1 | Ginsenoside Rg1 | Ginsenoside Re | Ginsenoside Rb1 | Ginsenoside Rd | |||||
| 1 | 0.34 | 4.0 | 400 | 88.8 | 88.4 | 90.0 | 90.5 | 97.7 | 43.2 |
| 2 | 0.34 | 5.5 | 300 | 91.5 | 91.6 | 94.1 | 94.4 | 99.6 | 41.8 |
| 3 | 0.34 | 2.5 | 300 | 86.7 | 87.2 | 89.9 | 90.5 | 95.8 | 41.5 |
| 4 | 0.42 | 2.5 | 200 | 86.2 | 85.3 | 88.0 | 89.5 | 90.7 | 40.8 |
| 5 | 0.42 | 2.5 | 400 | 87.6 | 85.7 | 86.1 | 88.7 | 92.2 | 43.1 |
| 6 | 0.34 | 4.0 | 200 | 89.9 | 90.3 | 89.0 | 93.8 | 94.3 | 38.9 |
| 7 | 0.52 | 4.0 | 200 | 87.5 | 86.8 | 90.6 | 91.2 | 93.2 | 41.1 |
| 8 | 0.52 | 2.5 | 300 | 80.8 | 78.7 | 81.2 | 81.9 | 83.7 | 40.2 |
| 9 | 0.52 | 5.5 | 300 | 87.0 | 85.4 | 87.3 | 88.0 | 90.6 | 39.8 |
| 10 | 0.42 | 4.0 | 300 | 89.4 | 88.9 | 89.7 | 91.9 | 88.9 | 41.5 |
| 11 | 0.42 | 5.5 | 400 | 89.7 | 89.4 | 91.4 | 92.0 | 93.9 | 41.1 |
| 12 | 0.42 | 5.5 | 200 | 92.1 | 91.6 | 92.9 | 93.4 | 96.4 | 41.2 |
| 13 | 0.52 | 4.0 | 400 | 84.9 | 82.8 | 84.2 | 87.0 | 88.8 | 39.4 |
| 14 | 0.42 | 4.0 | 300 | 88.2 | 88.1 | 89.7 | 90.9 | 90.9 | 41.9 |
| 15 | 0.42 | 4.0 | 300 | 88.2 | 87.5 | 88.8 | 88.9 | 88.0 | 40.2 |
| 16 | 0.42 | 4.0 | 300 | 90.2 | 88.4 | 88.3 | 91.3 | 91.9 | 41.3 |
Table 4. Conditions and results of verification experiments.
| No. | V1 | V2 | |
| Concentrated extract amount (g) | 3.0 | 3.0 | |
| DMCC (g/g) | 0.52 | 0.41 | |
| AWA (g/g) | 2.5 | 3.7 | |
| SS (rpm) | 400 | 320 | |
| Probability | 0.00 | 0.95 | |
| Within design space | No | Yes | |
| Notoginsenoside R1 recovery (%) | Experimental value | 84.1±1.1 | 89.2±0.5 |
| Predicted value | 82.3 | 88.6 | |
| Ginsenoside Rg1 recovery (%) | Experimental value | 84.6±1.0 | 87.1±0.2 |
| Predicted value | 79.3 | 87.7 | |
| Ginsenoside Re recovery (%) | Experimental value | 83.9±1.9 | 87.9±0.6 |
| Predicted value | 79.9 | 88.6 | |
| Ginsenoside Rb1 recovery (%) | Experimental value | 89.1±1.1 | 91.7±0.2 |
| Predicted value | 83.1 | 90.3 | |
| Ginsenoside Rd recovery (%) | Experimental value | 90.5±1.4 | 92.3±0.5 |
| Predicted value | 86.4 | 90.0 | |
| Total saponin purity in supernatant (%) | Experimental value | 40.4±0.2 | 40.3±0.5 |
| Predicted value | 40.3 | 41.5 |
Analytical methods
The quantitative analysis of saponins was performed using a UPLC method. A Waters Acquity UPLC system (Waters, Milford, MA) coupled with a Waters Acquity CSH C18 column (50 mm×2.1 mm, 1.7 µm) was used. The mobile phase consisted of 0.01% (v/v) formic acid in water (FA) and 0.01% (v/v) formic acid in acetonitrile (FB). The separation was achieved using a gradient elution program as follow: 19–20% FB at 0–6 min, 20–31% FB at 6–8.5 min, 31–33% FB at 8.5–11 min, 33–90% FB at 11–17 min, and 90–90% FB at 17–19 min. The solvent flow rate was 0.35 ml/min, and the column temperature was kept at 45°C. The detection wavelength was 203 nm. Dry matter content was determined gravimetrically using a precision electronic balance (AB204-N, Mettler Toledo Shanghai Co., Ltd). Before weighed, samples were dried at 105°C in an oven (DZF-6050, Shanghai Jing Hong Laboratory Instrument Co., Ltd.) for 3 h and then kept in a desiccator for 0.5 h.
Data processing
The recovery of saponins (SR) are defined as following equation.
| (1) |
where M and C refer to the mass and concentration, respectively; subscript SUP and CE are supernatant and concentrated extract, respectively; subscript i (i = 1 to 5) represents notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd, respectively. The content of total saponin (CTS) in supernatant or concentrated extract was calculated using Equation 2.
| (2) |
The purity of total saponin (TSP) was calculated using Equation 3.
| (3) |
where DM is the dry matter content of supernatant or concentrated extract.
Design Expert V8.0.6.1 (State-Ease Inc., MN) was used to analyze the results of fractional factorial design and Box-Behnken design. In the selection of CPPs, only the main effects of factors were considered, as shown in Equation 4.
| (4) |
where Y is the response; A, B, C, D, E, and F represent parameters; a0 is a constant; a1 to a6 are regression coefficients. The significance level was set to 0.05. Equation 5 was used to model the results of Box-Behnken experiments.
| (5) |
where b0 is a constant, and b1 to b9 are regression coefficients. All the parameters were coded before modeling.
The design space was calculated using a Monte-Carlo method with a self-written program of Matlab (R2010b,Version 7.11, MathWorks, USA). Considering the sampling error, saponin contents and dry matter contents in supernatant were assumed to follow a normal distribution. To generate random data of saponin contents and dry matter contents, the average values and standard deviations were required. The experimental values of saponin contents and dry matter contents were used as the average values in simulation. The relative standard deviations (RSD) of saponin contents and dry matter contents of supernatants were considered to be the same with the RSD values of the center point in experimental design. Then the standard deviations can be calculated according to average values and RSD values. Coded values were used in the calculation. Therefore the ranges of DMCC, AWA, and SS were within −1 and 1. The calculation step sizes for DMCC, AWA, and SS were 0.02, 0.02, and 0.02, respectively. Saponin recovery and TSP in supernatant were calculated in each simulation. Simulation was repeated 50000 times to calculate reliable probability values. The acceptable level of probability for design space was set as 0.90.
Supernatant remaining in the pores or surface of precipitation (SRP) was not collected. The mass of SRP (MSRP) was calculated using Equation 6.
| (6) |
The ratio of saponins in SRP (SRSRP) was calculated using Equation 7.
| (7) |
Results and Discussion
Process CQA determination
There are many CQAs for Xueshuantong powder, including color, water content, pH value, saponin purity in dry powder, fingerprint similarity, abnormal toxicity, residue on ignition, insoluble particles, bacterial content, heavy metals, and harmful elements. Heavy metals and harmful elements are generally considered to be affected by raw material quality. Bacterial content was controlled by producing Xueshuantong powder in sterile conditions. Process influences on other drug CQAs are identified using risk assessment based on experiences, as seen in Table 5. As one of the unit operations in the manufacturing of Xueshuantong powder, water precipitation is considered to affect saponin purity in dry powder and fingerprint similarity. Higher active ingredient recovery and higher TSP in supernatant both help to realize higher saponin purity in dry powder. Higher TSP in supernatant also means higher removal of impurities, which affects fingerprint similarity. Therefore saponin recovery and TSP in supernatant were identified as the process CQAs in this work. Their criteria are listed in Table 6.
Table 5. Risk assessment to identify process influences.
| Drug quality attributes | Extraction | Concentration | Water precipitation | First decolorization | Column chromatography | Second decolorization | Freeze drying |
| Color | + | − | − | ++ | − | ++ | − |
| Water content | − | − | − | − | − | − | ++ |
| pH value | − | − | − | − | + | − | − |
| Saponin content in dry powder | + | − | + | + | ++ | + | − |
| Fingerprint similarity | + | − | + | + | ++ | + | − |
| Abnormal toxicity | − | − | − | − | + | − | − |
| Residue on ignition | − | − | − | − | + | − | − |
| Insoluble particles | − | − | − | − | + | − | − |
++, +, and − refer to high influence, moderate influence, and low influence, respectively.
Table 6. Criteria of process CQAs.
| CQAs | Lower control limit (%) | Upper control limit (%) |
| Notoginsenoside R1 recovery | 85 | 92 |
| Ginsenoside Rg1 recovery | 85 | 91 |
| Ginsenoside Re recovery | 85 | 94 |
| Ginsenoside Rb1 recovery | 85 | 95 |
| Ginsenoside Rd recovery | 85 | 99 |
| TSP in supernatant | 40 | 43 |
Selection of CPPs
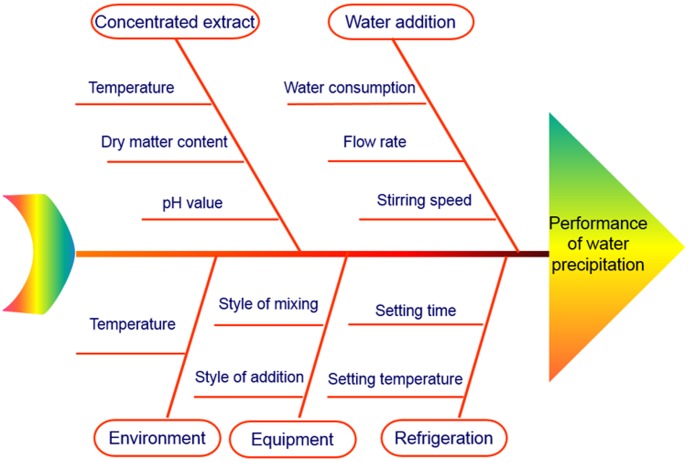
An Ishikawa diagram analysis was performed to find out process parameters that affect the CQAs of water precipitation, as seen in Figure 2. The risk severity of four parameters of concentrated extract temperature, concentrated extract pH value, environmental temperature, and water addition style is low. Parameter fail probability of water addition style, and style of mixing is very low. The detectability for concentrated extract temperature, and environmental temperature is high. Therefore, the risk caused by these five parameters are low.
Figure 2. Ishikawa diagram of water precipitation process.
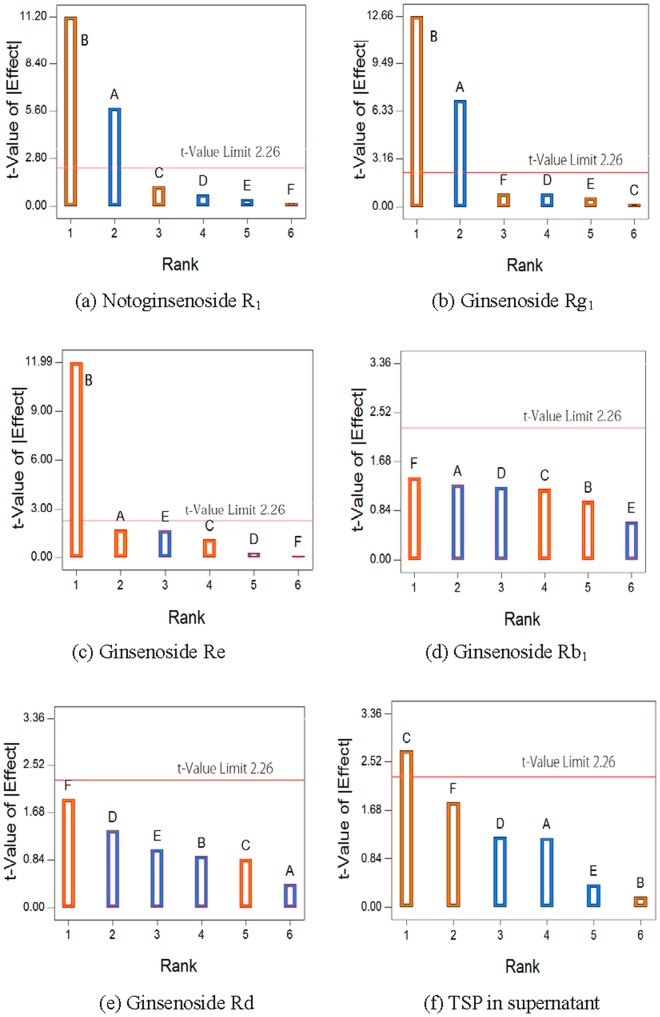
The other six parameters of AWA, SS, DMCC, refrigeration temperature, refrigeration time, and water addition flow rate were investigated using fractional factorial design. The results of fractional factorial design experiments are listed in Table 2. The Pareto chart for saponin recovery and TSP in supernatant are shown in Figure 3. In Figure 3(a) and 3(b), DMCC and AWA remarkably affected the recoveries of notoginsenoside R1 and ginsenoside Rg1. Ginsenoside Re recovery was mainly affected by AWA, as seen in Figure 3(c). In Figure 3(f), SS significantly affected TSP in supernatant. The other three factors, including refrigeration temperature, refrigeration time, and the flow rate of water were insignificant on process CQAs. Therefore DMCC, AWA, and SS were selected as CPPs.
Figure 3. Pareto chart of parameters.
The effects of CPPs on process CQAs
The experimental results of Box-Behnken design are displayed in Table 3. The recoveries of all the five saponins are higher than 78%, which means most active ingredients can be recovered in water precipitation. TSP in supernatant varied from 38.8% to 43.2%. Because TSP in concentrated extract was 36.7%, it can be concluded that TSP increases after water precipitation. Response surface models were developed to obtain quantitative relationships between CQAs and CPPs. The estimated regression coefficients are listed in Table 7. Analysis of variance (ANOVA) was performed, and p values of parameters are also listed in Table 7. For all the models, determination coefficients (R2) are larger than 0.86, which means most variations of process CQAs can be explained by DMCC, AWA, and SS. Models are significant with p-values less than 0.05. In Table 7, DMCC are significant for all the CQAs. AWA are significant for all the recoveries of saponins. The quadratic term of DMCC shows significant influences on the recovery of notoginsenoside R1 and ginsenoside Rg1. The purity of total saponin in supernatant is mainly affected by DMCC and the interaction term of DMCC and SS.
Table 7. Estimated parameter values and ANOVA results.
| Model terms | Recovery | TSP in supernatant | ||||||||||
| Notoginsenoside R1 | Ginsenoside Rg1 | Ginsenoside Re | Ginsenoside Rb1 | Ginsenoside Rd | Estimate | Prob>|t| | ||||||
| Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | |||
| Constant | 88.79 | -------- | 87.92 | -------- | 88.87 | -------- | 90.47 | -------- | 89.46 | -------- | 41.17 | -------- |
| A | −2.08 | 0.0016* | −2.98 | 0.0002* | −2.48 | 0.0063* | −2.63 | 0.0059* | −3.91 | 0.0018* | −0.60 | 0.0353* |
| B | 2.40 | 0.0008* | 2.68 | 0.0004* | 2.61 | 0.0051* | 2.19 | 0.0135* | 2.32 | 0.0197* | −0.23 | 0.3484 |
| C | −0.60 | 0.1667 | −0.99 | 0.0399* | −1.20 | 0.0948 | −1.23 | 0.1007 | −0.35 | 0.6500 | 0.51 | 0.0624 |
| A×B | 0.38 | 0.5076 | 0.58 | 0.3166 | 0.47 | 0.5974 | 0.57 | 0.5432 | 0.82 | 0.4573 | −0.18 | 0.5887 |
| A×C | −0.42 | 0.4675 | −0.59 | 0.3070 | −1.88 | 0.0697 | −0.33 | 0.7258 | −1.96 | 0.1070 | −1.52 | 0.0028* |
| B×C | −0.93 | 0.1343 | −0.63 | 0.2793 | 0.11 | 0.9058 | −0.17 | 0.8511 | −1.00 | 0.3734 | −0.58 | 0.1125 |
| A2 | −1.60 | 0.0269* | −1.42 | 0.0397* | −0.82 | 0.3824 | −0.88 | 0.3702 | 1.81 | 0.1365 | −0.58 | 0.1195 |
| B2 | −0.70 | 0.2439 | −0.79 | 0.1894 | −0.075 | 0.9326 | −0.87 | 0.3645 | 1.15 | 0.3096 | 0.24 | 0.4675 |
| C2 | 0.58 | 0.3233 | 0.58 | 0.3207 | 0.39 | 0.6601 | 1.03 | 0.2939 | 2.22 | 0.0758 | 0.059 | 0.8571 |
| Model P value | 0.0057* | 0.0018* | 0.0310* | 0.0487* | 0.0235* | 0.0277* | ||||||
| R 2 | 0.9367 | 0.9577 | 0.8833 | 0.8616 | 0.8947 | 0.8881 | ||||||
* p value less than 0.05.
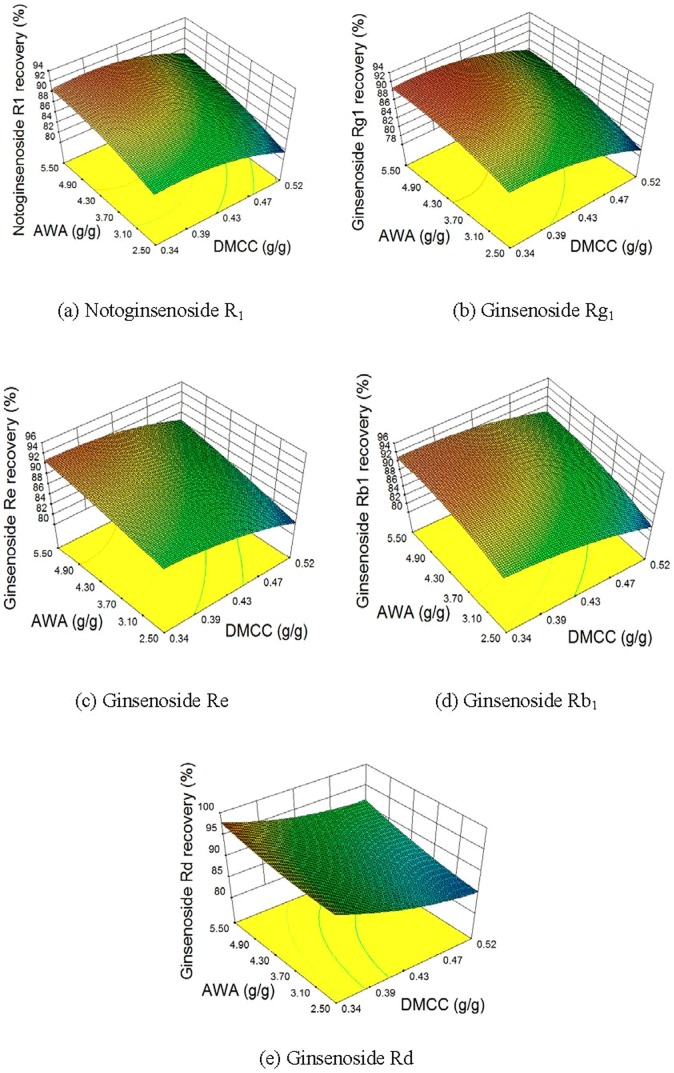
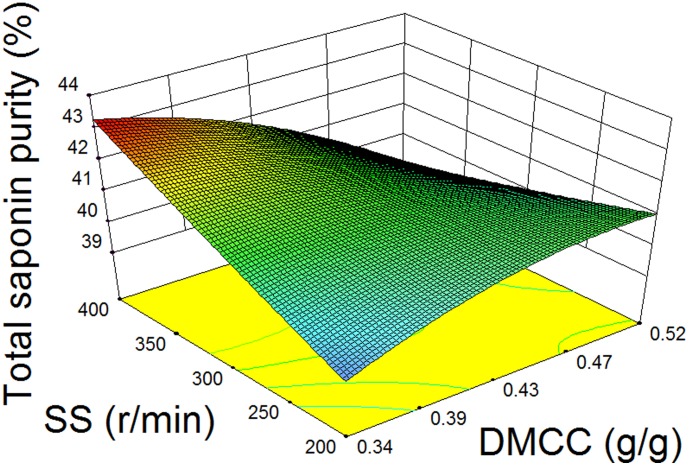
Figure 4 shows the effects of DMCC and AWA on the recoveries of saponins. The increase of AWA can reduce saponin loss. However, saponin recoveries decrease as DMCC increases. The effects of CPPs on TSP in supernatant are shown in Figure 5. High TSP in supernatant can be obtained when DMCC is low and SS is high.
Figure 4. Effects of DMCC and AWA on saponin recoveries.
Figure 5. Effects of DMCC, and SS on TSP in supernatant.
Loss mechanism of saponins
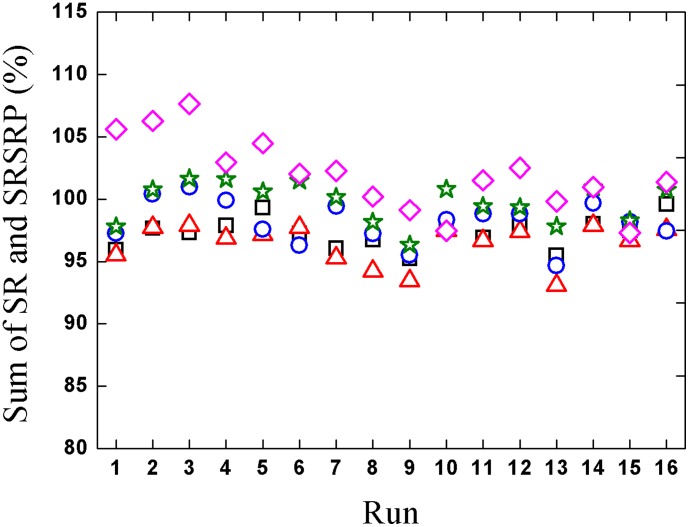
Saponins will degrade under acidic, basic, or thermal conditions [27]–[29]. The solubilities of some saponins were also reported [30], [31]. It can be concluded that saponin solubility in water is not large. Theoretically, the loss of saponins in water precipitation may be caused by chemical reactions, precipitation, or the incomplete collection of supernatant. The sums of SR and SRSRP for each saponin are plotted in Figure 6. Most values of the sums of SR and SRSRP are within 100%±5%. It indicates that the incomplete collection of supernatant is the main reason for the loss of saponins. The uncollected supernatant mainly remained in the pores and surface of precipitation.
Figure 6. Sum of SR and SRSRP for different saponins.
Design space development and verification
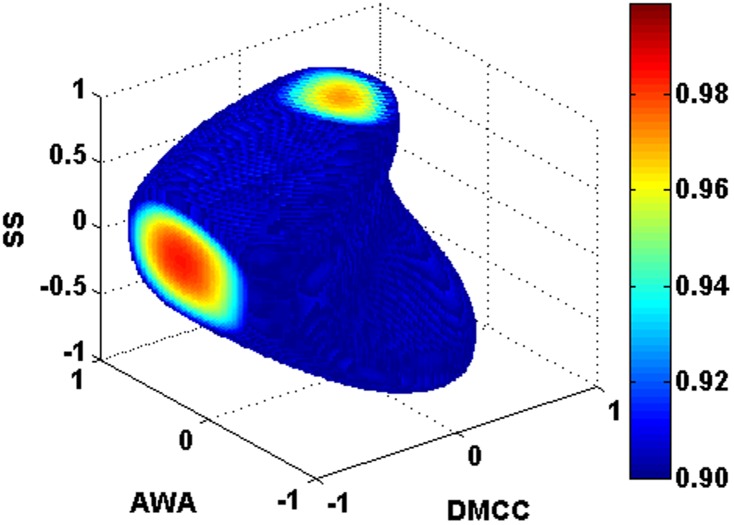
The calculated design space are shown in Figure 7. It can be concluded that the design space is an irregular polygon. Recommended normal operation region to attain CQA criteria are located in DMCC of 0.38–0.41 g/g, AWA of 3.7–4.9 g/g, and SS of 280–350 rpm with a probability more than 0.919. Verification experiments were carried out. The prediction results and experimental results of process CQAs are listed in Table 4. The recoveries of notoginsenoside R1, ginsenoside Rg1, and ginsenoside Re of Experiment V1 are lower than their criteria. While all the experimental values of Experiment V2 were in the ranges listed in Table 6. These results indicate that criteria of CQAs can be attained by operating CPPs within design space.
Figure 7. Design space obtained using Monte-Carlo simulations.
Conclusions
In this work, water precipitation process for the manufacturing of Xueshuantong injection was optimized using a design space approach as a sample. Saponin recovery and the total saponin purity in supernatant were identified as the process CQAs of water precipitation using risk assessment. An Ishikawa diagram was applied to find out potential CPPs. DMCC, SS, and AWA were identified as CPPs using a fractional factorial design. Models between CPPs and process CQAs were developed with experimental results of Box-Behnken design. Determination coefficients were higher than 0.86 for all the models. High TSP in supernatant can be obtained when DMCC is low and SS is high. Saponin recoveries decrease as DMCC increases. The loss of saponins was mainly caused by the incomplete collection of supernatant. Design space was calculated with a Monte-Carlo simulation method. In the simulation, 0.90 was adopted as the acceptable probability for the attainment of CQA criteria. Recommended normal operation region are located in DMCC of 0.38–0.41 g/g, AWA of 3.7–4.9 g/g, and SS of 280–350 rpm, with a probability more than 0.919 to attain CQA criteria. Design space was verified and verification experimental results showed that operating DMCC, SS, and AWA within design space helps to attain process CQA criteria with high probability. The design space applied in this work can also be used to optimize other unit operations in pharmaceutical engineering.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National S&T Major Project of China (2012ZX09101201-003) and Research Fund for the Doctoral Program of Higher Education of China (20110101120149). Haibin Qu received the first fund. The website is http://www.most.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Xingchu Gong received the second fund. The website is http://www.cutech.edu.cn/cn/kyjj/gdxxbsdkyjj/A010301index_1.htm. Fund were used to buy materials, chemicals, and pay labor costs.
References
- 1. Yu LX (2008) Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm Res-Dordr 25: 781–791. [DOI] [PubMed] [Google Scholar]
- 2. Gong XC, Wang SS, Li Y, Qu HB (2013) Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep Purif Technol 107: 273–280. [Google Scholar]
- 3. Korakianiti E, Rekkas D (2011) Statistical Thinking and Knowledge Management for Quality-Driven Design and Manufacturing in Pharmaceuticals. Pharm Res-Dordr 28: 1465–1479. [DOI] [PubMed] [Google Scholar]
- 4. Rozet E, Ziemons E, Marini RD, Boulanger B, Hubert P (2012) Quality by Design Compliant Analytical Method Validation. Anal Chem 84: 106–112. [DOI] [PubMed] [Google Scholar]
- 5. Nistor I, Lebrun P, Ceccato A, Lecomte F, Slama I, et al. (2013) Implementation of a design space approach for enantiomeric separations in polar organic solvent chromatography. J Pharmaceut Biomed 74: 273–283. [DOI] [PubMed] [Google Scholar]
- 6. Nistor I, Cao M, Debrus B, Lebrun P, Lecomte F, et al. (2011) Application of a new optimization strategy for the separation of tertiary alkaloids extracted from Strychnos usambarensis leaves. J Pharmaceut Biomed 56: 30–37. [DOI] [PubMed] [Google Scholar]
- 7. Xu XM, Costa AP, Khan MA, Burgess DJ (2012) Application of quality by design to formulation and processing of protein liposomes. Int J Pharmaceut 434: 349–359. [DOI] [PubMed] [Google Scholar]
- 8. Charoo NA, Shamsher AAA, Zidan AS, Rahman Z (2012) Quality by design approach for formulation development: A case study of dispersible tablets. Int J Pharmaceut 423: 167–178. [DOI] [PubMed] [Google Scholar]
- 9. Bhambure R, Rathore AS (2013) Chromatography Process Development in the Quality by Design Paradigm I: Establishing a High-Throughput Process Development Platform as a Tool for Estimating “Characterization Space” for an Ion Exchange Chromatography Step. Biotechnol Progr 29: 403–414. [DOI] [PubMed] [Google Scholar]
- 10. Ingvarsson PT, Yang M, Mulvad H, Nielsen HM, Rantanen J, et al. (2013) Engineering of an Inhalable DDA/TDB Liposomal Adjuvant: A Quality-by-Design Approach Towards Optimization of the Spray Drying Process. Pharm Res [DOI] [PubMed] [Google Scholar]
- 11. Mockus LN, Paul TW, Pease NA, Harper NJ, Basu PK, et al. (2011) Quality by design in formulation and process development for a freeze-dried, small molecule parenteral product: a case study. Pharm Dev Technol 16: 549–576. [DOI] [PubMed] [Google Scholar]
- 12. Looby M, Ibarra N, Pierce JJ, Buckley K, O'Donovan E, et al. (2011) Application of quality by design principles to the development and technology transfer of a major process improvement for the manufacture of a recombinant protein. Biotechnol Progr 27: 1718–1729. [DOI] [PubMed] [Google Scholar]
- 13. Lourenco V, Lochmann D, Reich G, Menezes JC, Herdling T, et al. (2012) A quality by design study applied to an industrial pharmaceutical fluid bed granulation. Eur J Pharm Biopharm 81: 438–447. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Zhao L, Li M, Feng Y, Xu D, et al. (2012) Applications and characteristics of mathematical models to study compression process of pharmaceutical powder. Chinese Journal of New Drugs 21: 1362–1366. [Google Scholar]
- 15. Zhang L, Yan B, Gong X, Yu LX, Qu H (2013) Application of quality by design to the process development of botanical drug products: a case study. AAPS PharmSciTech 14: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rozet E, Lebrun P, Debrus B, Boulanger B, Hubert P (2013) Design Spaces for analytical methods. Trac-Trend Anal Chem 42: 157–167. [Google Scholar]
- 17. Martins PF, Carmona C, Martinez EL, Sbaite P, Maciel R, et al. (2012) Evaluation of methyl chavicol concentration by different evaporation processes using central composite experimental design. Sep Purif Technol 98: 464–471. [Google Scholar]
- 18. Duan MH, Luo M, Zhao CJ, Wang W, Zu YG, et al. (2013) Ionic liquid-based negative pressure cavitation-assisted extraction of three main flavonoids from the pigeonpea roots and its pilot-scale application. Sep Purif Technol 107: 26–36. [Google Scholar]
- 19. Mbinze JK, Lebrun P, Debrus B, Dispas A, Kalenda N, et al. (2012) Application of an innovative design space optimization strategy to the development of liquid chromatographic methods to combat potentially counterfeit nonsteroidal anti-inflammatory drugs. J Chromatogr A 1263: 113–124. [DOI] [PubMed] [Google Scholar]
- 20. Debrus B, Lebrun P, Ceccato A, Caliaro G, Rozet E, et al. (2011) Application of new methodologies based on design of experiments, independent component analysis and design space for robust optimization in liquid chromatography. Anal Chim Acta 691: 33–42. [DOI] [PubMed] [Google Scholar]
- 21. Gui QF, Yang YM, Ying SH, Zhang MM (2013) Xueshuantong improves cerebral blood perfusion in elderly patients with lacunar infarction. Neural Regen Res 8: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinese Pharmacopoeia Commission (2011) Zhusheyong Xueshuantong (donggan). Available: http://www.chp.org.cn/export/sites/chp/resource/publicity/110613/2011061313465003968.doc. Accessed 2011 Jun 13.
- 23. Kim SJ, Murthy HN, Hahn EJ, Lee HL, Paek KY (2007) Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng CA Meyer). Sep Purif Technol 56: 401–406. [Google Scholar]
- 24. Xiao WH, Han LJ, Shi B (2008) Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Purif Technol 62: 614–618. [Google Scholar]
- 25. Xiao XH, Guo ZN, Deng JC, Li GK (2009) Separation and purification of isofraxidin from Sarcandra glabra by microwave-assisted extraction coupled with high-speed counter-current chromatography. Sep Purif Technol 68: 250–254. [Google Scholar]
- 26. Zheng XZ, Wang X, Lan YB, Shi J, Xue SJ, et al. (2009) Application of response surface methodology to optimize microwave-assisted extraction of silymarin from milk thistle seeds. Sep Purif Technol 70: 34–40. [Google Scholar]
- 27. Han BH, Park MH, Han YN, Woo LK, Sankawa U, et al. (1982) Degradation of Ginseng Saponins under Mild Acidic Conditions. Planta Med 44: 146–149. [DOI] [PubMed] [Google Scholar]
- 28. Popovich DG, Kitts DD (2004) Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry 65: 337–344. [DOI] [PubMed] [Google Scholar]
- 29. Wang YT, You JY, Yu Y, Qu CF, Zhang HR, et al. (2008) Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem 110: 161–167. [DOI] [PubMed] [Google Scholar]
- 30. Chen X, Zhu J (2007) On solubility of ginsenoside Rg1 and liposome entrapment efficiency. China Pharmacy 18: 41–43. [Google Scholar]
- 31. Li RY, Yan H, Wang Z, Gong JB (2012) Correlation of Solubility and Prediction of the Mixing Properties of Ginsenoside Compound K in Various Solvents. Ind Eng Chem Res 51: 8141–8148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.