Abstract
Human IgG1 antibody responses are associated with protection against Schistosoma haematobium infection and are now a target for schistosome vaccine development. This study aimed to investigate the relationship between total IgG and the IgG subclasses and the monocyte IgG receptor, known as FcγRIIIa or CD16, in schistosome exposed people. Systemic levels of schistosome-specific anti-adult worm total IgG and IgG subclass titres were measured by ELISA in 100 individuals from an S. haematobium endemic area in Zimbabwe and, using parametric statistical methods and regression analysis, related to the levels of CD16 expression on individuals' circulating monocytes, determined via flow cytometry. Monocyte CD16 expression rose with parasite-specific total IgG and IgG1 in healthy participants, but not in schistosome infected patients. Similar to parasite-specific IgG and IgG1, CD16 expression in healthy individuals is associated with protection against schistosome infection. This relationship indicates a mechanistic link between the innate and adaptive immune responses to helminth infection in protection against infection. Further understanding the elements of a protective immune response in schistosomiasis may aid in efforts to develop a protective vaccine against this disease.
Author Summary
Schistosomiasis is a parasitic disease caused by the parasite Schistosoma spp. Over 240 million people are infected worldwide, mainly in Sub-Saharan Africa, but an efficacious, protective vaccine has yet to be found. Protection against schistosome infection in individuals living in endemic areas is mediated by antibodies. In particular, IgG1 antibody has been shown to be protective against infection in individuals living in endemic areas, and eliciting IgG1 production has become a cornerstone of vaccine development efforts. However, little is known about the mechanisms by which IgG1 induces protection. The cell surface molecule CD16 is an IgG antibody receptor expressed on monocytes and binds preferentially to IgG antibody subclasses. The work presented here thus investigates the relationship between IgG levels and the monocyte CD16 receptor in a population endemically exposed to infection with schistosomes. We present results linking CD16 expression with IgG1 levels, whereby uninfected individuals have a positive relationship between IgG1 and CD16 expression levels, while schistosome infected individuals did not show any statistically significant relationship between the two. Thus we provide evidence to suggest a mechanistic link between the innate and adaptive immune response in parasitic infection, associating monocyte CD16 expression with a protective immune response.
Introduction
An estimated 200 million people worldwide are infected with helminths of the genus Schistosoma, with the heaviest burden of disease occurring in sub-Saharan Africa, where both Schistosoma haematobium and Schistosoma mansoni are endemic, causing significant morbidity amongst affected communities [1]. Infection and disease are controlled by treatment with the drug praziquantel (PZQ), and the World Health Organization (WHO) recommends protective chemotherapy via mass drug administration (MDA) with PZQ in endemic areas [2]. There is mounting pressure to develop a vaccine against schistosomiasis, which would provide long term protection to the 650 million people at risk of exposure [3], and pre-empt the development of drug resistance. Current vaccine development research focuses on determining which naturally developed immune responses are associated with protective immunity that develops in the context of endemic exposure to infection, and investigate ways of inducing those responses artificially whilst avoiding a pathological response [4], [5]. While significant progress has been made in characterising humoral and cellular responses in experimental models, relatively less work has been conducted relating the innate and adaptive arms of the immune system in schistosome infected versus uninfected humans. In particular, there is a paucity of studies simultaneously determining cellular and related humoral responses associated with natural protection against schistosome infection.
Experimental studies have shown links between innate cells from the myeloid lineage and resistance to helminth infection. For example, murine macrophages and are involved with tissue repair and fibrosis [6], [7], as well as in limiting pathology by regulating Type 2 cytokine production [8], [9] and inhibiting T cell proliferation [10]. This current study focused on circulating monocytes, myeloid cells related developmentally to macrophages, which are present in the blood vessels and are thus easily accessible for investigation in humans. Studies from several decades ago showed a direct role ex vivo for human PBMC-derived monocytes in the killing of schistosomula [11]–[13]. Similar to macrophages, monocytes display phagocytic capabilities and express varying levels of the FcγRIIIa (also known as the CD16 receptor) [14], which is related to distinctions in their phenotype and function in a range of pro-inflammatory conditions [15], [16]. The Fcγ receptors have a critical role in immune regulation, acting as a link between the humoral and innate cellular arms of the immune response [17]. In humans, the CD16 receptor exhibits high affinity binding to the Fc portion of IgG antibodies, with high affinity binding demonstrated to IgG1 and IgG3, which leads to phagocytosis, release of inflammatory mediators and clearance of immune complexes [14]. The importance of the interaction between IgG and Fcγ receptors has been demonstrated in experimental models, whereby there is a diminished macrophage effector function induced after IgG1-mediated phagocytosis in Fcγ chain knock-out mice [18]. Furthermore, S. mansoni infection exacerbated granuloma formation and fibrosis in both Fcγ receptor and in B cell deficient mice [19], highlighting the importance of antibody signalling via the Fcγ receptor in protection against pathology associated with schistosomiasis infection. However, there are few studies relating IgG subclasses to the Fcγ receptors in human schistosomiasis. To address this knowledge gap, the present study focuses on the relationship between CD16 and the IgG subclasses.
Our previous studies, and those of others, have shown that, in humans, schistosome-specific IgG1 and IgG3 antibodies are associated with natural resistance to infection [20]–[22]. Induction of helminth-specific IgG1 and IgG3 through vaccination is now preferred over IgE to avoid generating IgE-mediated pathological responses to vaccination [4], [23] and, in particular, this study focuses on these protective subclasses.
This study, therefore, investigated the relationship between levels of the IgG receptor, FcγRIIIa (CD16) and schistosome specific IgG subclasses in uninfected, healthy individuals versus schistosome infected patients. The healthy individuals comprised of young people, who had yet to acquire schistosome infection, and older people who were putatively resistant to infection, as they were infection free despite being lifelong residents of the schistosome endemic areas and experiencing regular exposure to infective water. The study focused on adult schistosome-specific IgG responses since adult worms reside in the circulating blood, and thus are in direct contact with monocytes in this compartment. In addition, our studies, as well as those of others, have highlighted the importance of the adult worm stage in stimulating protective immune responses [24]–[27].
Methods
Ethical approval
Ethical and institutional approval was granted by the Medical Research Council of Zimbabwe and the University of Zimbabwe's Institutional Review Board. Local permission for the study was granted by the Provincial Medical Director. The study design, aims and procedures were explained in the local language, Shona, prior to enrolment. Participants were free to drop out of the study at any time and informed written consent/assent was obtained from all participants and/or their guardians prior to taking part in the study and to receiving antihelminthic treatment.
Study design
The study presented here was part of a larger on-going immuno-epidemiological study based in Mashonaland East, Zimbabwe where S. haematobium is endemic [28]. The area has a low prevalence of soil transmitted helminths (STH) and Schistosoma mansoni [29], and the residents are subsistence farmers with frequent contact with infected water for purposes of bathing, washing and collecting water. Recruitment into the study was school based and the wider community was also invited to participate. Residential history, antihelminthic treatment history and water contact habits of the participants were captured through questionnaire. Following sample collection, participants were offered treatment with the antihelminthic drug praziquantel at the recommended dose of 40 mg/kg of body weight [3].
Inclusion criteria
In order to be included in this study participants had to meet the following criteria: 1) be lifelong residents of the study area to allow age to be used as a proxy for history of exposure to schistosome infection, 2) have provided a minimum of two urine and two stool samples on consecutive days for parasite detection, 3) not have previously received antihelminthic treatment, 4) be negative for co-infection with malaria, STH, S. mansoni and HIV and 5) have provided a blood sample for serological and cellular assays. Further to this, participant's PBMC sample must have yielded at least 106 cells to allow enough cells for all experimental conditions. From an initial cohort of 633 recruited individuals, 68 were excluded for not meeting criteria 1–4 above and a further 184 did not provide sufficient blood sample for both serological assays and cell phenotyping. From the remaining 381 individuals, a cohort of 100 individuals was further selected to allow for, as far as possible, equal numbers of females to males and an even distribution of ages and infection prevalence. Individuals with one or more S. haematobium eggs found in their urine samples were classified as infected. The final study group was divided into three age groups and is described in Table 1.
Table 1. Characteristics of study cohort.
| Age Group | ||||||
| 5–10 years | 11–15 years | >16 years | ||||
| Infection Status | Sh− | Sh+ | Sh− | Sh+ | Sh− | Sh+ |
| Sample size (no.) | 28 | 16 | 18 | 13 | 16 | 9 |
| Mean Age (years) | 7.68 | 7.62 | 13.17 | 12.54 | 30.12 | 27.11 |
| Infection intensity | 0 | 56.78 | 0 | 99.44 | 0 | 59.26 |
| Infection range (SD) | 0 | 1.33–297 (83.7) | 0 | 0.33–523 (165.7) | 0 | 0.33–550 (109.5) |
| Males∶Females | 8∶20 | 11∶5 | 9∶9 | 8∶5 | 2∶14 | 3∶6 |
All selected people of the cohort were negative for HIV, soil transmitted helminths and S. mansoni; infection intensity: eggs/10 mL urine; Sh− negative for S. haematobium, Sh+ positive for S. haematobium.
Sample collection
From each participant a stool and urine sample was collected on three consecutive days and examined microscopically for the presence of S. haematobium eggs in urine, and S. mansoni and STH eggs in stool using standard techniques [30], [31]. A random sample of 100 stool samples was also processed via the formol ether concentration technique, and these confirmed Kato Katz diagnosis [32]. Up to 20 millilitres of venous blood was collected from each participant in heparinised tubes or silicone –coated tubes (both from BD Biosciences, San Jose, CA), for purposes of processing for PBMC purification (heparin tubes), or serum (silicone tubes) using routine methods. An additional drop of blood was collected from each participant for microscopic detection of malaria parasites and for HIV detection using DoubleCheckGold HIV 1&2 Whole Blood Test (Orgenics Ltd., Yavne, Israel). Peripheral blood mononuclear cells (PBMC) were isolated from the remaining tubes via density gradient centrifugation using Lymphoprep (Axis-Shield, Cambridgeshire, UK). Isolated PBMCs were cryopreserved and stored in liquid nitrogen in Zimbabwe prior to freighting to Edinburgh in dry shippers where the overall viability of isolated PBMCs was estimated in a sample of 84 individuals' PBMCs using propidium iodide (PI) (Sigma-Aldrich, Dorset, UK) exclusion. Mean PI uptake in this sample was 15.3% (+/−1.06%), which is within the range considered viable.
Antibody assays
Schistosome soluble worm antigen preparation (SWAP)-specific antibody serum levels for total IgG, IgG1, IgG2, IgG3 and IgG4 were quantified using antibody ELISA. Lyophilized SWAP (Theodor Bilharz Institute, Giza, Egypt) was reconstituted as recommended by the manufacturer and as described by Mutapi et al. [25]. ELISAs were conducted as previously reported [22], using 5 µg/ml of SWAP antigen in carbonate bicarbonate buffer to coat all ELISA plates, and adding sample at a 1∶100 dilution in 5% skimmed milk. Secondary IgG HRP-conjugated antibody was added at a 1∶1000 dilution for total IgG and IgG1, and at a 1∶500 dilution for IgG2, IgG3 and IgG4. The colorimetric reaction was left for 10 minutes for total IgG, and 15 minutes for the IgG subclasses, and quantified with an ELISA reader at 405 nm. Each antibody ELISA was performed in duplicate on the same day for all samples with positive and negative controls on each plate.
Phenotyping of monocytes
Cryopreserved PBMCs were thawed as previously described [33], and resuspended at 5×106 cells/ml in PBS. Cells were incubated with 10% FCS at 4°C for 10 minutes prior to staining for 30 minutes with Alexa488 conjugated anti-CD14 (clone M5E2), PE-Cy7 conjugated HLA-DR (clone L243) (both from BD Biosciences, San Jose, CA), and Pacific Blue conjugated anti-CD16 (clone CB16) (eBiosciences, San Diego, CA). Unbound antibodies were washed off and cells were resuspended in PBS prior to acquisition of at least 50,000 live events on a BD FACS LSR II (BD Biosciences, San Jose, CA). Compensation was performed prior to acquisition of each experiment using BD FacsComp beads (BD Biosciences, San Jose, CA). Analysis was performed using FlowJo software (TreeStar, USA) and mean fluorescence intensity (MFI) was calculated for CD16.
To ensure that only CD14 positive cells representing monocytes were analysed, only cells expressing both HLA-DR and CD14 were selected for analysis, in a gating strategy previously described [28], [34]. Briefly, a live gate to include all leukocytes was drawn based on forward scatter (FSC) and side scatter (SSC), HLA-DR positive cells were gated to exclude any CD16+ expressing natural killer (NK) cells as well as other non-MHC expressing cells. Monocytes were defined as CD14 or CD16 expressing cells. The expression level of CD14 and CD16 was reported as MFI.
Statistical analysis
All statistical analyses were conducted using the statistical package SPSS version 19 (IBM Corp, NY, USA). Due to the possibility of gender and age dependent exposure patterns in this population [35], [36], appropriate statistical techniques were necessary to adjust for this variation prior to investigating the relationship of interest [37]. Parametric statistical modelling in the form of analysis of variance (ANOVA) and linear regression was therefore used. Data were transformed in order to meet assumptions of parametric tests. Surface marker expression (measured as MFI) was log transformed (log10(x+1)). Antibody level (after subtraction of the blank control) was square root transformed. Categorical variables were sex (male/female), infection status (uninfected/infected) and age group (5–10 years [age group where infection is rising], 11–15 years [age group where infection is peaking] or >16 years [age group where infection is declining]).
To determine the extent of changes in the proportion of monocytes relative to the rest of the PBMCs, ANOVA with sequential sums of squares (SS) was used with the total number of live monocytes as the dependent variable, and the independent variables were sex (male or female), age group (5–10 years, 11–15 years or >16 years) and infection status (uninfected or infected).
Monocytes from different individuals show varying levels of CD14 and CD16 expression intensity dependent on various factors including age [38], [39] and presence of inflammation [40]. Therefore, to test the hypothesis that CD14 and CD16 receptor expression levels changed with infection and age group, a multivariate analysis of variance (MANOVA) with sequential SS was used with receptor expression (CD14 and CD16) as the dependent variable, and the independent variables were sex, age group and infection status. The model was extended to include an interaction term between age and infection to assess patterns of surface receptor expression co-dependent on age and infection status. The relationship between CD16 expression and age group dependent on infection status was investigated further using a partial correlation, controlling for variation due to sex, and followed with Fisher's z transformation, which tests for a significance in the difference between two correlations.
The relationship between each of the anti-SWAP antibody titres (total IgG, IgG1, IgG2, IgG3, IgG4) with infection and age group was tested using a univariate ANOVA with sequential SS, entering sex and age group before infection in the analysis. In addition, the interaction between infection and age was tested and all appropriate post-hoc tests were conducted.
Finally to determine the relationship between CD16 expression on monocytes and antibody levels according to infection status, a linear regression analysis was used. The relationship between antibody levels and CD16 expression according to infection status was investigated after accounting for sex and age differences, and significant interactions were followed up with a correlation analysis of the relationship between the two variables in uninfected and infected individuals separately.
For all statistical tests significance of p≤0.05 was considered significant.
Results
The proportion of total circulating monocytes in schistosome patients is similar to that in healthy participants
The proportion of monocytes in PBMCs did not vary significantly with host age or infection as shown in Figure 1A. Thus, any changes observed in subsequent analyses will be a result of changes in monocyte phenotype between individuals, rather than as a result of changes in monocyte proportions, for example due to migration of monocytes into or out of the vasculature.
Figure 1. CD16 but not CD14 expression intensity on circulating monocytes differs between uninfected and infected older patients.
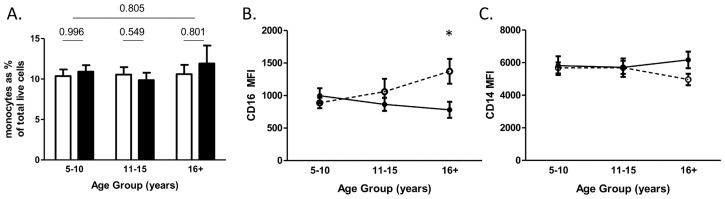
Proportion of (A) total circulating monocytes, (B) MFI of CD16, and (C) MFI of CD14 on monocytes according to age group and infection status and as determined by flow cytometry. Open bars and open circles with dashed lines, healthy individuals; filled bars and closed circles with solid lines, schistosome infected patients. Bars represent standard error of mean. Significant differences between infected and uninfected participants within age groups according to sub-group analysis are indicated by *(p≤0.05).
Healthy older individuals express significantly higher monocyte surface CD16 than schistosome infected patients of the same age
The expression levels of CD14 and CD16 on monocytes were investigated with relation to infection status. CD16 expression levels were shown to vary dependent on age and infection status (Table 2 and Figure 1B). Thus, while expression of CD16 on monocytes was similar between infected (schistosome patients) and uninfected (healthy) individuals in the younger age groups, with increasing age there was an increasing intensity of expression in the healthy individuals compared to a decreasing intensity of expression with age in the infected individuals as shown in Figure 1B. Consistent with the heterogeneous relationship between age and infection status, the correlation coefficient was significantly different (z = 2.97, p = 0.003) with schistosome patients having a positive relationship (r = 0.350, p = 0.006) and healthy individuals having a negative relationship (r = −0.287, p = 0.085). The oldest age group demonstrated significant differences in monocyte CD16 expression dependent on infection status (Figure 1B). In contrast, levels of the monocyte marker CD14 did not vary with any of the investigated variables (Table 2 and Figure 1C).
Table 2. Relationship between the MFI of CD14 or CD16 expression on monocytes with infection status and age group.
| Monocyte marker | ||
| Independent variable | CD16 | CD14 |
| Sex | 5.370 (M<F) | 0.235 (M>F) |
| Age group | 0.813 (1<2<3) | 0.157 (1>2<3) |
| Infection status | 0.60 (sh−>sh+) | 0.80 (sh−<sh+) |
| Infection status * Age group | 4.946 | 0.772 |
F values of output of MANOVA for relationship between CD14 or CD16 and each factor. Model accounts for variation in sex (male (M) or female (F)), age group (1: 5–10 years; 2: 11–15 years; 3: >16 years), and infection status (uninfected: sh−; infected: sh+). The interaction term is designated by *. Direction of differences between variables is indicated in brackets below F value. Significant values (p≤0.05) are indicated in bold. N = 100.
Parasite-specific IgG levels increase with age in healthy people, but not in schistosome patients
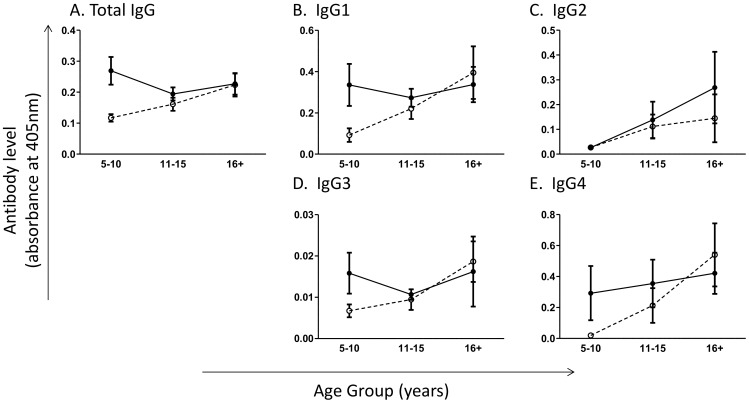
The relationship between SWAP-specific total IgG, as well as the IgG subclasses and infection status dependent on age was investigated. Total IgG, IgG1, IgG2 and IgG4, but not IgG3, significantly varied with host age, with total schistosome-specific IgG levels lowest in the age of peak schistosome infection, but the adult worm-specific subclasses IgG1, IgG2 and IgG4 highest in the oldest age group (Table 3). Overall, levels of parasite-specific IgG and IgG1 varied between healthy participants compared to schistosome infected people, with infected individuals having greater antibody levels compared to uninfected individuals (Table 3). However, only for total IgG, the effects of infection status varied with host age as indicated by the significant age group-infection status interaction term (Table 3). Figure 2 demonstrates the gradual increase in levels of schistosome-specific total IgG in healthy participants, while in schistosome infected patients, total IgG levels did not change significantly with age. For total IgG, IgG1, IgG3 and IgG4, but not IgG2, the youngest age group showed significant differences in antibody levels dependent on infection, with the infected individuals showing greater antibody levels compared to the uninfected individuals (Figure 2).
Table 3. Variables affecting adult worm specific IgG levels.
| Dependent variable | |||||
| Independent variable | Total IgG | IgG1 | IgG2 | IgG3 | IgG4 |
| Sex | 2.695 (M>F) | 0.687 (M>F) | 0.377 (M<F) | 0.006 (M<F) | 1.063 (M>F) |
| Age group | 4.382 (1>2<3) | 8.068 (1<2<3) | 3.632 (1<2<3) | 2.699 (1<2<3) | 8.772 (1<2<3) |
| Infection status | 9.040 (sh−<sh+) | 5.302 (sh−<sh+) | 0.782 (sh−<sh+) | 1.520 (sh−<sh+) | 2.152 (sh−<sh+) |
| Age group* Infection status | 3.525 | 2.718 | 0.256 | 2.200 | 0.564 |
F values from ANOVA for relationship between antibody and variables. Model accounts for variation in sex (male (M) or female (F)), age group (1∶5–10 years; 2: 11–15 years; 3: >16 years), and infection status (uninfected: sh−; infected: sh+). The interaction term is designated by *. Direction of differences between variables is indicated in brackets below F value. Significant F values (p≤0.05) are indicated in bold.
Figure 2. Total adult worm-specific IgG levels differ by age group in healthy participants but not schistosome patients.
Adult worm specific (A) total IgG, (B) IgG1, (C) IgG2, (D) IgG3, (E) IgG4 responses by age group and infection status measured by ELISA. Open circles and dashed lines, healthy individuals; closed circles and solid lines, schistosome patients. Bars represent standard error of mean. Significant differences between schistosome infected and uninfected participants within age groups according to sub-group analysis are indicated by p: ** p≤0.005, *p≤0.05.
CD16 expression level is related to parasite-specific IgG and IgG1 levels in healthy participants but not in schistosome patients
The relationship between intensity of CD16 expression on all monocytes and IgG levels against SWAP was investigated to test the hypothesis that innate cell (monocyte) phenotype is related to schistosome-specific acquired immune markers (IgG). As both CD16 expression and IgG antibody titres showed significant relationships with infection status, the population was partitioned by infection status prior to analysing the relationship between IgG and CD16 by regression analysis. This analysis showed that in healthy, uninfected individuals, expression levels of CD16 rose significantly with levels of total IgG and IgG1 after allowing for variation due to age and sex (Table 4). Expression levels of CD16 in infected individuals did not show a relationship with parasite-specific total IgG or IgG1. In addition, levels of IgG2, IgG3 and IgG4 did not show a relationship with CD16 expression levels in either infected or uninfected participants (Table 4).
Table 4. Relationship between SWAP specific IgG and CD16 expression according to infection status.
| CD16 MFI | ||||
| Uninfected | Infected | |||
| Antibody subclass | β (SE) | p-value | β (SE) | p-value |
| Total IgG | 0.346 (0.268) | 0.007 | −0.188 (0.280) | 0.280 |
| IgG1 | 0.282 (0.127) | 0.032 | −0.069 (0.157) | 0.704 |
| IgG2 | −0.001 (0.148) | 0.993 | 0.231 (0.129) | 0.196 |
| IgG3 | 0.083 (0.661) | 0.538 | 0.053 (0.610) | 0.771 |
| IgG4 | 0.101 (0.095) | 0.448 | −0.149 (0.091) | 0.408 |
Regression (β) coefficients and the standard error (SE) and p-values from linear regression after accounting for sex and age differences. Significant p values and their associated β coefficients are indicated in bold.
Discussion
Human schistosome-specific IgE and IgG1 responses have been shown to be associated with resistance to infection [41]–[43]. In order to understand naturally developed protective immune responses that can be targets for artificial induction through vaccination, previous studies in schistosomiasis have focussed on describing the interaction between IgE and cellular mediators of protective effector responses, such as eosinophils and macrophages. However, following Phase 1b vaccination trials on the human hookworm vaccine candidate, it was found that inducing an IgE response in naturally exposed people caused a pathological immune response, compromising the vaccine's safety [23]. Subsequently, there has been a shift from developing IgE-mediated helminth vaccines towards vaccines that induce the IgG1 and IgG3 subclasses. This study focused on determining the relationship between schistosome-specific IgG subclasses and IgG FcγRIIIa (CD16) on human monocytes, a cell type related developmentally to the macrophage and which has been shown to have a significant role in immune responses to experimental schistosome infection. Monocytes have previously been demonstrated to be involved in immune response to schistosome larvae [11], [12], [44], and as circulating cells interacting with adult schistosome stages in the venules, monocytes are a highly relevant, but under studied innate immune cell type in human schistosomiasis.
The relationship of increasing IgG subtypes with age, as well as the higher expression of IgG against adult worm antigen in infection, has previously been reported [20], [45]. Anti-SWAP IgG1 and IgG4 are both dominant antibody subclasses in human schistosomiasis, while IgG2 and IgG3 are detected at lower levels [46]. In particular, IgG1 is associated with protection or developing immunity [43] and IgG4 is associated with infection [47]. These schistosome specific responses observed here in both the older uninfected and infected groups confirm that both age groups have been exposed to schistosome infection. Although the age at which this exposure occurred in the uninfected group is not clear, the increasing levels of all the antibody subclasses with age represent the cumulative exposure to adult schistosome antigens throughout the population's lifetime. In human immuno-epidemiological studies, the uninfected, older individuals are classified as putatively resistant to infection via an immune-mediated mechanism [43], [48], and the gradual increase in parasite-specific total IgG and IgG1 in this group is consistent with schistosome-specific immune responses associated with protection [20], [22].
The presence of infection in the older individuals who are lifelong residents of this schistosome endemic area suggests that they are carrying chronic infections, a fact that has been corroborated by other studies conducted in the same area and including some of the same participants [49]. In particular, chitinase 3-like 1 protein, a marker of inflammation that has been linked to schistosome-related hepatic fibrosis [50], was found to be highest in the older people harbouring infection [49]. Indeed, with increasing age, and thus duration of exposure to schistosomes, differences in the immune system become more apparent, and studies investigating myeloid derived dendritic cells from members of the same residential area have, similar to results presented here, shown age related changes with infection [33]. In this study, members of the younger patient cohort will have had a shorter infection history compared to the older age groups, and therefore have experienced much less schistosomiasis associated pathology [5], [42], [51]. The differences in CD16 expression dependent on age and infection may therefore be indicating an altered immune activation status in relation to schistosome infection, and may indicate a potential link between CD16 expression and pathology. Indeed, the pattern of increasing CD16 expression with age, observed here in the healthy individuals, has previously been noted in other populations [38], [39], and CD16 expression has previously been reported to be upregulated with monocyte maturation and activation [15], [52].
The absence of a relationship between monocyte CD14 expression levels and schistosome infection is likely related to CD14 being a lipopolysaccharide (LPS) receptor, which is involved in immunity against bacterial challenge [53], [54], and therefore playing a less significant role in schistosome-specific responses induced by adult worm antigens.
In contrast to the significant relationship between CD16 and infection status shown here, we found no relationship between infection intensity and either CD14 or CD16 expression (data not shown), indicating that it was the presence of infection in the host that was important in this relationship rather than the burden of infection, a pattern that has been demonstrated in other immunological and pathological features of human schistosomiasis [49], [55].
The positive correlation observed between the protective IgG antibodies (total IgG and IgG1) and monocyte CD16 in uninfected individuals, indicates that the CD16 expression level on monocytes may be associated with protection against infection, in association with an activated monocyte phenotype. Observations from research into monocyte involvement in human HIV infection report on CD16 - IgG mediated ADCC activity [56], [57], and there may be a similar mechanism mediating protection in schistosomiasis, in particular involving CD16-IgG1 interactions. However, the precise role of any ADCC mechanism warrants further investigation. Importantly, consistent with results from mechanistic mouse experimental studies of schistosomiasis [19], this relationship suggests a link between the innate and adaptive arms of the immune system in the response to schistosomiasis, which may be important for furthering vaccine research efforts.
The lack of association between the other IgG subclasses, IgG2, IgG3, and IgG4 may be due to antibody properties such as length of memory response [58], [59] and lack of affinity between the Fcγ receptor and the antibody, as is the case with IgG4; or the relatively low levels of expression associated with schistosome infection, as is the case with IgG3; or a combination of these factors, as may be the case for IgG2.
Although the study cannot determine causality from the observations made here, transfection studies of a macrophage cell line demonstrated that chronic inflammation inhibits FcyRIIIa (CD16) glycosylation, in turn reducing the ability for CD16 receptor activation following IgG binding [60]. Thus, chronically infected individuals in this study may have deficiencies in their CD16 receptor contributing to continued infection. In addition to the Fcγ receptor, CD16, monocytes also express CD32 (also known as FcγRIIb) an inhibitory receptor [61], and CD64 (also known as FcγR1a), a high affinity receptor for IgG [62]. Differential expression of these receptors may further indicate the capability of monocytes to activate in response to infection, and may enlighten on the role of other IgG subtypes in schistosome infection.
Altogether, our study demonstrated that monocyte CD16 expression is associated with protection against schistosome infection. The level of CD16 in healthy individuals is positively associated with levels of total IgG and IgG1, antibodies which have previously been associated with resistance to infection. Conversely, in schistosome infected patients who are lifelong residents of a schistosome endemic area, CD16 expression is significantly reduced. This decrease in expression of a monocyte activation marker, combined with the lack of association with protective IgG, may be a result of an altered immune activation state in chronic schistosomiasis infection.
Acknowledgments
We are grateful for the cooperation of the Ministry of Health and Child Welfare in Zimbabwe, the Provincial Medical Director of Mashonaland East, the Environmental Health Workers, and nursing staff at Chitate and Chitowa Clinics and Murehwa Hospital, and the residents, teachers and school children in Magaya, Chitate and Chipinda Schools. We also thank members of the National Institutes of Health in Zimbabwe and the Biochemistry Department at University of Zimbabwe for technical support. We are grateful to Professor Judith Allen (University of Edinburgh) for help with protocol development and discussions on the monocyte work.
Funding Statement
This work was supported by the World Health Organisation and the Wellcome Trust grant WT082028MA, the Thrasher Research Fund and the Medical Research Council grant LJA-544. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2012) WHA65.21 Elimination of schistosomiasis. Geneva: World Health Organization. [Google Scholar]
- 2.WHO (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 3.WHO (2006) Preventative Chemotherapy in Helminthiasis: coordinated use of antihelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization. [Google Scholar]
- 4. Kupferschmidt K (2013) A Worm Vaccine, Coming at a Snail's Pace. Science 339: 502–503. [DOI] [PubMed] [Google Scholar]
- 5. Woolhouse M, Taylor P, Matanhire D, Chandiwana S (1991) Acquired immunity and epidemiology of Schistosoma haematobium . Nature 351: 757–759. [DOI] [PubMed] [Google Scholar]
- 6. Loke Pn, Gallagher I, Nair MG, Zang X, Brombacher F, et al. (2007) Alternative Activation Is an Innate Response to Injury That Requires CD4+ T Cells to be Sustained during Chronic Infection. J Immunol 179: 3926–3936. [DOI] [PubMed] [Google Scholar]
- 7. Wynn TA, Barron L (2010) Macrophages: master regulators of inflammation and fibrosis. Seminars in liver disease 30: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nair M, Du Y, Perrigoue J, Zaph C, Taylor J, et al. (2009) Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. The Journal of Experimental Medicine 206: 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, et al. (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P (2003) L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends in Immunology 24: 302–306. [DOI] [PubMed] [Google Scholar]
- 11. Ellner JJ, Mahmoud AAF (1979) Killing of Schistosomula of Schistosoma mansoni by Normal Human Monocytes. The Journal of Immunology 123: 949–951. [PubMed] [Google Scholar]
- 12. Olds GR, Ellner JJ, el-Kholy A, Mahmoud AA (1981) Monocyte-mediated killing of schistosomula of Schistosoma mansoni: alterations in human Schistosomiasis mansoni and tuberculosis. The Journal of Immunology 127: 1538–1542. [PubMed] [Google Scholar]
- 13. Xu X, Remold HG, Caulfield JP (1993) Potential role for scavenger receptors of human monocytes in the killing of Schistosoma mansoni . The American journal of pathology 142: 685–689. [PMC free article] [PubMed] [Google Scholar]
- 14. van de Winkel JGJ, Capel PJA (1993) Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunology Today 14: 215–221. [DOI] [PubMed] [Google Scholar]
- 15. Zawada AM, Rogacev KS, Rotter Br, Winter P, Marell R-R, et al. (2011) SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118: e50–e61. [DOI] [PubMed] [Google Scholar]
- 16. Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, et al. (2012) The three human monocyte subsets: implications for health and disease. Immunol Res 53: 41–57. [DOI] [PubMed] [Google Scholar]
- 17. Gessner JE, Heiken H, Tamm A, Schmidt RE (1998) The IgG Fc receptor family. Annals of Hematology 76: 231–248. [DOI] [PubMed] [Google Scholar]
- 18. Hazenbos WLW, Heijnen IAFM, Meyer D, Hofhuis FMA, Renardel de Lavalette C, et al. (1998) Murine IgG1 Complexes Trigger Immune Effector Functions Predominantly via FcγRIII (CD16). The Journal of Immunology 161: 3026–3032. [PubMed] [Google Scholar]
- 19. Jankovic D, Cheever AW, Kullberg MC, Wynn TA, Yap G, et al. (1998) CD4+ T Cell–mediated Granulomatous Pathology in Schistosomiasis Is Downregulated by a B Cell–dependent Mechanism Requiring Fc Receptor Signaling. The Journal of Experimental Medicine 187: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mutapi F, Ndhlovu PD, Hagan P, Woolhouse ME (1997) A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol 19: 255–263. [DOI] [PubMed] [Google Scholar]
- 21. Khalife J, Dunne DW, Richardson BA, Mazza G, Thorne KJ, et al. (1989) Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni . The Journal of Immunology 142: 4422–4427. [PubMed] [Google Scholar]
- 22. Mutapi F, Mduluza T, Gomez-Escobar N, Gregory WF, Fernandez C, et al. (2006) Immuno-epidemiology of human Schistosoma haematobium infection: Preferential lgG3 antibody responsiveness to a recombinant antigen dependent on age and parasite burden. BMC Infectious Diseases 6: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotez P, Bethony J, Diemert D, Pearson M, Loukas A (2011) Developing Vaccines to Combat Hookworm Infection and Intestinal Schistosomiasis. The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies. Washington D.C.: National Academies Press. [Google Scholar]
- 24. Mitchell KM, Mutapi F, Savill NJ, Woolhouse ME (2012) Protective immunity to Schistosoma haematobium infection is primarily an anti-fecundity response stimulated by the death of adult worms. Proc Natl Acad Sci U S A 109: 13347–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutapi F, Burchmore R, Foucher A, Harcus Y, Nicoll G, et al. (2005) Praziquantel treatment of people exposed to Schistosoma haematobium enhances serological recognition of defined parasite antigens. Journal of Infectious Diseases 192: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 26. Mutapi F, Burchmore R, Mduluza T, Midzi N, Turner CM, et al. (2008) Age-related and infection intensity-related shifts in antibody recognition of defined protein antigens in a schistosome-exposed population. J Infect Dis 198: 167–175. [DOI] [PubMed] [Google Scholar]
- 27. Joseph S, Jones FM, Walter K, Fulford AJ, Kimani G, et al. (2004) Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. Journal of Infectious Diseases 190: 835–842. [DOI] [PubMed] [Google Scholar]
- 28. Appleby LJ, Nausch N, Midzi N, Mduluza T, Allen JE, et al. (2013) Sources of heterogeneity in human monocyte subsets. Immunology Letters 152: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Midzi N, Sangweme D, Zinyowera S, Mapingure MP, Brouwer KC, et al. (2008) The burden of polyparasitism among primary schoolchildren in rural and farming areas in Zimbabwe. Transactions of the Royal Society of Tropical Medicine and Hygiene 102: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 30. Mott K (1983) A reusable polyamide filter for diagnosis of S. haematobium infection by urine filtration. Bull Soc Pathol Exot Filiales 76: 101–104. [PubMed] [Google Scholar]
- 31. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de Sao Paulo 14: 397–400. [PubMed] [Google Scholar]
- 32. Knight WB, Hiatt RA, Cline BL, Ritchie LS (1976) A Modification of the Formol-Ether Concentration Technique for Increased Sensitivity in Detecting Schistosoma Mansoni Eggs. The American Journal of Tropical Medicine and Hygiene 25: 818–823. [DOI] [PubMed] [Google Scholar]
- 33. Nausch N, Louis D, Lantz O, Peguillet I, Trottein F, et al. (2012) Age-related patterns in human myeloid dendritic cell populations in people exposed to Schistosoma haematobium infection. PLoS Neglected Tropical Diseases 6: e1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, et al. (2012) CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi)/CD16(neg) monocytes: Expansion of CD14(hi)/CD16(pos) and contraction of CD14(lo)/CD16(pos) monocytes in acute liver failure. Cytometry Part A : the journal of the International Society for Analytical Cytology 81: 823–834. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RM, May RM (1991) Infectious diseases of humans : dynamics and control/Roy M. Anderson and Robert M. May: Oxford: Oxford University Press, 1991. [Google Scholar]
- 36. Chandiwana SK, Woolhouse MEJ (1991) Heterogeneities in water contact patterns and the epidemiology of Schistosoma haematobium . Parasitology 103: 363–370. [DOI] [PubMed] [Google Scholar]
- 37. Mutapi F, Roddam A (2002) p-values for pathogens: statistical inference from infectious-disease data. Lancet Infect Dis 2: 219–230. [DOI] [PubMed] [Google Scholar]
- 38. Nyugen J, Agrawal S, Gollapudi S, Gupta S (2010) Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol 30: 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F (2010) Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunology 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, et al. (1993) The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 82: 3170–3176. [PubMed] [Google Scholar]
- 41. Hagan P, Blumenthal UJ, Dunn D, Simpson AJG, Wilkins HA (1991) Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium . Nature 349: 243–245. [DOI] [PubMed] [Google Scholar]
- 42. Capron A, Dessaint JP (1992) Immunologic aspects of schistosomiasis. Annual review of medicine 43: 209–218. [DOI] [PubMed] [Google Scholar]
- 43. Mutapi F, Ndhlovu P, Hagan P, Spicer J, Mduluza T, et al. (1998) Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. Journal of Infectious Diseases 178: 289–293. [DOI] [PubMed] [Google Scholar]
- 44. Turner JD, Bourke CD, Meurs L, Mbow M, Dièye TN, et al. (2014) Circulating CD14brightCD16+ ‘Intermediate’ Monocytes Exhibit Enhanced Parasite Pattern Recognition in Human Helminth Infection. PLoS Negl Trop Dis 8: e2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mutapi F, Bourke C, Harcus Y, Midzi N, Mduluza T, et al. (2011) Differential recognition patterns of Schistosoma haematobium adult worm antigens by the human antibodies IgA, IgE, IgG1 and IgG4. Parasite Immunology 33: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boctor FN, Peter JB (1990) IgG subclasses in human chronic schistosomiasis: over-production of schistosome-specific and non-specific IgG4. Clinical and experimental immunology 82: 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caldas IR, Correa-Oliveira R, Colosimo E, Carvalho OS, Massara CL, et al. (2000) Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. The American Journal of Tropical Medicine and Hygiene 62: 57–64. [DOI] [PubMed] [Google Scholar]
- 48. Black CL, Mwinzi PN, Muok EM, Abudho B, Fitzsimmons CM, et al. (2010) Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni . PLoS Neglected Tropical Diseases 4: e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Appleby LJ, Nausch N, Bourke CD, Rujeni N, Midzi N, et al. (2012) Chitinase 3-like 1 protein levels are elevated in Schistosoma haematobium infected children. PLoS Neglected Tropical Diseases 6: e1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng M, Cai WM, Zhao JK, Zhu SM, Liu RH (2005) Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop 96: 148–152. [DOI] [PubMed] [Google Scholar]
- 51. Jankovic D, Wynn TA, Kullberg MC, Hieny S, Caspar P, et al. (1999) Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. Journal of Immunology 162: 345–351. [PubMed] [Google Scholar]
- 52. Clarkson SB, Ory PA (1988) CD16. Developmentally regulated IgG Fc receptors on cultured human monocytes. The Journal of Experimental Medicine 167: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziegler-Heitbrock HW, Ulevitch RJ (1993) CD14: cell surface receptor and differentiation marker. Immunology Today 14: 121–125. [DOI] [PubMed] [Google Scholar]
- 54. Landmann R, Knopf HP, Link S, Sansano S, Schumann R, et al. (1996) Human monocyte CD14 is upregulated by lipopolysaccharide. Infection and Immunity 64: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. King CH (2007) Lifting the burden of schistosomiasis–defining elements of infection-associated disease and the benefits of antiparasite treatment. J Infect Dis 196: 653–655. [DOI] [PubMed] [Google Scholar]
- 56. Kramski M, Schorcht A, Johnston AP, Lichtfuss GF, Jegaskanda S, et al. (2012) Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. Journal of immunological methods 384: 51–61. [DOI] [PubMed] [Google Scholar]
- 57. Webster NL, Kedzierska K, Azzam R, Paukovics G, Wilson J, et al. (2006) Phagocytosis stimulates mobilization and shedding of intracellular CD16A in human monocytes and macrophages: inhibition by HIV-1 infection. Journal of Leukocyte Biology 79: 294–302. [DOI] [PubMed] [Google Scholar]
- 58. Isa MB, Martinez L, Giordano M, Passeggi C, de Wolff MC, et al. (2002) Comparison of immunoglobulin G subclass profiles induced by measles virus in vaccinated and naturally infected individuals. Clinical and diagnostic laboratory immunology 9: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Woolhouse M, Hagan P (1999) Seeking the ghost of worms past. Nature Medicine 5: 1225–1227. [DOI] [PubMed] [Google Scholar]
- 60. Drescher B, Witte T, Schmidt RE (2003) Glycosylation of FcγRIII in N163 as mechanism of regulating receptor affinity. Immunology 110: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ravetch JV, Bolland S (2001) IgG Fc receptors. Annu Rev Immunol 19: 275–290. [DOI] [PubMed] [Google Scholar]
- 62. Li J, Pritchard DK, Wang X, Park DR, Bumgarner RE, et al. (2007) cDNA microarray analysis reveals fundamental differences in the expression profiles of primary human monocytes, monocyte-derived macrophages, and alveolar macrophages. J Leukoc Biol 81: 328–335. [DOI] [PubMed] [Google Scholar]