Abstract
Chinese honeybee Apis cerana (Ac) is one of the major Asian honeybee species for local apiculture. However, Ac is frequently damaged by Chinese sacbrood virus (CSBV), whereas Apis mellifera (Am) is usually resistant to it. Heterospecific royal jelly (RJ) breeding in two honeybee species may result in morphological and genetic modification. Nevertheless, knowledge on the resistant mechanism of Am to this deadly disease is still unknown. In the present study, heterospecific RJ breeding was conducted to determine the effects of food change on the larval mortality after CSBV infection at early larval stage. 2-DE and MALDI-TOF/TOF MS proteomic technology was employed to unravel the molecular event of the bees under heterospecific RJ breeding and CSBV challenge. The change of Ac larval food from RJC to RJM could enhance the bee resistance to CSBV. The mortality rate of Ac larvae after CSBV infection was much higher when the larvae were fed with RJC compared with the larvae fed with RJM. There were 101 proteins with altered expressions after heterospecific RJ breeding and viral infection. In Ac larvae, 6 differential expression proteins were identified from heterospecific RJ breeding only, 21 differential expression proteins from CSBV challenge only and 7 differential expression proteins from heterospecific RJ breeding plus CSBV challenge. In Am larvae, 17 differential expression proteins were identified from heterospecific RJ breeding only, 26 differential expression proteins from CSBV challenge only and 24 differential expression proteins from heterospecific RJ breeding plus CSBV challenge. The RJM may protect Ac larvae from CSBV infection, probably by activating the genes in energy metabolism pathways, antioxidation and ubiquitin-proteasome system. The present results, for the first time, comprehensively descript the molecular events of the viral infection of Ac and Am after heterospecific RJ breeding and are potentially useful for establishing CSBV resistant populations of Ac for apiculture.
Introduction
Apis mellifera (Am) and A. cerana (Ac) are major honey bee species in the global beekeeping industry [1], [2]. They are heavily infected by different vital viruses [3], [4]. Chinese sacbrood virus (CSBV) is the most stricken pathogen of Ac, which results in severe and deadly infections of the colony and eventually losses of the entire colony [5]. As a small RNA virus (picorna-like virus) that has an icosahedral virion with a diameter of 26–30 nm, CSBV genome consists of a single positive-strand RNA molecule with 8.8 kilo bases (kb) [7]. Although CSBV shows a close genetic relationship to its western counterpart sacbrood virus (SBV), there is no cross infection [8]. Since this viral disease broke out in 1972 in southern China [8], [9], some efforts have been made to study this virus, such as diagnostic methods (electron microscopy, enzyme-linked immunosorbent assay and reverse transcription-polymerase chain reaction (RT-PCR)) [10], and the control of this disease by RNA interference [11]. It is interesting that A. mellifera is not sensitive to CSBV in general beekeeping practice [12]. Similar to all other insects, the honeybees lack a classically adaptive immune system as in the case of mammalian [6]. To survive, they have evolved cellular and humoral immune responses to cope with microbial infections. A. mellifera may develop cellular and humoral immune responses to various pathogens such as bacteria [13], [14], viruses [15], microsporidian [16]–[18] and Varroa mites [19], [20]. Usually, the viral infection causes cell apoptosis, tissue damage and even functional disorder of the whole organism and all these changes can be further reflected in proteome alteration [21]. Recent study revealed the pathological mechanism of Chinese sacbrood disease to Ac [6]. However, no information on the difference in the immune responses of two bee species to CSBV is reported.
In the honey bees, queen and workers have different behavior and reproductive capacity despite possessing the same genome. The primary substance that leads to this differentiation is royal jelly (RJ), which contains a range of proteins, carbohydrates, lipids, minerals, vitamins, and a large number of bioactive substances, especially immunological peptides and antibacterial proteins [22]–[24]. Major Royal Jelly Proteins (MRJPs) are the prime RJ ingredients, which are crucial in regulating reproductive maturation [22]. There are quantitative differences in nucleic acids and protein composition in fresh RJ between Am and Ac [24]. The most recent discovery is that RJ contains microRNAs which may play a role in caste differentiation [25]–[27]. Whether the RJ also influences the resistant behavior of Am to CSBV is unknown. The molecular event for CSBV resistance of Am needs to be also determined.
Two-dimensional gel electrophoresis (2-DE) based proteomics technology and matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) are powerful molecular tools. In recent years, proteomic techniques have been successfully used to profile the proteome change in insect growth and development [6], [28]–[30]. The present study employed gel-based (2-DE) and shotgun proteomic (label-free LC-MS based) strategies, which have complementary natures, to gain an in-depth understanding of the resistant mechanism of Am to the fatal CSBV disease by comparison of the proteome-wide change of the healthy and sick worker larvae of both Am and Ac fed with RJ either from Am (RJM) or Ac (RJC).
In the present study, we performed comparison analyses of the proteome in Ac and Am after heterospecific RJ breeding (i.e. Am larvae fed with RJC or Ac larvae fed with RJM), and then CSBV challenge, by two-dimensional electrophoresis and MALDI-TOF MS analysis, to systematically search for the molecular basis of the crossbreeding and CSBV challenge. A total of 101 differentially expressed nonredundant proteins (≥3 folds change) were identified. Furthermore, gene expression patterns in two bee species were investigated at mRNA levels. The results show that differential expression proteins are involved in the regulation of metabolism and response to CSBV challenge.
Materials and Methods
Ethics Statement
Honey bee colonies (Am and Ac) were raised at Conghua, Guangdong Province, China (113°17' E, 23°8'' N), by Guangdong Entomological Institute, by standard beekeeping techniques. According to the present regulation from Ministry of Agriculture Am and Ac used in this studies are not endangered or protected species. No specific permissions were required for performing these experiments.
Honey Bees
To obtain age controlled second instar larvae, the queen was caged on a comb and left to lay eggs for 6 h. Twenty hours after larval eclosion, or 92 h after oviposition, the comb containing second instar larvae was retrieved from the colony, and placed in the laboratory for treatments at 32–34°C. All the larvae used in this study were detected by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) method for the absence of the following viruses, black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), deformed wing virus (DWV), kashmir bee virus (KBV), Chinese sacbrood virus (CSBV) and Israeli acute paralysis virus (IAPV), with the primers from Ai et al. [4]. RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, USA) and cDNA synthesis was performed using PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Kyoto, Japan). Amplification profile of PCR consisted of an initial 2-min denaturation at 94°C, followed by 35 cycles of 30 s at 95°C, 30 s at 55°C, 1 min at 72°C and finally 7-min cycle at 72°C. No signs of clinical American foulbrood or other viral disease were observed in these larvae.
Virus
CSBV-infected larvae with typical symptom were collected from an apiary at Xinhua Village from Hunan Province, and kept at - 80°C for less than 10 days before use. The presence of CSBV virus was confirmed by observation of the morphological symptom under electronic microscopy and RT-PCR according to Chen et al. and Yan et al. [3], [10].
To obtain CSBV, the larvae (approximately 0.2 g) infected by CSBV were ground in 6 mL sterile phosphate-buffered solution (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) with a sterile grinder. The resulting solution was centrifuged at 14 000 g at 4°C for 10 min, and the supernatant was further passed through a 0.45 µm cell filter first, then through a 0.22 µm cell filter [31]. The CSBV concentration was quantitated by absolute quantification assay [11]. CSBV polyprotein gene (SBV1) was amplified to detect copy number of CSBV according to Liu et al. [11]. PCR reactions were carried out in triplicate in an Mx3000P Real-Time PCR System (Stratagene, California, USA), using SYBR_Green (Brilliant II SYBR-Green QPCR Master Mix; Stratagene, California, USA). The CSBV concentration for the experiments was approximately 7.9×105 copies/mL.
Harvest of RJM and RJC
RJM and RJC were produced according to standard practices in China [32]. Briefly, the queen was confined inside a queen excluding cage. Queen cups with young larvae (one-day old) were introduced into the colony and allowed to be fed by workers for 2 days. 3-day old larvae were first carefully removed by using either a grafting tool or a pair of forceps, then the royal jelly was removed by using a spatula.
Larvae Fed with RJM or RJC and Challenged with CSBV
To determine the effects of the changing food (RJs) on the mortality and protein expression of the third instar larvae, which are the most sensitive stage to CSBV infection, the second instar larvae of Ac and Am were collected carefully from the combs, respectively transferred to 96-cell culture plates containing 30 µL RJC or RJM, in each cell, then placed in a cabinet (Sanyo, Tokyo, Japan) at 80% relative humidity at 32–34°C. After 24 h, the third instar larvae of both bee species fed with RJM or RJC were transferred to new plates, and challenged with 30 µL CSBV solution per cell, prepared as described above. After 8 h, the larvae were washed with sterile distilled water for 3 times, and kept at −80°C for use. 8 treatments (Table 1) were established with 3 replicates containing 20 larvae in each replicate. Each 20 larvae were pooled as one biological replicate and three independent biological replicates were produced.
Table 1. Treatments of the two honey bee species by RJs and CSBV.
| No. | Honey bees | Treats | Abbreviation |
| A | A. cerana | fed on A. cerana royal jelly | AC-RJC |
| B | fed on A. cerana royal jelly, and challenged with CSBV | AC-RJC+CSBV | |
| C | fed on A. mellifera royal jelly | AC-RJM | |
| D | fed on A. mellifera royal jelly, and challenged with CSBV | AC-RJM+CSBV | |
| E | A. mellifera | fed on A. cerana royal jelly | AM-RJC |
| F | fed on A. cerana royal jelly, and challenged with CSBV | AM-RJC+CSBV | |
| G | fed on A. mellifera royal jelly | AM-RJM | |
| H | fed on A. mellifera royal jelly, and challenged with CSBV | AM-RJM+CSBV |
The same treatments were also established to examine the mortality of bee larvae fed with RJM or RJC and challenged by CSBV after 24 h. The death of the larvae was confirmed when they had no response after stimulating with a soft tip, and by the color change of their bodies (from white to yellow or even dark).
Protein Extraction and 2-DE
Larval protein extractions were carried out according to the previously described method with some modifications [33], [34].
Bee larvae were manually homogenized for 30 min and sonicated for 2 min on ice in lysis buffer (8 M urea, 0.2% w/v Bio-Lyte 3/10 Ampholyte (Bio-Rad, USA), 4% CHAPS, 65 mM DTT) containing Protease Inhibitor Cocktail (Calbiochem, Germany)(w/l = 1 mg/4 µL). The mixture was centrifuged 15 000 g at 4°C for 30 min, and the supernatant was collected. The resulting precipitates were treated three times with the same procedures. All the supernatants were centrifuged 15 000 g at 4°C for 60 min. The final supernatant was used or stored at −80°C for 2-DE. Protein concentrations were determined by the Bradford method using Modified Bradford Protein Assay Kit (Sangon, Shanghai, China).
The 2-DE was performed according to the methods described previouslyand the manufacturer's instruction [35]. The first dimension (isoelectric focusing) was conducted using the IPGphor IEF system (Bio-Rad, California, USA) at 20°C. For analytical gels, 350 µg protein was solubilized in 400 µL rehydration solution (8 M urea, 0.2% w/v Bio-Lyte 3/10 Ampholyte, 4% CHAPS, 65 mM DTT, 0.001% w/v bromophenol blue), and loaded onto a 17 cm pH 3–10 NL IPG strip (Bio-Rad). Focusing was performed for 13 h at 50 V, 1 h at 500 V, 1 h at 1000 V, and 30 min and 6 h at 8000 V (total = 48 kVh). The IPG strips were equilibrated as previously described. The second dimension was performed with 13% (w/v) SDS-polyacrylamide gels using the Protean II xi 2D Multicell system (Bio-Rad, California, USA). Proteins were stained with silver nitrate, and gels were digitized using Image ScannerII (Amersham Biosciences, USA). Digitized 2-DE gel patterns were edited and matched using the PDQUEST V8.0.1 software package (PDI, Humington Station). Triplicate experiments were run to confirm the reproducibility of results.
Protein Identification by MALDI-TOF/TOF MS
Protein digestion and peptide extraction were done according to our previously established protocol [36]. Spots of interest in gels staining with silver nitrate were cut out, washed, reduced, S-alkylated with iodoacetamide and in-gel digested at 37°C overnight with sequencing grade porcine trypsin (Promega, USA). After extraction in extractant of 50% ACN (Fisher, Waltham, MA,USA) and 2.5% TFA (Sigma, St Louis, MO, USA), peptide mixtures were analyzed using a saturated solution of 5 mg/mL α-cyano-4-hydroxycinnamic acid (CHCA, Sigma, St Louis, MO, USA) in ACN containing 0.1% TFA (Sigma, St Louis, MO, USA) (50/50 v/v) using a 4800 Proteomics Analyzer equipped with matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Applied Biosystems, Framingham, MA, USA). For MS calibration, the trypsin autolysis peptides were used as internal calibrants. Monoisotopic peak masses were automatically determined within the mass range of 800–4000 Da, with a minimum S/N of 50. Five of the most intense ion signals were selected as precursors for MS/MS acquisition. Combined MS and MS/MS queries were performed with the MASCOT search engine (V2.1, Matrix Science, UK) embedded in GPS-Explorer Software (V3.6, Applied Biosystems, Framingham, MA, USA), using the A. mellifera database (Gene DB). MASCOT protein scores (based on combined MS and MS/MS spectra) of greater than 61 were considered statistically significant (P≤0.05). The individual MS/MS spectrum with statistically significant (confidence interval >95%) best ion score (based on MS/MS spectra) were also accepted.
Based on the UniProt Knowledgebase (http://www.uniprot. org/), identified proteins were submitted to KEGG (Kyoto Encyclopedia of Genes and Genomes) and classified according to KEGG pathway maps (http://www.genome.jp/kegg/pathway.html) and Gene Ontology (http://www.geneontology.org/), grouped on the basis of their biological process of GO terms (http://www.blast2go.com) [19]. Protein interaction networks of the differentially regulated proteins were analyzed using the online database resource Search Tool for the Retrieval of Interacting Genes (STRING: http://string-db.org) [37], [38].
Quantitative Real-Time PCR (qRT-PCR)
To verify the gene expression of differentially expressed proteins, 11 proteins (FBA, LOC408516, MDH, SOD, Jafrac1, LOC725646, AGO1, Che-3, Ald, Pxd and Tpi) were randomly selected for qRT-PCR analysis of their encoding genes by quantitative real time PCR, according to our previously method with some modifications [19]. Briefly, total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, USA). The RNA was dissolved in diethylpyrocarbonate (DEPC) treated water and quantified by measuring ultraviolet absorbance at 260 and 280 nm. Reverse transcription was performed on 1 µg of total RNA using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Kyoto, Japan). The gene-specific primers for the analysis are listed in Table 2. Expression of actin gene (GI: 406122) was used as an internal control. The efficiency of each primer set was first validated by constructing a standard curve through five serial dilutions. PCR reactions were carried out in triplicate in an Mx3000P Real-Time PCR System (Stratagene, California, USA), using SYBRGreen (SYBR Premix Ex Taq II, Takara, Kyoto, Japan). A control without template was included in all batches. The PCR program began with a single cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s and 48°C for 60 s. Afterwards, the PCR products were heated to 95°C for 15 s, cooled to 48°C for 15 s and heated to 95°C for 15 s, in order to measure the dissociation curves and to determine a unique PCR product for each gene. mRNA levels were calculated relative to actin expression using the Mx3000P Software (version 4.1) (Agilent, California, USA). The fold change was calculated using the 2−ΔΔCt method. Each sample was analyzed independently and processed in triplicate.
Table 2. Primers for qRT-CR analysis of 12 differentially expressed genes.
| Product name | Gene ID | Forward primer sequence | Reverse primer sequence | Amplicon size (bp) |
| Actin | gi|406122 | ATGCCAACACTGTCCTTTCTGG | GACCCACCAATCCATACGGA | 150 |
| FBA | gi|66526635 | TTTCTAGTATCTAAAGCATG | ACTGCTATTGCTACTGCT | 228 |
| LOC408516 | gi|66524882 | GATCCAGATGCACCAAG | GGACAGAATCGTGGAAA | 235 |
| MDH | gi|66513092 | AAGGCTGGCACAGGTTC | TAAATGCAGCGATCCCA | 239 |
| SOD | gi|33089104 | ACTTGTCGTTCCGTGTA | CACATTTCAACCCATTA | 229 |
| Jafrac1 | gi|66548188 | AAACTCATTGCAGCATC | GGGAGGTCTGTTGATGA | 250 |
| LOC725646 | gi|110755974 | ACCCGAATTGTACTTTA | TAGATGAAGGTGCTCAA | 221 |
| AGO1 | gi|110777044 | TGGCCCAGATCAAGTAGAGC | AATTTGATAGCGTTTGTGGTGAT | 200 |
| Che-3 | gi|110757336 | TCCTGTCCGAATTTTTACCTGT | GAAGCTGCGTTTGCGTCTA | 250 |
| Ald | gi|110748949 | TGCGTACTGTTCCACCT | GAGGGCTAAGGCTAACA | 233 |
| Pxd | gi|110757934 | CGCTGTTCCAAGAGGCT | ACGACAAACGCCAGATC | 250 |
| Tpi | gi|148224276 | TCAGTAACAGCAGGAAAT | TTTATGTCATGTTCCTGCAA | 269 |
Western Blot
To further verify the variation tendency of differentially expressed proteins identified by the proteomic approaches, glyceradehyde-3-phosphate dehydrogenase 2 isofome 1(GAPDH), fmarylacetoacetase hydrolase (FAH), heat shock protein (Hsp)60, phosphoglycerate kinase (PGK), triosephosphate isomerase 1 (Tpi), CuZn superoxide dismutase (SOD) and fructose-bisphophate aldolase (FBA) were randomly selected for Western Blot analysis by the method of Han et al. [6] with minor modifications. Briefly, equal amount of protein sample (30 µg/lane) were separated by stacking (5%) and separating (12%) SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gels and then transferred to a nitrocellulose transfer membrane (0.2 µm pore size) (Invitrogen, Eugene, OR) using the iBlot apparatus (Invitrogen, Eugene, OR). After blocking, the membranes were incubated over night at 4°C with primary rabbit polyclonal antibodies of anti-GAPDH, FAH, Hsp60, PGK, Tpi, SOD, and FBA antibodies (Abcam, Cambridge, MA) at a dilution of 1∶1000. Following three washes, the membranes were further incubated with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (BOSTER, Wuhan, China) at a dilution of 1∶2000 for 1 h. Immunoreactive protein bands were detected using the DAB Western Blotting Substrate (BOSTER, Wuhan, China) and quantified by densitometry using Quantity-one image analysis system (Bio-Rad, Hercules, CA). Actin was detected simultaneously as loading control of the analysis, which immunoreactived by BCIP/NBT Western Blotting Substrate (BOSTER, Wuhan, China). Quantification of the protein bands was conducted by scanning the films and importing the images into the Quantity One 1-D analysis software (Bio-Rad, Hercules, CA). Scanning densitometry was used for semiquantitative analysis of the data.
Data Analysis
The statistical analysis of gene expression was performed by one-way ANOVA test followed by Tukey's test for pair comparisons (SPSS version 16.0, SPSS, Inc.). Comparison of larval mortality differences between different groups was performed by independent samples t test. An error probability p<0.05 was considered statistically significant.
Results
Effects of RJM and RJC on the Mortality of Two Bee Species after CSBV Challenge
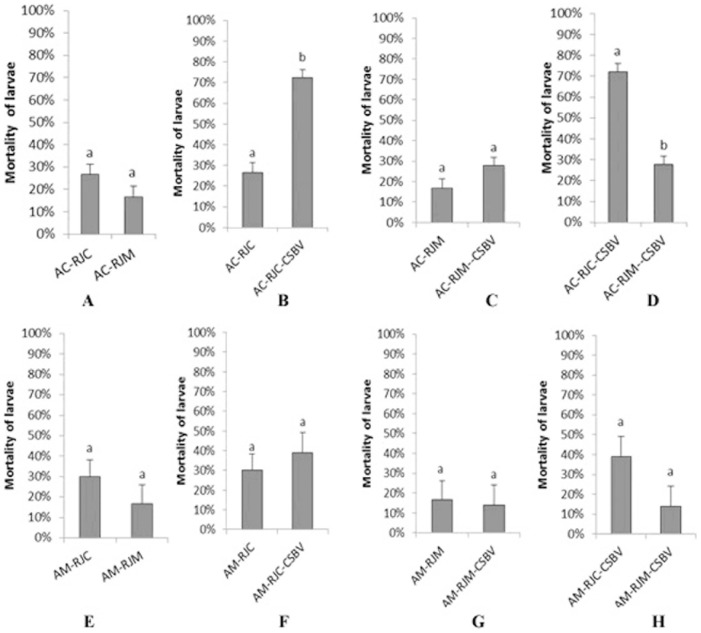
Experiments were carried out to examine whether RJM would enhance the resistant ability of Ac larvae to CSBV infection. The larval mortalities of the treatments after 56 h were indicated in Figure 1. The dead larvae showed the symptom of CSBV infection. Without CSBV infection, the mortalities of Ac larvae fed with RJC or RJM were not significantly different (t = 2.040, df = 4, p = 0.111; Figure 1 A); meanwhile, the mortalities of Am larvae fed with RJM or RJC were also not significantly different (t = 1.572, df = 4, p = 0.191; Figure 1 E). This indicated that the RJs did not significantly influence the mortalities of the larvae of two bee species, at least at key early larval stage.
Figure 1. The larval mortalities of A. cerana and A. mellifera under different treatments after 56 hours.
RJC: royal jelly from A. cerana; RJM: royal jelly from A. mellifera; AC-RJC: 3-day Ac larvae fed with RJC. AC-RJM: 3-day Ac larvae fed with RJM. AC-RJC+CSBV: 3-day Ac larvae fed with RJC, and infected with CSBV. AC-RJM+CSBV: 3-day Ac larvae fed with RJM, and infected with CSBV. AM-RJC: 3-day Am larvae fed with RJC. AM-RJM: 3-day Am larvae fed with RJM. AM-RJC+CSBV: 3-day Am larvae fed with RJC, and infected with CSBV. AM-RJM+CSBV: 3-day Am larvae fed with RJM, and infected with CSBV. The data are averages from three replicates. The error bars indicate standard deviations. Bars with different letters mean significantly different (P<0.05, independent samples t test).
However, the mortality (72.2%) of Ac larvae fed with RJC and subsequent CSBV infection was significantly higher than that of the larvae fed with RJC (26.7%) but without CSBV (t = −9.642, df = 3.801, p = 0.001; Figure 1 B); When Ac larvae were fed with RJM and subsequently challenged by CSBV, their mortality was not significantly different than that of those larvae fed with RJM and without CSBV (t = −2.447, df = 4, p = 0.071; Figure 1 C); Interestingly, when Ac larvae were fed with RJC or RJM and subsequently challenged with CSBV, the mortality (72.2%) of Ac larvae fed with RJC was significantly higher than that of those larvae fed with RJM (27.8%) (t = 10.674, df = 4, p = 0.000; Figure 1 D). This indicated that RJM conferred antiviral activity for Ac larvae.
When Am larvae were fed with RJC or RJM, and subsequently challenged with CSBV, no significant differences in mortalities of Am larvae were observed (t = 1.572, df = 4, p = 0.191, Figure 1 E; t = −0.941, df = 4, p = 0.400, Figure 1 F; t = −0.512, df = 4, p = 0.636, Figure 1 G; t = 2.000, df = 4, p = 0.116, Figure 1 H). So the RJs did not influence the mortality of Am larvae after CSBV challenge. As usual, Am is not sensitive to CSBV infection.
Qualitative Comparisons and Identification of Differentially Expressed Proteins
To determine the effects of the RJ on the protein expression of two bee species after CSBV infection, the protein spots were visualized by 2-DE from the bee larvae of Ac and Am fed with RJM or RJC, and with RJM or RJC for 24 h and then challenged by CSBV for 8 h. 8 h after CSBV challenged, there was no dead larvae, although the virus was detected in the challenged larvae.
The total proteins were separated on 2-DE gels spanning pH 3–10, silver stained, and analyzed by MS. Protein levels were expressed as percentage volume, which corresponds to the percentage ratio between the volume of a single spot and the total volume of all spots present in a gel. The mean values of spot intensity were calculated using at least three gels. Spots showing more than 15% variation were not considered (Student's test, with 7 degrees of freedom, P<0.05). Little deviation was observed in the patterns on replica gels.
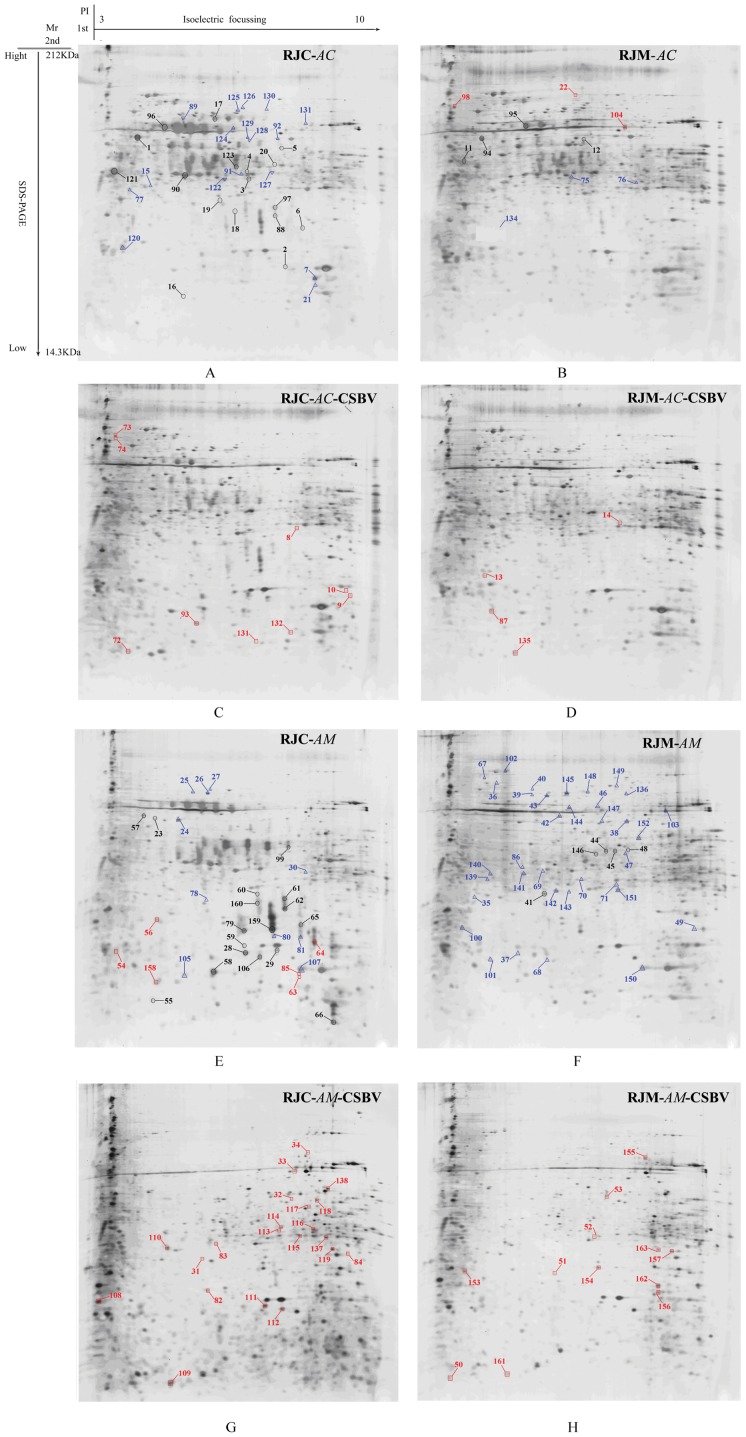
530–670 spots were revealed on the silver-stained 2-DE patterns of the total proteins from different larvae, depending on bee species and the treatments (Figure 2). Protein spots were distributed over the 3–10 pH range.
Figure 2. 2-DE pictures of A. cerana and A. mellifera 3-day old larvae fed with RJs and by CSBV infection.
RJC: royal jelly from A. cerana; RJM: royal jelly from A. mellifera; A: 3-day old Ac larvae fed with RJC. B: 3-day old Ac larvae fed with RJM. C: 3-day old Ac larvae fed with RJC and by CSBV infection. D: 3-day old Ac larvae fed with RJM and CSBV infection. E: 3-day old Am larvae fed with RJC. F: 3-day old Am larvae fed with RJM. G: 3-day old Am larvae fed with RJC and CSBV infection. H: 3-day old Am larvae fed with RJM and by CSBV infection.
Quantitatively, 296 total spots showed significant changes of expression (by a factor of ≥3-folds and p<0.05). All differentially expressed spots were identified by a MALDI-TOF/TOF mass spectrometer, and submitted to the NCBI nr database or the NCBI EST_ database. 163 differentially expressed proteins were successfully identified (Figure 2), among which 44 were identified as MRJPs, and 119 were clustered to 101 non-redundant proteins. The up-regulated and down-regulated proteins from the 3-day old Ac and Am larvae fed with RJC or RJM, and with CSBV infection were listed (Table 3).
Table 3. Identification of differentially expressed proteins.
| No. | Protein Name | Gene | Accession No. | Protein MW (kDa)/PI | Protein Score C.I. % | GO | EC No. | Fold change | ||||||||
| AC-RJM-up | ||||||||||||||||
| 22 | SF2 CG6987-PA | SF2 | gi|66548276 | 28.4/9.88 | 100 | F:nucleotide binding | - | ↑∞ | ||||||||
| 98 | vasa intronic gene CG4170-PA, isoform A | Vig | gi|66565092 | 46.9/8.85 | 99.69 | P:RNA interference | - | 3.22 | ||||||||
| 104 | glyceraldehyde-3-phosphate dehydrogenase 2 isoform 1 | Gapdh | gi|48142692 | 35.8/8.11 | 100 | F:NAD/NADP binding; P:glucose metabolic process | EC:1.2.1.12 | 3.21 | ||||||||
| AC-RJM-down | ||||||||||||||||
| 15 | Gamma-actin | Act5C | gi|178045 | 25.8/5.65 | 100 | P:adherens junction organization; F:protein kinase binding; | - | ↓∞ | ||||||||
| 21 | ubiquitin conjugating enzyme E2 | ben | gi|156551253 | 17.3/5.71 | 100 | P:response to anesthetic; F:ubiquitin-protein ligase activity; | EC:6.3.2.19 | ↓∞ | ||||||||
| 77 | abnormal CHEmotaxis family member (che-3) isoform 1 | LOC411639 | gi|110757336 | 451.7/6.14 | 97.72 | P:regulation of transcription, DNA-dependent; F:microtubule motor activity; | EC:3.6.1.3 | ↓∞ | ||||||||
| AM-RJC-up | ||||||||||||||||
| 54 | pORF2 | pORF2 | gi|16508047 | 149.5/9.7 | 99.97 | P:RNA-dependent DNA replication; F:RNA-directed DNA polymerase activity; | - | ↑∞ | ||||||||
| 56 | yellow-e2 CG17044-PA | yellow-e2 | gi|110776421 | 39.6/8.29 | 99.39 | P:defense response to fungus/G+/G-; caste determination, influence by environmental factors; | - | ↑∞ | ||||||||
| 63 | Hypothetical protein Bd0095 | Bd0095 | gi|42521740 | 41.4/6.17 | 95 | - | - | ↑∞ | ||||||||
| 64 | mitochondrial malate dehydrogenase precursor isoform 1 | LOC408950 | gi|66513092 | 35.8/9.33 | 100 | P:tricarboxylic acid cycle; F:L-malate dehydrogenase activity; | EC:1.1.1.37 | ↑∞ | ||||||||
| 85 | Ornithine aminotransferase precursor CG8782-PA | Oat | gi|66524972 | 47.3/8.5 | 99.99 | P:metabolic process; F:ornithine-oxo-acid transaminase activity; | EC:2.6.1.13 | ↑∞ | ||||||||
| 158 | 40S ribosomal protein S12 | RpS12 | gi|66548338 | 15.3/5.48 | 100 | P:translation; F:structural constituent of ribosome; | - | 5.14 | ||||||||
| AM-RJC-down | ||||||||||||||||
| 67 | Heat shock protein cognate 5 CG8542-PA | Hsc70-5 | gi|66501507 | 75.4/6.38 | 99.9 | P:protein folding; F:2-alkenal reductase [NAD(P)] activity; | EC: 1.3 | ↓∞ | ||||||||
| 68 | thioredoxin peroxidase 1 CG1633-PA, isoform 1 | Tpx-1 | gi|66548188 | 21.7/5.65 | 100 | P:oxidation-reduction process; F:glutathione/thioredoxin peroxidase activity; | EC: 1.1 | ↓∞ | ||||||||
| 69 | Elongation factor 2 (EF-2) isoform 1 | Ef2b | gi|66508439 | 94.5/6.11 | 100 | P: translational elongation; F:translation elongation factor activity; | - | ↓∞ | ||||||||
| 70 | Fumarylacetoacetase (Fumarylacetoacetate- hydrolase)(Beta-diketonase) | Faa | gi|66522657 | 41.6/6.09 | 100 | P:aromatic amino acid family metabolic process; F:fumarylacetoacetase activity; | EC: 3.7 | ↓∞ | ||||||||
| 71 | Putative invasion protein | invA | gi|16266003 | 7.4/9.64 | 97.68 | P:nucleobase-containing compound metabolic process; F:hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides | EC: 3.6 | ↓∞ | ||||||||
| 86 | T-complex Chaperonin 5 CG8439-PA, isoform A | Cct5 | gi|66522349 | 59.4/5.7 | 97.50 | - | ↓∞ | |||||||||
| 100 | hypothetical protein LOC726980 | CG13321 | gi|110755727 | 14.4/5.52 | 99.99 | F:transferase activity | - | 3.21 | ||||||||
| 101 | 40S ribosomal protein S12 | Rps12 | gi|66548338 | 15.3/5.48 | 100 | P:translation; F:structural constituent of ribosome; | - | 3.19 | ||||||||
| 102 | 60 kDa heat shock protein, mitochondrial-like | Hsp60 | gi|66547450 | 60.4/5.64 | 100 | P:response to stress; F:solute:hydrogen antiporter activity; | - | 6.64 | ||||||||
| 103 | Glyceraldehyde 3 phosphate dehydrogenase 1 | Gapdh1 | gi|66517066 | 31.6/7.6 | 100 | P:glucose metabolic process; F:glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity; | EC: 1.2 | 3.27 | ||||||||
| 136 | hexamerin 110 | Hex110 | gi|155369750 | 112.1/6.43 | 99.98 | - | - | 3.21 | ||||||||
| AC-RJC-CSBV-up | ||||||||||||||||
| 8 | Vacuolar h | Vha55 | gi|66531434 | 55.1/5.41 | 99.57 | P:ATP metabolic process; F:hydrogen-exporting ATPase activity, phosphorylative mechanism; | EC: 3.6 | ↑∞ | ||||||||
| 9 | HSP70 | hsp70 | gi|92430370 | 70.8/5.42 | 99.97 | P:response to stress; F:ATP binding; | - | ↑∞ | ||||||||
| 10 | 40S ribosomal protein S3a (C3 protein) | RpS3A | gi|66547340 | 30.0/9.67 | 100 | F:structural constituent of ribosome; P:translation | - | ↑∞ | ||||||||
| 72 | MTA1-like CG2244-PB, isoform B | MTA1-like | gi|110756803 | 94.4/9.68 | 95.86 | F:zinc ion binding; P:regulation of transcription, DNA-dependent | - | ↑∞ | ||||||||
| 73 | peroxidase-like isoform 2 | pxd | gi|110757934 | 87.6/7.11 | 99.053 | P:response to oxidative stress; F:peroxidase activity; | EC: 1.1 | ↑∞ | ||||||||
| 74 | Argonaute 1 CG6671-PB, isoform B | AGO1 | gi|110777044 | 103.6/9.35 | 98.599 | P:targeting of mRNA for destruction involved in RNA interference; F:miRNA binding; | - | ↑∞ | ||||||||
| 93 | phosphatidylethanolamine-binding protein homolog F40A3.3-like isoform 1 | LOC408516 | gi|66524882 | 20.6/8.84 | 99.793 | - | - | 3.55 | ||||||||
| 133 | 60S ribosomal protein L9 | RpL9 | gi|66565444 | 21507.7/9.88 | 99.962 | - | 3.17 | |||||||||
| 132 | 10 kDa heat shock protein, mitochondrial-like | LOC552531 | gi|66547447 | 11.4/8.01 | 100 | P:response to stress; F:ATP binding; | - | 5.25 | ||||||||
| AC-RJC-CSBV-down | ||||||||||||||||
| 7 | Hypothetical protein V12B01_09746 | V12B01_09746 | gi|84388955 | 20.5/5.61 | 99.616 | - | - | ↓∞ | ||||||||
| 89 | tyrosyl-trna cytoplasmic | Aats-tyr | gi|110762892 | 73.7/8.91 | 99.548 | P:tyrosyl-tRNA aminoacylation; F:tyrosine-tRNA ligase activity; | EC: 6.1 | 3.64 | ||||||||
| 91,128 | fructose-bisphosphate aldolase | FBA | gi|110748949 | 39.6/7.57 | 99.959 | P:glycolysis; F:fructose-bisphosphate aldolase activity; | EC: 4.1 | 3.79 3.50 | ||||||||
| 92 | aldose reductase-like isoform 1 | AKR-1 | gi|66525576 | 36.1/6.26 | 99.997 | P:oxidation-reduction process; F:oxidoreductase activity | - | 3.97 | ||||||||
| 120 | ribosomal protein s9 | RpS9 | gi|48101950 | 22.5/10.74 | 97.835 | P:translation; F:rRNA binding | - | 4.47 | ||||||||
| 122 | peroxiredoxin 1 | Jafrac1 | gi|66548188 | 21.7/5.65 | 95.152 | P:hydrogen peroxide catabolic process; F: thioredoxin/glutathione peroxidase activity; | EC: 1.1 | 8.45 | ||||||||
| 124 | phosphoglycerate kinase | Pgk | gi|110763826 | 44.9/8.15 | 100 | P:phosphorylation; F:phosphoglycerate kinase activity; | EC: 2.7 | 9.74 | ||||||||
| 125 | 60 kDa heat shock protein, mitochondrial-like | Hsp60 | gi|66547450 | 60.4/5.64 | 100 | P:response to stress; F:solute:hydrogen antiporter activity; | - | 3.45 | ||||||||
| 126 | t-box protein h15 | mid | gi|110757297 | 9.7/9.36 | 99.914 | F:sequence-specific DNA binding transcription factor activity; P:regulation of transcription, DNA-dependent; | - | 14.10 | ||||||||
| 127 | prohibitin protein wph | wph | gi|48097857 | 29.9/6.54 | 100 | Required for larval metabolism or for the progression of the larva into a pupa | - | 3.72 | ||||||||
| 129 | pyruvate kinase | LOC552007 | gi|66548684 | 55.8/6.92 | 100 | P:phosphorylation; F:potassium ion binding; | EC: 2.7 | 3.93 | ||||||||
| 131 | similar to CG31531-PA, isoform A isoform 1 | LOC409705 | gi|66500352 | 113.1/6.62 | 98.889 | P:imaginal disc-derived wing morphogenesis; F:zinc ion binding | - | 3.09 | ||||||||
| AC-RJM-CSBV-up | ||||||||||||||||
| 13 | eukaryotic translation initiation factor 5a | eIF-5A | gi|110767655 | 17.6/5.19 | 97.017 | F:translation initiation factor activity; P:translational initiation; | - | ↑∞ | ||||||||
| 14 | Triosephosphate isomerase1 | Tpi | gi|148224276 | 26.9/7.77 | 99.999 | P:gluconeogenesis; F:triose-phosphate isomerase activity | EC: 5.3 | ↑∞ | ||||||||
| 87 | neurochondrin homolog | CG2330RA | gi|66509434 | 84.2/5.89 | 99.177 | P:metabolic process; F:phosphoglycolate phosphatase activity | EC: 3.1 | 3.24 | ||||||||
| 135 | actin, indirect flight muscle-like | Act88F | gi|110775533 | 37.3/5.37 | 99.948 | P:phagocytosis, engulfment; F:ATP binding; | - | 3.04 | ||||||||
| AC-RJM-CSBV-down | ||||||||||||||||
| 75 | grip and coiled-coil domain-containing protein 1 | CG10703 | gi|110750733 | 58.3/8.69 | 95.142. | P:cellular process | - | ↓∞ | ||||||||
| 76 | 60 kDa heat shock protein, mitochondrial precursor (Hsp60) | HSP60 | gi|66547450 | 60.4/5.64 | 100 | P:response to stress; F:transporter activity; | - | ↓∞ | ||||||||
| 134 | yellow-e2 CG17044-PA | CG15040 | gi|110776421 | 39.6/8.29 | 99.773 | P:killing of cells of other organism; | - | 3.36 | ||||||||
| AM-RJC-CSBV-up | ||||||||||||||||
| 31 | lambda-crystallin homolog | Cryl1 | gi|66521282 | 34.4/6.3 | 100 | F:3-hydroxyacyl-CoA dehydrogenase activity; P:oxidation-reduction process | EC:1.1.1.35 | ↑∞ | ||||||||
| 32 | GL19229 | Dper\GL19229 | gi|194106483 | 17.3/6.05 | 99.505 | - | - | ↑∞ | ||||||||
| 34 | Catalase | Cat | gi|25990773 | 57.9/8.39 | 100 | P:hydrogen peroxide catabolic process; F:protein kinase activity; | EC:1.11.1.6 | ↑∞ | ||||||||
| 82 | metallo-beta-lactamase domain-containing protein 1-like | CG9117 | gi|110775625 | 16.1/5.86 | 95.029 | F:hydrolase activity | - | ↑∞ | ||||||||
| 83 | transposase | LOC725919 | gi|110766128 | 22.6/9.6 | 98.985 | P:transposition, DNA-mediated; F:transposase activity; | - | ↑∞ | ||||||||
| 84 | Translation elongation factor eEF-1 alpha chain | Eif | gi|58585198 | 50.5/9.16 | 99.948 | F:translation elongation factor activity; P:translational elongation; | - | ↑∞ | ||||||||
| 108 | 40S ribosomal protein S20 | RpS20 | gi|110766823 | 13.7/9.95 | 100 | P:translation; F:structural constituent of ribosome; | - | 6.08 | ||||||||
| 109 | serine protease easter | Sp7 | gi|110757145 | 41.0/7.83 | 98.199 | P:proteolysis; F:serine-type endopeptidase activity; | EC:3.4.21.0 | 5.08 | ||||||||
| 110 | atp synthase subunit mitochondrial | LOC551766 | gi|110762902 | 55.1/5.25 | 100 | P:ATP synthesis coupled proton transport; F:ATP binding; | EC:3.6.3.6 | 3.80 | ||||||||
| 111 | CuZn superoxide dismutase | Sod | gi|33089104 | 15.6/6.21 | 100 | P:superoxide metabolic process; F:superoxide dismutase activity; | EC:1.15.1.1; EC:1.11.1.7 | 4.13 | ||||||||
| 112 | Nucleoside diphosphate kinase (NDK) (NDP kinase) (Abnormal wing disks protein) (Killer of prune protein) | Awd | gi|66520497 | 17.6/6.75 | 100 | P:nucleoside diphosphate phosphorylation; F:nucleoside diphosphate kinase activity; | EC:2.7.4.6 | 4.15 | ||||||||
| 113 | fructose-bisphosphate aldolase-like | FBA | gi|110748949 | 39.6/7.57 | 100 | P:glycolysis; F:fructose-bisphosphate aldolase activity | EC:4.1.2.13 | 3.49 | ||||||||
| 114 | ornithine aminotransferase, mitochondrial | Oat | gi|66524972 | 47.3/8.5 | 100 | P:metabolic process; F:ornithine-oxo-acid transaminase activity; | EC:2.6.1.13 | 4.46 | ||||||||
| 33, 115,116,118 | glyceraldehyde-3-phosphate dehydrogenase | LOC410122 | gi|66517066 | 31.6/7.6 | 100 | F:NAD/NADP binding; P:glucose metabolic process; | EC:1.2.1.12 | ↑∞ 4.29 3.16 3.89 | ||||||||
| 117 | larval cuticle protein a3a-like | CPR31A | gi|110764443 | 34.3/5.54 | 100 | F:structural constituent of cuticle | - | 3.25 | ||||||||
| 119,137,138 | glyceraldehyde-3-phosphate dehydrogenase 2 isoform 1 | Gapdh | gi|48142692 | 35.8/8.11 | 100 | F:NAD/NADP binding; P:glucose metabolic process; | EC:1.2.1.12 | 8.54 3.08 3.14 | ||||||||
| AM-RJC-CSBV-down | ||||||||||||||||
| 24, 25, 26 | 60 kDa heat shock protein, mitochondrial precursor | Hsp60 | gi|66547450 | 60.4/5.64 | 100 | P:response to stress; F:transporter activity | - | ↓∞ ↓∞ ↓∞ | ||||||||
| 27 | 60 kDa heat shock protein, mitochondrial | Hsp60 | gi|170045840 | 60.4/5.39 | 100 | P: protein metabolic process; response to obiotic stimulus; F: nucleotide/protein binding; catalytic activity; | EC: 4.2 | ↓∞ | ||||||||
| 30 | GI16304 | Hsp60 | gi|193906980 | 60.7/5.65 | 99.812 | P: protein metabolic process; response to obiotic stimulus; F: nucleotide/protein binding; catalytic activity; | EC: 4.2 | ↓∞ | ||||||||
| 78 | Spectrin beta chain | Beta-Spec | gi|110759783 | 268.6/5.37 | 97.139 | F:phosphoprotein phosphatase activity; | EC: 3.1 | ↓∞ | ||||||||
| 80 | Stretchin-Mlck CG18255-PA, isoform A | Strn-Mlck | gi|110757372 | 38.1/5.45 | 99.212 | - | - | ↓∞ | ||||||||
| 81 | yellow-h CG1629-PA | Yellow-h | gi|110761428 | 33.7/5.71 | 100 | - | - | ↓∞ | ||||||||
| 105 | fatty acid binding protein | FABP | gi|27531027 | 15.5/5.46 | 100 | F:lipid binding; P:transport; | - | 8.52 | ||||||||
| 107 | protein NPC2 homolog | NPC2 | gi|110756609 | 16.1/7.55 | 100 | - | - | 3.22 | ||||||||
| AM-RJM-CSBV-up | ||||||||||||||||
| 50 | c6 transcription | SS1G_11931 | gi|156039589 | 50.2/8.99 | 97.085 | F:sequence-specific DNA binding RNA polymerase II transcription factor activity; P:regulation of transcription from RNA polymerase II promoter; | - | ↑∞ | ||||||||
| 51,161 | protein disulfide-isomerase | Pdi | gi|110768510 | 24.3/4.74 | 100 | F:electron carrier activity; P:cell redox homeostasis; | EC: 5.3; EC: 2.4 | ↑∞ 18.95 | ||||||||
| 52 | aldehyde dehydrogenase mitochondrial-like | Aldh | gi|66526635 | 45.4/5.25 | 100 | F:retinal dehydrogenase activity; P:oxidation-reduction process | EC: 1.2 | ↑∞ | ||||||||
| 53 | hydroxysteroid dehydrogenase-like protein 2-like | CG5590 | gi|66530010 | 45.2/8.69 | 100 | P:oxidation-reduction process; F:glucose 1-dehydrogenase [NAD(P)] activity; | EC: 1.1 | ↑∞ | ||||||||
| 153 | heat shock protein cognate 3 | Hsc70-3 | gi|229892214 | 72.8/5.29 | 100 | F:metal ion binding; P:porphyrin-containing compound biosynthetic process; | EC: 4.2 | 4.15 | ||||||||
| 154 | phosphoglycerate kinase isoform 1 | PGK | gi|110763826 | 44.9/8.15 | 100 | P:glycolysis; phosphorylation; F:phosphoglycerate kinase activity | EC: 2.7 | 6.13 | ||||||||
| 155 | a-kinase anchor protein 9 | Cp309 | gi|110758909 | 81.4/8.39 | 98.602 | P:microtubule cytoskeleton organization; F:protein binding; | - | 7.52 | ||||||||
| 156 | heat shock protein cognate 4 | Hsc70-4 | gi|229892210 | 71.0/5.43 | 100 | F:ATP binding; P:response to stress; | - | 4.99 | ||||||||
| 157 | glyceraldehyde-3-phosphate dehydrogenase 2 isoform 1 | Gapdh | gi|48142692 | 35.9/8.11 | 100 | F:NAD/NADP binding; glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity; P:oxidation-reduction process; | EC: 1.2 | 3.68 | ||||||||
| 161 | 26S proteasome non-ATPase regulatory subunit 13 | Rpn9 | gi|66547365 | 43.6/6.07 | 95.252 | - | - | 18.95 | ||||||||
| 163 | myosin heavy muscle isoform 1 | Mhc | gi|110759191 | 224.7/5.78 | 99.86 | P:ecdysone-mediated induction of salivary gland cell autophagic cell death; F:2-alkenal reductase [NAD(P)] activity; | EC: 1.3 | 3.14 | ||||||||
| AM-RJM-CSBV-down | ||||||||||||||||
| 35 | 30S ribosomal protein S3 | RpS3 | gi|73661978 | 24.0/9.8 | 99.657 | P:translation; F:RNA binding; | - | ↓∞ | ||||||||
| 37, 142,143 | thioredoxin peroxidase 1 CG1633-PA | Jafrac1 | gi|66548188 | 21.8/5.65 | 100 | P:response to stress; F:antioxidant activity; | EC: 1.1 | ↓∞, 3.36, 4.50 | ||||||||
| 38 | aldo-keto reductase | CG6084 | gi|66525576 | 36.1/6.26 | 100 | P:metabolic process; F:catalytic activity | - | ↓∞ | ||||||||
| 36,39,42 141, 144145148 | 60 kDa heat shock protein, mitochondrial precursor | Hsp60 | gi|66547450 | 60.4/5.64 | 100 | P:response to stress; F:transporter activity; | - | ↓∞ ↓∞ ↓∞ 7.55 3.95 29.95 3.24 | ||||||||
| 40 | tyrosyl-trna cytoplasmic | Aats-tyr | gi|110762892 | 73.7/8.91 | 100 | P:translation; F:catalytic activity; RNA binding; | EC: 6.1 | ↓∞ | ||||||||
| 43, 46 | heat shock protein 60 | Hsp60 | gi|156541962 | 60.5/5.5 | 100 | P:response to stress; F:transporter activity; | - | ↓∞ ↓∞ | ||||||||
| 47 | fructose-bisphosphate aldolase | FBA | gi|110748949 | 39.6/7.57 | 100 | P:carbohydrate metabolic process; F:catalytic activity | EC: 4.1 | ↓∞ | ||||||||
| 49 | heat shock cognate 70 | Hsp70 | gi|66537940 | 71.7/5.43 | 100 | P:response to stress; F:nucleotide binding; | - | ↓∞ | ||||||||
| 139 | arylphorin subunit alpha | Lsp2 | gi|149939401 | 79.5/6.72 | 100 | - | - | 4.00 | ||||||||
| 140 | atp synthase subunit mitochondrial | Vha68 | gi|110762902 | 55.1/5.25 | 100 | P:generation of precursor metabolites and energy; F:hydrolase activity; | EC: 3.6 | 3.07 | ||||||||
| 147 | similar to Pyruvate kinase CG7070-PB, isoform B | LOC552007 | gi|66548684 | 55.8/6.92 | 100 | 3.99 | ||||||||||
| 149 | hexamerin 110 | Hex110 | gi|155369750 | 112.1/6.43 | 100 | - | - | 4.27 | ||||||||
| 150 | protein NPC2 homolog | NPC2 | gi|110756609 | 16.1/7.55 | 100 | - | - | 6.33 | ||||||||
| 151 | grpe protein mitochondrial-like | Roe1 | gi|66525522 | 26.4/7.82 | 100 | P:protein metabolic process; F:protein binding; | - | 3.66 | ||||||||
| 152 | n-acetylneuraminate lyase-like | LOC725646 | gi|110755974 | 34.0/8.3 | 100 | P:metabolic process; F:catalytic activity | - | 4.19 | ||||||||
Spot numbers and folds change correspond to the number of the protein spots in Figure 2. The theoretical molecular weight (Mr) and isoelectric point (pI) of the identified proteins were retrieved from the protein database of NCBInr limited to Apis mellifera. The Mascot score was searched against the A. mellifera database. Protein name is given when proteins were identified by MALDI-TOF/TOF-MS. The taxonomy is A. mellifera. Accession number is the unique number given to mark the entry of a protein in the NCBInr database. GO molecular function and biology process result in the Blast2GO analysis, P, Biological process; F, Molecular function.
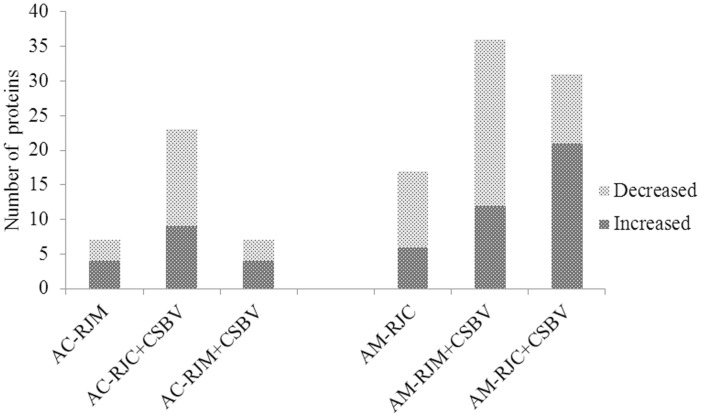
In Ac larvae, 6 differential expression proteins (6 spots) were identified from heterospecific RJ breeding only, 21 differential expression proteins (22 spots) from CSBV challenge only and 7 differential expression proteins (7 spots) from heterospecific RJ breeding plus CSBV challenge. In Am larvae, 17 differential expression proteins (17 spots) were identified from heterospecific RJ breeding only, 26 differential expression proteins (36 spots) from CSBV challenge only and 24 differential expression proteins (31 spots) from heterospecific RJ breeding plus CSBV challenge (Figure 3).
Figure 3. Numbers of up-regulated and down-regulated proteins from the bee larvae fed with RJs and challenged by CSBV.
On the basis of triplicate replications analyses, only the proteins that changed ≥3.0-fold in relative ratios (p<0.05) were considered. RJC: royal jelly from A. cerana; RJM: royal jelly from A. mellifera; AC-RJM: 3-day old Ac larvae fed with RJM. AC-RJC+CSBV: 3-day old Ac larvae fed with RJC and infected with CSBV. AC-RJM+CSBV: 3-day old Ac larvae fed with RJM and infected with CSBV. AM-RJC: 3-day old Am larvae fed with RJC. AM-RJC+CSBV: 3-day old Am larvae fed with RJC and infected with CSBV. AM-RJM+CSBV: 3-day old Am larvae fed with RJM and infected with CSBV.
Some proteins were not identified either because their abundance was too low to produce enough spectra or because the database search scores can not yield unambiguous results (>95%).
GO (Gene Ontology) Functional Term Enrichment
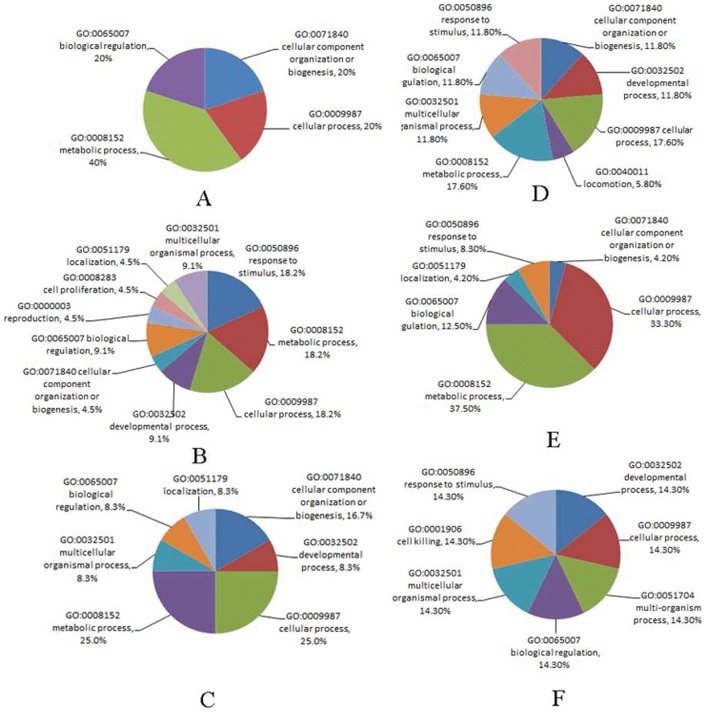
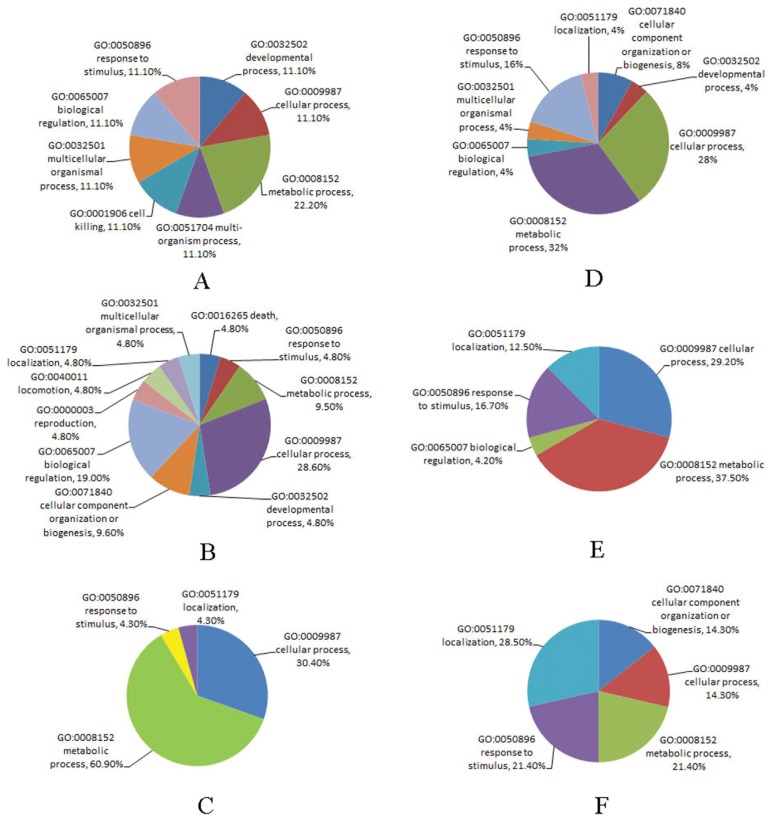
To understand the functions of differentially expressed proteins from the bee larvae, which resulted from heterospecific RJ breeding or CSBV infection, 101 identified proteins were analysed by gene ontology (GO) mapping respectively. At least one GO term could be assigned to 88 of 101 detected differentially expressed proteins (Table 3). Thirteen (12.8%) proteins were deemed of unknown function after blast searches against the nr databases and annotation augmentation. The GO terms assigned to individual proteins were shown in Table 3. Based on the GO annotations, proteins were grouped by their broad biological processes (BP) (Figure 4, 5).
Figure 4. Based on the GO annotations, the proteins from A. cerana larvae fed with RJM or RJC, and by CSBV challenge, were grouped by the broad biological processes (BP).
A, B, C: up-regulated proteins; D, E, F: down-regulated proteins. RJC: royal jelly from A. cerana; RJM: royal jelly from A. mellifera; A, D: 3-day Ac larvae fed with RJM; B, E: 3-day Ac larvae fed with RJC, and infected with CSBV; C, F: 3-day Ac larvae fed with RJM, and infected with CSBV.
Figure 5. Based on the GO annotations, the proteins from A. mellifera larvae fed with RJM or RJC, and by CSBV challenge, were grouped by the broad biological processes (BP).
A, B, C: up-regulated proteins; D, E, F: down-regulated proteins. RJC: royal jelly from A. cerana; RJM: royal jelly from A. mellifera; A, D: 3-day Am larvae fed with RJC; B, E: 3-day Am larvae fed with RJM, and infected with CSBV; C, F: 3-day Am larvae fed with RJC, and infected with CSBV.
Three major BPs of the up-regulated proteins from the Ac larvae fed with RJM were metabolic processes (40.0%), cellular processes (20%) and biological regulation (20%), whereas those of down-regulated proteins were mainly metabolic processes (17.6%), cellular processes (17.6%) and developmental process (11.8%). The major BPs of the increasing proteins from Ac fed with RJC, and then by CSBV challenge were metabolic process (18.2%), cellular processes (18.2%) and response to stimulus (18.2%), whereas the major BPs of the decreasing proteins were metabolic processes (37.5%), cellular processes (33.3%) and biological regulation (12.5%). The major BPs of the increasing proteins from Ac fed with RJM, and then by CSBV challenge were metabolic processes (25.0%), cellular processes (25.0%) and cellular component organization or biogenesis (16.7%), whereas the major BPs of the decreasing proteins were metabolic processes (25.0%), cellular processes (25.0%) and biological regulation (12.5%).
Three major BPs of the increasing proteins from the Am larvae fed with RJM were metabolic processes (22.2%), cellular processes (11.1%) and cell killing (11.1%), whereas those of decreasing proteins were metabolic processes (32.0%), cellular processes (28.0%) and response to stimulus (16%). The major BPs of the increasing proteins from Am fed with RJM, and then by CSBV challenge were cellular processes (28.6%), biological regulation (19.0%) and cellular component organization (9.6%), whereas the major BPs of the decreasing proteins were metabolic processes (37.5%), cellular processes (29.2%) and response to stimulus (16.7%). The major BPs of the increasing proteins from Am fed with RJC, and then by CSBV challenge were metabolic processes (60.9%), cellular processes (30.4%) and response to stimulus (4.3%), whereas the major BPs of the decreasing proteins were localization (28.5%), response to stimulus (21.4%) and metabolic processes (21.4%).
Network Analysis of Differentially Expressed Proteins
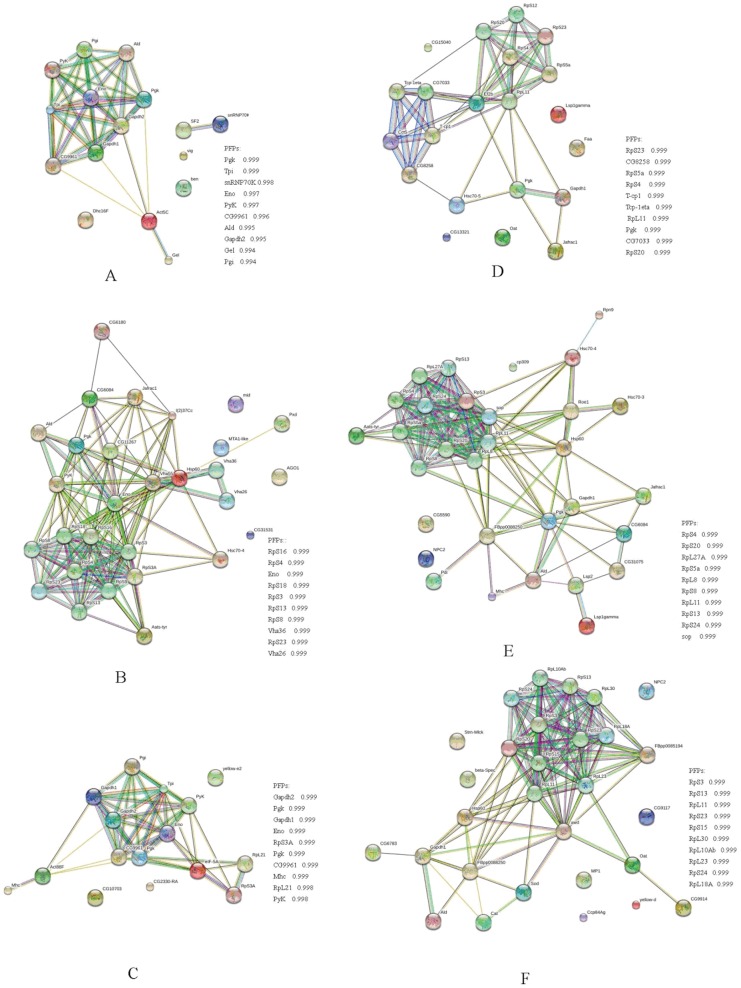
Proteins function jointly in a living cell through networks by forming protein- protein interactions (PPI), modifications and regulation of expression relationships. Of all the identified proteins in this study, 107 proteins with the established functions based on Drosophila melanogaster were selected and their PPI mapping was constructed using STRING. The result showed that all the treats almost divided into two distinct PPI clusters with several others operating without connectivity. The first cluster primarily consisted of cytoskeletal proteins, while the second cluster mainly consisted of proteins involved in metabolism (Figure 6).
Figure 6. Protein-protein interaction networks of identified differentially expressed proteins from nutritional crossbreeding and CSBV challenge were constructed by Search Tool for the Retrieval of Interacting Genes (STRING).
Lines represent the existence of the different types of evidence used in predicting the associations. A red line indicates the presence of fusion evidence; a green line, neighborhood evidence; a blue line, co-occurrence evidence; a purple line, experimental evidence; a yellow line, text mining evidence; a light blue line, database evidence; a black line, coexpression evidence. PFPs: Predicted Functional Partners. A: 3-day Ac larvae fed with RJM. B: 3-day Ac larvae fed with RJC, and infected with CSBV. C: 3-day Ac larvae fed with RJM, and infected with CSBV. D: 3-day Am larvae fed with RJC. E: 3-day Am larvae fed with RJM, and infected with CSBV; F: 3-day Am larvae fed with RJC, and infected with CSBV.
Verification of Differentially Expressed Proteins
To test the tendency of protein expression between its encoding gene at the transcript level, 11 proteins (FBA, LOC408516, MDH, SOD, Jafrac1, LOC725646, AGO1, Che-3, Ald, Pxd and Tpi) were randomly selected for qRT-PCR analysis of their encoding genes. The trend of mRNA expression of the selected genes showed consistent patterns with their protein expression (Figure 7).
Figure 7. Verification of 11 differentially expressed proteins at mRNA level by qRT-PCR analysis.
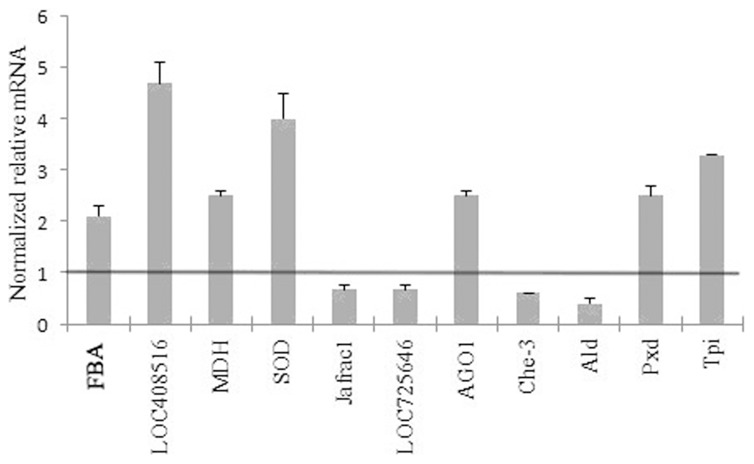
The normalized relative mRNA levels (>1) of the genes of the corresponding differentially expressed proteins indicate up-regulation and those (<1) indicate down-regulation. Error bar is standard deviation. The names of the proteins are referred in Table 2.
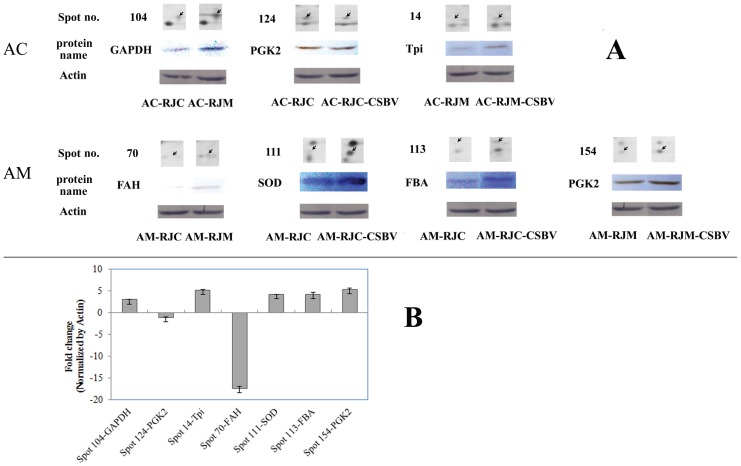
Western Blot Analysis
Western blot analysis was conducted to verify the expression of the proteins which are closely related to energy metabolism of heterospecific royal jelly and CSBV resistance, such as GAPDH, PGK, Tpi, FAH, SOD, and FBA. The results are consistent well with the proteomic data (Figure 8).
Figure 8. Western blot Analysis of differentially expressed proteins.
Six proteins were selected. Proteins were separated on a 12% SDS-PAGE gel and immunoblotted with anti-GAPDH, anti-PKG2, anti-Tpi, anti-FAH, anti-SOD, and anti-FBA. β-actin was used as an internal control. (A) The Western blot images of GAPDH, PKG2, TPI, FAH, SOD, FBA and Actin. The protein spots from Figure 2. (B) Quantification of target protein expression.The relative fold change of GAPDH, PKG2, Tpi, FAH, SOD, FBA (normalized by Actin) was visulized by using Quantity One Software. The expression of GAPDH, SOD, FBA and Tpi were up-regulatedd. On the other hand, FAH and PGK were down-regulated. Error bar is standard deviation.
Discussion
Heterospecific RJ breeding means during the growth or reproduction period of the colony or individual, changing the comb from Ac to Am or from Am to Ac or feeding RJ artificially. In recent years, heterospecific RJ breeding between Ac and Am has been concerned in the bee industry [39]. This technology was used between different species of western honeybees in 1957. Am fed with RJC showed some morphological characteristic of Ac, such as proboscis, forewings, abdomen and thorax, and body weight of new adults [23], [40], and even influenced the genotype of malate dehydrogenase II [41]. It was reported that Am could be resistant to varroa mites when fed with RJC [42]–[44]. Interestingly, the present results showed that the change of Ac larval food from RJC to RJM could enhance Ac resistance to CSBV, under the laboratory condition. The mortality of Ac larvae after CSBV infection was much lower when they were fed with RJM compared with those fed with RJC. However, the change of RJM to RJC did not lower the viral resistance of Am larvae. Whether RJM stimulates the viral resistance of Ac by inducing the antiviral protein expression or RJM molecules can directly inhibit the viral replication in bee larvae needs further study.
To determine whether the proteins induced by the food change are probably involved in the bee resistance to CSBV, 2-DE and MALDI-TOF-MS based proteomic strategies were used. Accordingly, 101 nonredundant proteins were identified as being differentially expressed among Ac and Am larvae by heterospecific RJ breeding and CSBV challenge.
Differentially Expressed Proteins from Hererospecific RJ Breeding
The results showed that protein expression was influenced by the RJ. Within 6 differentially expressed proteins from 3-day old Ac larvae fed with RJM, 3 proteins CG6987-PA (SF2), vasa intronic gene (Vig), and GAPDH were up-regulated, and 3 proteins (Act5C, ben, Che-3) down-regulated. SF2 is a RNA recognition motif, a highly abundant domain in eukaryotes found in proteins involved in post-transcriptional gene expression processes [45]. Vig has been identified as a component of Drosophila RISC involved in RNA interference [46]. GAPDH is a key enzyme in the glycolytic pathway. So the up-regulated proteins are associated with gene expression process, RNA interference and glycolytic metabolism. Of the down-regulated proteins, Gamma-actin (Act5C) is an ubiquitous protein involved in the formation of filaments that are a major component of the cytoskeleton. Ubiquitin conjugating enzyme E2 (E2) is an ubiquitin conjugating enzyme [47], [48]. The ubiquitin proteasome system enables cells to selectively recognize and degrade damaged or potentially harmful proteins [49]. Abnormal CHEmotaxis family member (Che-3) is one of the AAA+ superfamily. In Caenorhabditis elegans Che-3 is specifically responsible for the retrograde transport of the anterograde motor, kinesin-II, and its cargo within sensory in chemosensory neurons [50]. Therefore, the down-regulated proteins are related with the cytoskeleton arrangement, protein metabolism and cellular processes.
Correspondingly, 17 differentially expressed proteins from 3-day old larvae of Am fed with RJC included 6 up-regulated (pORF2, yellow-e2, MDH, Oat, RpS12, one unknown protein), and 11 down-regulated proteins (Hsc70-5, Tpx-1, Ef2b, FAH, invA, Cct5, Rps12, Hsp60,Gapdh1, Hex110, one unknown protein). For the up-regulated proteins, pORF2 is a retrovirus reverse transcriptase with RNA-directed DNA polymerase activity [39]. Yellow-e2 responses to fungus/G+/G−, and functions in caste determination, and is influenced by environmental factors [51]. MDH is the key enzymes in the citric acid cycle, and possess L-malate dehydrogenase activity elevated by oxidative stress [52]. Ornithine aminotransferase precursor (Oat) possesses ornithine-oxo-acid transaminase activity in metabolic process [53]. For the down-regulated proteins, T-complex Chaperonin 5 (Cct5) is involved in productive folding of proteins [54]. The methylation of the Hex110 gene in A. mellifera is regulated at the developmental stage and in a caste-dependent manner [55]. HSP60 and Hsc70-5 are heat shock proteins responding to stress. Generally, HSPs act as molecular chaperones facillitating protein maturation in cells and activating innate and acquired immune responses [6]. They are excellent immunomodulators against a wide variety of pathogens in protecting host [56]. Thioredoxin peroxidase 1 (Jafrac1) is in response to oxidative stress [57]. Elongation factor 2 (Ef2b) is a GTPase, involved in the translocation of the peptidyl-tRNA [58]. Fumarylacetoacetase hydrolysis (FAH) is responsible for the hydrolysis of fumarylacetoacetate to generate fumarate and acetoacetic acid [59]. Putative invasion protein (InvA) is a prominent inner-membrane component of the Salmonella type III secretion system (T3SS) apparatus, which is responsible for regulating virulence protein export in pathogenic bacteria [60].
From the above description, differentially expressed proteins from heterospecific RJ breeding were related to metabolism mainly in TCA and glycolytic pathway and response to microbe and oxidative stress. RJ protein difference between Ac and Am was reported [24]. The majority of the identified proteins were major royal jelly proteins (MRJPs) with MRJP1 being the most abundant. Peroxiredoxin 2540, glutathione S-transferase S1, and MRJP5 were detected only in the RJ of A. mellifera ligustica, and MRJP7 was found only in the RJ of A. cerana cerana [24]. Maybe different components of two royal jellies changed the metabolism pathways of honey bees, conferring to the resistant difference to viral infection.
Differentially Expressed Proteins from Ac Larvae Fed with RJC and by CSBV Challenge
Comparing to the 3-day old Ac larvae fed with RJC and without CSBV infection, 21 altered proteins were detected from 3-day old Ac larvae fed with RJC and CSBV infection, in which 9 proteins being up-regulated (Vha55,Hsp70, RpS3A, MTA1-like, pxd, AGO1, phosphatidylethanolamine-binding protein, Hsp10, an unknown protein), and 12 proteins down-regulated (V12B01_09746, Aats-tyr, Ald, AKR-1, RpS9, Jafrac1, PGK, Hsp60, mid, wph, LOC552007, LOC409705).
The up-regulated proteins were mainly involved in the stress response and in the defense from viral infection. Such as, vacuolar h (Vha55) couples ATP hydrolysis to the build up of a H+ gradient. Metastasis associated gene 1 (MTA1) is part of the NURD (nucleosome remodeling and deacetylating) complex and plays a role in cellular transformation and metastasis [61]. Peroxidase-like (Pxd) is response to oxidative stress. Argonaute 1 (AGO1) is part of the RNA-induced silencing complex (RISC) in the RNA interference pathway which can defense insects from virus infection [62], [63].
The down-regulated proteins were mainly involved in the energy process and in the development regulation. For example, tyrosyl-trna cytoplasmic (Aats-tyr) are involved in amino acid synthesis, cell cycle control, RNA shear modification and transport of tRNA [64], [65]. Fructose-bisphosphate aldolase (FBA) and aldose reductase-like isoform 1 (AKR-1) are involved in glycolysis. PGK and pyruvate kinase are involved in energy metablism. PGK is the enzyme responsible for the first ATP generating step of glycolysis and the in vivo activation of l-nucleoside pro-drugs effective against retroviruses such as HIV and hepatitis [66]. Pyruvate kinase (PK) regulates the final rate-limiting step of glycolysis. In tumor cells, the glycolytic pyruvate kinase isoenzyme M2 (PKM2, M2-PK) determines whether glucose is converted to lactate for regeneration of energy or used for the synthesis of cell building blocks [67]. Cell proliferation only proceeds when metabolism is capable of providing a budget of metabolic intermediates that is adequate to ensure both energy regeneration and the synthesis of cell building blocks in sufficient amounts. In this study, the three important enzymes of glycolysis were significantly down regulated after Ac larvae fed with RJC and by CSBV challenge, indicating that the energy metabolism pathway may be important in antiviral activity of the bees. Prohibitin protein wph (Wph) is required for larval metabolism or for the progression of the larva into a pupa. LOC409705 is responsible for imaginal disc-derived wing morphogenesis. These proteins were significantly down-regulated, probably directly impacting the larval development.
As indicated in Figure 1, the Ac larvae fed with RJC were not resistant to CSBV infection. Although the up-and down-regulated protein expressions were detected from those bee larvae, it seemed that these proteins were not actively involved in effectively protecting the larvae from CSBV infection. Whether some Ac expressed proteins were active for helping viral infection needs further study.
Differentially Expressed Proteins from Ac Larvae Fed with RJM and by CSBV Challenge
Comparing to the 3-day old Ac larvae fed with RJM but without CSBV infection, 4 highly expressed protein spots (eIF-5A, Tpi, CG2330RA, Act88F) and 3 down-regulated proteins (CG10703, HSP60, yellow-e2) were found from the Ac larvae fed with RJM and by CSBV infection.
Eukaryotic translation initiation factor 5a (eIF-5A) plays an important role in protein translation extending, and in stress tolerance [68]. Triosephosphate isomerase (Tpi) is a glycolytic enzyme [69]. Act88F (actin, indirect flight muscle-like) is involved in phagocytosis [51]. HSP 60 and yellow-e2 are both activated by environmental factors as discussed above. They were down-regulated may indicated that the influence of CSBV were weakened when the Ac larvae fed with RJM.
Interestingly, CSBV infection did not significantly increase the mortality of Ac larvae fed with RJM comparing to those fed with RJM and without viral challenge (Figure. 1). So RJM may protect Ac larvae from viral infection probably by promoting energy metabolism and activating phagocytosis. Another possibility is that the specific components from RJM, such as Peroxiredoxin 2540, glutathione S-transferase S1, and MRJP5, which are detected only in the RJ of A. mellifera ligustica [24], may inhibit the viral replication.
Differentially Expressed Proteins from Am Larvae Fed with RJC and by CSBV Challenge
In comparison to 3-day old Am larvae fed with RJC and without CSBV infection, 24 proteins were detected from 3-day old Am larvae fed with RJC and CSBV infection, in which 17 proteins were up-regulated (Cryl1 (lambda-crystallin homolog), Dper\GL19229, Cat, CG9117 (metallo-beta-lactamase), LOC725919 (transposase), Eif, RpS20, serine protease easter (Sp7), LOC551766 (atp synthase subunit mitochondrial), SOD, Abnormal wing disks protein (Awd), FBA, ornithine aminotransferase (Oat), GADPH, larval cuticle protein a3a-like (CPR31A)) and 7 proteins down-regulated (Hsp60, Beta-Spec, Strn-Mlck, Yellow-h, FABP, NPC2, an unknown protein).
Most of the up-regulated proteins were involved in energy metabolism, such as Cryl1, CG9117, LOC725919, Sp7, LOC551766, FBA, Oat, LOC410122, and GAPDH. The regulation pattern of FBA and GADPH was opposite to that in the Ac larvae fed with RJC and challenged with CSBV, maybe due to different species of honey bees. But in response to viral invasion, two bee species showed the same way in regulation of antioxidative enzymes, such as SOD, CuZn superoxide dismutase and Catalase, which were biomarkers to evaluate the toxic effects of organisms [70]. The proteins (Awd and CPR31A) related to development were up-regulated, to some degree, but the cytoskeletal proteins (Beta-Spec and Strn-Mlck) were significantly down-regulated. These differentially expressed proteins might be involved in protecting Am larvae from CSBV infection, although these larvae were fed with RJC (Figure 1). Am larvae are not sensitive to CSBV infection [6]. The food change from RJM to RJC did not lower the resistant ability of Am to CSBV.
Differentially Expressed Proteins from Am Larvae Fed with RJM and by CSBV Challenge
In response to CSBV challenge, 11 up-regulated proteins (SS1G_11931, Pdi, FBA, CG5590, Hsc70-3, PGK, Cp309, Hsc70-4, GAPDH, Rpn9, Mhc), and 15 down-regulated proteins (RpS3, Jafrac1, CG6084, Hsp60, Aats-tyr, ALD, Hsp70, Lsp2, Vha68, LOC552007, Hex110, NPC2, Roe1, LOC725646) were found from the larvae fed with RJM and with CSBV infection.
Among the up-regulated proteins, those involved in energy metabolism were the majority, including PDI, FBA, ALD, PGK and GAPDH. This was in according with the expression pattern of Ac larvae fed with RJM. 26S proteasome (Rpn9) plays a fundamental role in eukaryotic homeostasis [71], [72]. The ubiquitin-proteasome system mediated viral protein degradation constitutes a host defense process against some RNA viral infections [73]. In the present study Rpn9 and HSPs were both up-regulated, indicating the possible involvement of these proteins in antiviral activity. Correspondingly, chaperonins or store proteins were down-regulated. This may guarantee the normal growth of Am larvae. As a source of amino acids for tissue reconstruction during pupal development, hexamerins are first synthesized by a larval fat body and released into the hemolymph where they accumulate to extraordinarily high concentrations to build adult structures [74].
Conclusions
Although Ac is frequently damaged by CSBV, Am is usually resistant to this virus. The present study showed that the change of Ac larval food from RJC to RJM could enhance the bee resistance to CSBV, at least at early larval stage. Proteomic technology was employed to unravel the molecular events of the bees under heterospecific RJ breeding and CSBV challenge. There were 101 proteins with altered expressions after heterospecific RJ breeding and viral infection in two bee species. The RJM may protect Ac larvae from CSBV infection, probably by activating the genes in energy metabolism pathways, antioxidation and ubiquitin-proteasome system. The results, for the first time, comprehensively descript the molecular events of the viral infection of Ac and Am, and are potentially useful for establishing CSBV resistant populations of Ac for apiculture.
Funding Statement
This work was supported by Guangdong Department of Science and Technology (2011B01050002), Guangzhou Agricultural Bureau ([2011]584), Guangdong Academy of Sciences (Sf201208), and National Natural Science Foundation of China (31301924). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gallai N, Salles JM, Settele J, Vaissieère BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68: 810–821. [Google Scholar]
- 2. Garibaldi LA, Steffan-Dewenter I, Kremen C, Morales JM, et al. (2011) Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol Lett 14: 1062–1072. [DOI] [PubMed] [Google Scholar]
- 3. Chen Y, Zhao Y, Hammond J, Hsu HT, Evans JD, et al. (2004) Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J Invert Pathol 87: 84–93. [DOI] [PubMed] [Google Scholar]
- 4. Ai HX, Yan X, Han RC (2012) Occurrence and prevalence of seven bee viruses in Apis mellifera and Apis cerana apiaries in China. J Invertebr Pathol 109: 160–164. [DOI] [PubMed] [Google Scholar]
- 5. Li ZL, Ou X, Hu JJ (2006) Investigation and control of Chinese bee sacbrood. Guangxi J Animal Husbandry & Veterinary Medicine 22: 276–277. [Google Scholar]
- 6. Han B, Zhang L, Feng M, Fang Y, Li JK (2013) An integrated proteomics reveals pathological mechanism of honeybee (Apis cerena) sacbrood disease. J Proteome Res 12: 1881–1897. [DOI] [PubMed] [Google Scholar]
- 7.Ma MX, Li M, Cheng J, Yang S, Wang SD, et al.. (2011) Molecular and biological characterization of Chinese sacbrood virus LN isolate. Comp Funct Genomics doi:10.1155/2011/409386 [DOI] [PMC free article] [PubMed]
- 8. Choe SE, Nguyen LT, Noh JH, Kweon CH, Reddy KE, et al. (2012) Analysis of the complete genome sequence of two Korean sacbrood viruses in the Honey bee, Apis mellifera . Virology 432: 155–161. [DOI] [PubMed] [Google Scholar]
- 9. Zhang GZ, Han RC (2008) Advances on sacbrood of honeybees. China J Biologic Control 24: 130–137. [Google Scholar]
- 10. Yan X, Chen JH, Han RC (2009) Detection of Chinese sacbrood virus (CSBV) in Apis cerana by RT-PCR method. Sociobiology 53: 687–694. [Google Scholar]
- 11. Liu XJ, Zhang Y, Yan X, Han RC (2010) Prevention of Chinese sacbrood virus infection in Apis cerana using RNA interference. Curr Microbiol 61: 422–428. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Feng J, Liang Y, Cheng D, Zhou ZH, et al. (2001) Three- dimensional structure of the Chinese sacbrood bee virus. Sci China 44: 443–449. [DOI] [PubMed] [Google Scholar]
- 13. Chan QW, Melathopoulos AP, Pernal SF, Foster LJ (2009) The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae . BMC Genomics 10: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lourenço AP, Guidugli-Lazzarini KR, Freitas FC, Bitondi MM, Simões ZL, et al.. (2013) Bacterial infection activates the immune system response and dysregulates microRNA expression in honey bees. Insect Biochem Mol Biol doi: 10.1016/j.ibmb.2013.03.001 [DOI] [PubMed]
- 15. Azzami K, Ritter W, Tautz J, Beier H (2012) Infection of honey bees with acute bee paralysis virus does not trigger humoral or cellular immune responses. Arch Virol 157: 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, et al. (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environm Microbiol 11: 2284–2290. [DOI] [PubMed] [Google Scholar]
- 17. Chaimanee V, Chantawannakul P, Chen Y, Evans JD, Pettis JS (2012) Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae . J Insect Physiol 58: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz RS, Evans JD (2013) Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev Comp Immunol doi: 10.1016/j.dci.2013.03.010 [DOI] [PubMed]
- 19. Zhang Y, Liu XJ, Zhang WQ, Han RC (2010) Differential gene expression of the honey bees Apis mellifera and A. cerana induced by Varroa destructor infection. J Insect Physiol 56: 1207–1218. [DOI] [PubMed] [Google Scholar]
- 20. Gregorc A, Evans JD, Scharf M, Ellis JD (2012) Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J Insect Physiol 58: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 21. Tan SL, Ganji G, Paeper B, Proll S, Katze MG (2007) Systems biology and the host response to viral infection. Nat Biotechnol 25: 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drapeau MD, Albert S, Kucharski R, Prusko C, Maleszka R (2006) Evolution of the yellow/major royal jelly protein family and the emergence of social behavior in honey bees. Genome Res 16: 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng ZJ, Xie XB, Xue YB, Yan WY, Fan ZB, et al. (2005) Effects of nutritional crossbreeding between Apis cerana cerana and Apis mellifera ligustica on morphological characters of worker bees. Acta Agriculturae Universitatis Jiangxiensis 27: 454–457. [Google Scholar]
- 24. Fang Y, Feng M, Li JK (2010) Royal jelly proteome comparison between A. mellifera ligustica and A. cerana cerana . J Proteome Res 9: 2207–2215. [DOI] [PubMed] [Google Scholar]
- 25.Guo XQ (2010) The development and molecular mechanism of queen-worker differentiation, the miRNAs of royal jelly make a difference to queen-worker differentiation. National Science Library, Chinese Academy of Sciences. 156p.
- 26. Kamakura M (2011) Royalactin induces queen differentiation in honeybees. Nature 473: 478–483. [DOI] [PubMed] [Google Scholar]
- 27. Shi YY, Wu XB, Huang ZY, Wang ZL, Yan WY, et al. (2012) Epigenetic modification of gene expression in honey bees by heterospecific gland secretions. PLoS ONE 7: e43727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao XF, He HJ, Dong DJ, Wang JX (2006) Identification of differentially expressed proteins during larval molting of Helicoverpa armigera . J Proteome Res 5: 164–169. [DOI] [PubMed] [Google Scholar]
- 29. Sun Y, An S, Henrich VC, Sun X, Song Q (2007) Proteomic identification of PKC-mediated expression of 20E-induced protein in Drosophila melanogaster . J Proteome Res 6: 4478–88. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Q, Lu YX, Xu WH (2012) Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. . J Proteome Res 11: 1042–1053. [DOI] [PubMed] [Google Scholar]
- 31. Ghosh RC, Ball BV, Willcocks MM, Carter MJ (1999) The nucleotide sequence of sacbrood virus of the honey bee: an insect picorna-like virus. J Gen Virol 80: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 32.Zeng ZJ, Jiang YS, Hu FL, Xu BH, Su SK, et al.. (2009) Apiculture. China Agriculture Press. 123 p. [Google Scholar]
- 33. Scharlaken B, de Graaf DC, Memmi S, Devreese B, Beeumen JV, et al. (2007) Differential protein expression in the honey bee head after a bacterial challenge. Arch Insect Biochem Physiol 65: 223–237. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Zhang L, Feng M, Zhang Z, Pan Y (2009) Identification of the proteome composition occurring during the course of embryonic development of bees (Apis mellifera). Insect Mol Biol 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 35. Gorg A, Postel W, Domscheit A, Günther S (1988) Two-dimensional electrophoresis with immobilized pH gradients of leaf proteins from barley (Hordeum vulgare): method, reproducibility and genetic aspects. Electrophoresis 9: 681–692. [DOI] [PubMed] [Google Scholar]
- 36. Qiu XH, Yan X, Liu MX, Han RC (2012) Genetic and proteomic characterization of rpoB mutations and their effect on nematicidal activity in Photorhabdus luminescens LN2. PLoS ONE 7: e43114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Begna D, Han B, Feng M, Fang Y, Li JK (2011) Differential expressions of nuclear proteomes between honeybee (Apis mellifera L.) queen and worker larvae: A deep insight into caste pathway decisions. J Proteome Res 11: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 38. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, et al. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucl Acids Res 39: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Sun W, Li Z, Wang X, Lv Z (2011) Identification and characterization of two critical sequences in SV40PolyA that activate the green fluorescent protein reporter gene. Genet Mol Biol 34: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He XJ, Wang ZP, Chen LH, Dai SZ, Sun J, et al. (2010) Effects of morphological characters of worker bees on nutritional crossbreed between Apis cerana cerana and Apis mellifera ligustica . J bee 6: 3–5. [Google Scholar]
- 41. Zeng ZJ, Xie XB, Xue YB, Yan WY, Guo DS (2006) Effects of malate dehydrogenase II of worker bees on nutritional crossbreed between Apis cerana cerana and Apis mellifera ligustica . J Shanghai Jiaotong University (Agricultural Science) 24: 75–79. [Google Scholar]
- 42. He XJ, Wang ZP, Chen LH, Dai SZ, Yan WY (2010) Effects of nutritional crossbreed of Apis cerana cerana and Apis mellifera ligustica on resistibility against mite and hygienic behavior ability of Apis mellifera ligustica . Acta Agricult Universitatis Jiangxiensis 32: 1245–1247. [Google Scholar]
- 43. Xie XB, Zeng ZJ, Zou Y, Yan WY, Guo DS (2005) Effects of nutritional crossbreed between Apis cerana cerana and Apis mellifera ligustica on the resistibility against mite of Apis mellifera ligustica . Acta Agricult Universitatis Jiangxiensis 27: 607–610. [Google Scholar]
- 44. Xie XB, Peng WJ, Zeng ZJ (2008) Breeding of mite-resistant honeybee by using nutritional crossbreed technology. Scientia Agricult Sinica 41: 1530–1535. [Google Scholar]
- 45. Kaplan N, Linial M (2006) ProtoBee: hierarchical classification and annotation of the honey bee proteome. Genome Res 16: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin KH, Choi B, Park YS, Cho NJ (2008) Analysis of C. elegans VIG-1 expression. Mol Cells 26: 554–557. [PubMed] [Google Scholar]
- 47. Huang L, Kinnucan E, Wang GL, Beaudenon S, Howley PM, et al. (1999) Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade. Science 286: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 48. Wenzel DM, Stoll KE, Klevit RE (2010) E2s: structurally economical and functionally replete. Biochem J 433: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rabut G (2012) Introduction to the pervasive role of ubiquitin-dependent protein degradation in cell regulation. Semin Cell Dev Biol 23: 481. [DOI] [PubMed] [Google Scholar]
- 50. Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, et al. (1999) Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans . J Cell Biol 147: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, et al. (2003) A protein interaction map of Drosophila melanogaster . Science 302: 1727–1736. [DOI] [PubMed] [Google Scholar]
- 52. Shi QL, Gibson GE (2011) Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J Neurochem 118: 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senthil-kumar M, Mysore S (2012) Ornithine-delta-aminotransferase and proline dehydrogenase genes play a role in non-host disease resistance by regulating pyrroline-5-carboxylate metabolism-induced hypersensitive response. Plant Cell Environ 35: 1329–1343. [DOI] [PubMed] [Google Scholar]
- 54. Kubota H (2002) Function and regulation of cytosolic molecular chaperone CCT. Vitam Horm 65: 313–331. [DOI] [PubMed] [Google Scholar]
- 55. Ikeda T, Furukawa S, Nakamura J, Sasaki M, Sasaki T (2011) CpG methylation in the hexamerin 110 gene in the European honeybee, Apis mellifera . J Insect Sci 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Misra RC, Verma AK, Verma SK, Kumar V, Siddiquic WA, et al. (2012) Heat shock protein 60 of filarial parasite Brugia malayi: cDNA cloning, expression, purification and in silico modeling and analysis of its ATP binding site. Exp Parasitol 132: 257–266. [DOI] [PubMed] [Google Scholar]
- 57. Ross SJ, Findlay VJ, Malakasi P, Morgan BA (2000) Thioredoxin peroxidase is required for the transcriptional response to oxidative stress in budding yeast. Mol Biol Cell 11: 2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaul G, Pattan G, Rafeequi T (2011) Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct 29: 227–234. [DOI] [PubMed] [Google Scholar]
- 59. Bateman RL, Bhanumoorthy P, Witte JF, McClard RW, Grompe M, et al. (2001) Mechanistic inferences from the crystal structure of fumarylacetoacetate hydrolase with a bound phosphorus-based inhibitor. J Biol Chem 276: 15284–15291. [DOI] [PubMed] [Google Scholar]
- 60. Worrall LJ, Vuckovic M, Strynadka NCJ (2010) Crystal structure of the C-terminal domain of the Salmonella type III secretion system export apparatus protein InvA. Protein Sci 19: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hofer MD, Tapia C, Browne TJ, Mirlacher M, Sauter G, et al. (2006) Comprehensive analysis of the expression of the metastasis-associated gene 1 in human neoplastic tissue. Arch Pathol Lab Med 130: 989–996. [DOI] [PubMed] [Google Scholar]
- 62. Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, et al. (2004) RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae . Proc Natl Acad Sci USA 101: 17240–17245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Van Rij RP, Saleh MC, Berry B, Foo C, Houk A, et al. (2006) The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster . Genes Dev 20: 2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Francklyn C (2003) tRNA synthetase paralogs: evolutionary links in the transition from tRNA-dependent amino acid biosynthesis to de novo biosynthesis. Proc Natl Acad Sci USA 100: 9650–9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Francklyn CS (2008) DNA polymerases and aminoacyl-tRNA synthetases: shared mechanisms for ensuring the fidelity of gene expression. Biochemistry 47: 11695–11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mathe C, Gosselin G (2006) l-Nucleoside enantiomers as antivirals drugs: a mini-review. Antiviral Res 71: 276–281. [DOI] [PubMed] [Google Scholar]
- 67. Mazureka S (2011) Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43: 969–980. [DOI] [PubMed] [Google Scholar]
- 68. Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, et al. (2006) eIF5A binds to translational machinery components and affects translation in yeast. Biochem Biophys Res Commun 348: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 69. Ostoa-Saloma P, Garza-Ramos G, Ramírez J, Becker I, Berzunza M (1997) Cloning, expression and characterization of triosephosphate isomerase from Trypanosoma cruzi . Eur J Biochem 244: 700–705. [DOI] [PubMed] [Google Scholar]
- 70. Mofeed J, Mosleh YY (2013) Toxic responses and antioxidative enzymes activity of Scenedesmus obliquus exposed to fenhexamid and atrazine, alone and in mixture. Ecotox Environ Safe 95: 234–240. [DOI] [PubMed] [Google Scholar]
- 71. Grune T, Catalgol B, Licht A, Ermak G, Pickering A, et al. (2011) HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med 51: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. da Fonseca PC, He J, Morris EP (2012) Molecular model of the human 26S proteasome. Mol Cell 46: 54–66. [DOI] [PubMed] [Google Scholar]
- 73. Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, et al. (2009) PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 46: 452–462. [DOI] [PubMed] [Google Scholar]
- 74. Burmester T (1999) Evolution and function of the insect hexamerins. Eur J Entomol 96: 213–225. [Google Scholar]