Abstract
Objective:
To compare associations between four measures of anticholinergic exposure (anticholinergic risk scale, ARS; anticholinergic drug burden, DBAC; number and use versus no use of anticholinergic drugs), Barthel Index (BI, physical function) and Abbreviated Mental Test (AMT, cognitive function) on admission in older hospitalized patients.
Methods:
Prospective observational study of a consecutive series of 271 older patients (age 83 ± 7 years) from community-dwelling and institutionalized settings, admitted to an acute geriatric admission unit between 28 September 2011 and 18 December 2011. The main outcome measures were BI quartiles (primary outcome) and AMT (secondary outcome) on admission.
Results:
Anticholinergic prevalence was 47%. Multinomial logistic regression showed higher DBAC was associated with a greater risk of being in the lower BI quartiles versus highest BI quartile (Q4). This risk was significant for Q3 (p = 0.04) and Q2 (p = 0.02) but not for Q1 (p = 0.06). A greater number of anticholinergic drugs was associated with a higher risk of being in Q2 (p = 0.02). This risk was not significant for either Q3 (p = 0.10) or Q1 (p = 0.06). No significant associations were observed either with use of anticholinergic medication or with ARS and BI quartiles. AMT did not show independent associations with any of the four measures of anticholinergic exposure.
Conclusion:
In older hospitalized patients, DBAC and some crude measures of anticholinergic exposure, but not ARS, showed independent associations with lower BI, but not AMT. These results highlight differences between various measures of anticholinergic drug exposure when studying their associations with functional status.
Keywords: Aged, Anticholinergic Risk Scale, Barthel Index, cholinergic antagonists, Drug Burden Index, drug toxicity, frail elderly, muscarinic antagonists
Introduction
Several commonly prescribed medications have anticholinergic (antimuscarinic) adverse effects [Bostock et al. 2010]. These are especially problematic for frail older patients and include central effects (e.g. confusion and falls) and peripheral effects (e.g. dry mouth and constipation) [Bostock et al. 2010]. However, it is difficult to predict the likelihood of adverse effects in any given patient or population [Bostock et al. 2010; Mangoni, 2011].
In early studies, anticholinergic drug exposure was assessed using relatively crude measures such as the use (or not) of such drugs [Lechevallier-Michel et al. 2005], or the total number of anticholinergic drugs taken. To enhance quantification, anticholinergic drug scoring systems have been developed in an attempt to account for important pharmacological factors such as drug affinity for muscarinic acetylcholine receptors and drug dosage [Bostock et al. 2010; Mangoni, 2011]. To be of value in clinical practice, scoring systems should be quick and simple to use with minimal training, valid in a variety of healthcare settings, and predict outcomes above crude measures [Bostock et al. 2010; Mangoni, 2011].
Examples of scoring systems to quantify anticholinergic drug exposure include the Anticholinergic Risk Scale (ARS) [Rudolph et al. 2008] and the anticholinergic burden (DBAC) component of the Drug Burden Index (DBI) [Hilmer et al. 2007].
Anticholinergic drug exposure, assessed using scoring systems, is independently associated with decreased functional status in older people. An inverse correlation between DBI and markers of cognitive and physical function has been demonstrated in a range of settings [Hilmer et al. 2007; Cao et al. 2008; Gnjidic et al. 2009, 2012a, 2012b; Lowry et al. 2012]. The ARS has been shown to be associated with confusion, constipation, falls [Rudolph et al. 2008] and reduced Barthel Index (BI) [Lowry et al. 2012], an established scale to assess activities of daily living and mobility [Mahoney and Barthel, 1965]. Little information is available on whether there are differences between scoring systems and crude measures of anticholinergic drug exposure on the strength of associations with functional status.
The objective of this study was to compare different measures of anticholinergic drug exposure, and the association of these measures with physical (primary outcome) and cognitive (secondary outcome) functional status in older hospitalized patients.
Methods
This was a prospective observational study of a consecutive series of older patients acutely admitted to the Department of Medicine for the Elderly, Woodend Hospital, NHS Grampian, Aberdeen, UK, between 28 September 2011 and 18 December 2011. Full ethical approval was obtained from the North of Scotland Research Ethics Committee, reference number 11/AL/0274.
The Department of Medicine for the Elderly admits frail older patients on the basis of need for comprehensive geriatric assessment and complex comorbidity rather than chronological age. Patients were admitted from community-dwelling settings (own home, sheltered housing), and institutions (care homes).
There were no exclusion criteria.
Variables
The following data were collected on admission: clinical and demographic variables, full medication exposure, biochemical parameters, BI and Abbreviated Mental Test (AMT).
Data sources/measurement
All data were collected by a single trained research nurse. A data collection sheet was completed for each participant from information routinely available from the participant’s medical and nursing notes. For consistency, the same portions of medical and nursing notes were used by the trained research nurse for data collection for each participant.
Demographic and clinical data were collected from the participants’ medical notes and clerking sheet. A full medication history was obtained from the medical notes, including dosage and timings of all medications taken at the time of admission. Laboratory results on admission are recorded on the clerking sheet.
The Modification of Diet in Renal Disease (MDRD) [Levey et al. 2006] equation was used to calculate estimated glomerular filtration rate (eGFR).
The Charlson Comorbidity Index (CCI) [de Groot et al. 2003; Hall et al. 2004], an established index for assessing comorbidity in clinical research, was calculated from the past medical history documented in the medical notes. CCI includes cardiac disease, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes, renal disease, solid organ tumours, lymphoma, leukaemia and acquired immunodeficiency syndrome.
The BI is a sensitive, valid and reliable measure of functional independence [Mahoney and Barthel, 1965]. It covers 10 activities including feeding, bathing, grooming, dressing, toileting, transfers mobility and stairs. All the activities are scored and the values are then added to give a total score ranging from 0 (totally dependent) to 100 (completely independent). The BI was calculated by the research nurse from information routinely collected by nursing staff in the nursing admission document.
The AMT is routinely measured on all admissions to the Department of Medicine for the Elderly and is recorded on the clerking sheet. The AMT is a validated, simple to administer 10-point bedside screening test of cognition [Hodkinson, 1972].
The medication history was used to calculate the total number of medications, the number of anticholinergic medications and the number of nonanticholinergic medications. Medications used on an as-required basis were excluded. Topical and ophthalmic medications were excluded as per the ARS derivation study [Rudolph et al. 2008]. Injections such as hydroxycobalamin and sex-hormone antagonists were excluded, but antipsychotic depot injections were included. Inhaled and nebulized medications and patches (e.g. fentanyl) prescribed for daily use were included.
Anticholinergic Risk Scale calculation
The ARS was calculated as per the derivation study [Rudolph et al. 2008]. Rudolph and colleagues developed the ARS, a ranked categorical list of commonly prescribed medications with anticholinergic potential. The included medications were derived from a list of the 500 most commonly prescribed medications in the Veterans Affairs Boston Healthcare System, USA. An expert group comprising one geriatrician and two pharmacists with an interest in geriatric prescribing independently reviewed the medications with known potential to cause anticholinergic adverse effects using the dissociation constant for the cholinergic receptor and published adverse effect rates from a number of databases. The three panel members assigned each medication a score based on its anticholinergic activity of 0 (limited or none), 1 (moderate), 2 (strong) or 3 (very strong). The ARS for the patient is the sum of the scores of all medications taken by that patient.
Whilst ipratropium and tiotropium were not assigned a score in the ARS derivation paper, both have been found to have a significant anticholinergic effect [Casarosa et al. 2010]. An adjudicating team [one clinical pharmacologist (AM) and one geriatrician (RS)] assigned ipratropium an ARS score of 3 and tiotropium a score of 2. The modified scale has been tested in previous studies by our group [Lowry et al. 2011b, 2012].
Anticholinergic drug burden calculation
DBAC was calculated as per the anticholinergic component of the DBI [Hilmer et al. 2007]. The total DBI for each patient is the sum of the sedative drug burden (DBS) and anticholinergic drug burden (DBAC):
The DBI for each anticholinergic drug was calculated by the following formula, where D is the daily drug dose and δ is the minimum recommended daily drug dose:
For example, a patient taking 45 mg mirtazapine, which has a minimum recommended daily dose of 15 mg [British Medical Association and Royal Pharmaceutical Society of Great Britain, 2012] would have a DBI score of 0.75.
In deciding which drugs to include as anticholinergic in calculating DBAC, we adapted the list of medications previously used by Hilmer and colleagues by expanding each existing drug class to include medications more commonly prescribed in the UK. The minimum recommended daily doses, specifically for older patients if stated, were obtained from the British National Formulary. For a full list of DBI medications used by this study population and the corresponding minimum daily doses, please see Table 1.
Table 1.
Medications included in Drug Burden Index (DBI) analysis in study population.
| Anticholinergic drugs | Minimum daily dose (mg) |
|---|---|
| Quetiapine | 25 |
| Citalopram | 10 |
| Citalopram drops | 16 drops |
| Escitalopram | 5 |
| Paroxetine | 20 |
| Fluoxetine | 20 |
| Sertraline | 50 |
| Mirtazapine | 15 |
| Duloxetine | 60 |
| Prochlorperazine | 10 |
| Trifluoperazine | 2 |
| Haloperidol | 1 |
| Oxybutynin | 5 |
| Tolterodine | 4 |
| Trospium | 40 |
| Solifenacin | 5 |
| Amitriptyline | 10 |
| Lofepramine | 140 |
| Trazodone | 100 |
| Risperidone | 1 |
| Ipratropium combination nebulizer | 1.5 |
| Tiotropium | 0.018 |
| Cetirizine | 10 |
| Cyclizine | 150 |
| Cinnarizine | 90 |
| Fexofenadine | 120 |
| Loratidine | 10 |
| Hydroxyzine | 25 |
| Chlorphenamine | 12 |
| Levodopa | 300 |
| Ropinirole | 2 |
| Pramipexole | 0.264 |
| Metoclopramide | 30 |
| Betahistine | 24 |
Anticholinergic medication calculation
Medications included in either ARS or DBAC, or both, were considered to be anticholinergic and were used to count the ‘number of anticholinergic medications’. Medications not included in either ARS or DBI were used to count the ‘number of nonanticholinergic medications’:
Statistical methods
As the data for the primary and secondary outcome measures (BI and AMT) and the measures of anticholinergic use were not normally distributed, correlations were assessed using Spearman’s correlation. The independent relationship between outcome measures and measures of anticholinergic use were assessed using multinomial logistic regression, controlling for comorbidities and demographic characteristics. Potential confounders were determined by assessing the univariate correlation between patient variables (e.g. age, CCI, history of dementia) and each of the outcome measures. Variables that correlated with the outcome measure with p < 0.2 were included in multinomial logistic regression. BI was split into quartiles (0–25, 26–50, 51–75, 76–100) and AMT into categories (0–6, 7–10).
The following 11 variables were considered a priori possibly or probably associated with BI: age, sex, living in an institution, CCI, history of dementia, delirium, eGFR, serum albumin, serum sodium, total number of medications and measures of anticholinergic exposure. A minimum of 20 participants per predictor variable in the multinomial logistic regression were included. Therefore a minimum of 220 patients with complete data were required. Data were analysed using SPSS V.20 (SPSS Inc, Chicago, IL, USA) and STATA IC11 (StataCorp, College Station, TX, USA). A p value <0.05 indicated statistical significance.
Results
A total number of 271 patients were admitted during the study period and all patients were included in the analysis.
Descriptive data
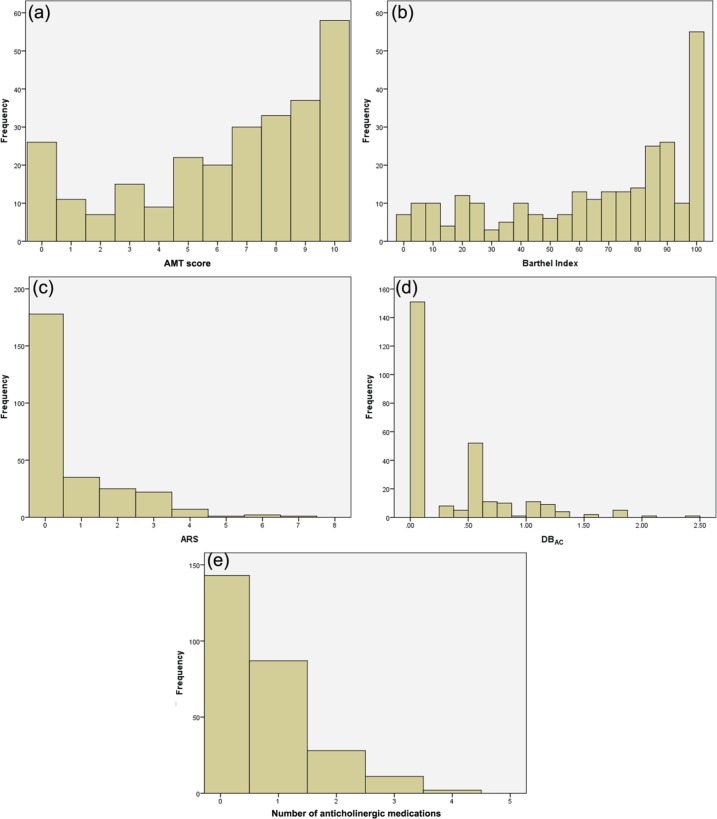
The sample demographics, clinical features and results of measures of anticholinergic use are displayed in Table 2 and Figure 1. AMT scores were missing for three individuals for the following reasons: one non-English speaker, one patient with dysphasia, one not performed. These values were treated as missing. Due to problems with data capture, creatinine value was not recorded for 28 participants and hence MDRD could only be calculated for 243 participants. Absent values were treated as missing. In a single case, the dose of an anticholinergic medication was unknown and DBAC was assigned as 0.5 for this medication in this case.
Table 2.
Clinical and demographic characteristics of the study population (n = 271).
| Age (mean ± SD, years) | 83 ± 7 |
| Sex (% men) | 40 |
| Living in an institution (%) | 11 |
| Charlson Comorbidity Index (mean ± SD) | 6 ± 2 |
| History of dementia (%) | 26 |
| Presenting with delirium (%) | 26 |
| Creatinine (mean ± SD) (n = 243) | 101 ± 60 |
| MDRD eGFR (mean ± SD) (n = 243) | 66 ± 31 |
| Serum albumin (mean ± SD) | 40 ± 4 |
| Serum sodium (mean ± SD) | 138 ± 5 |
| Barthel Index, (median [range]{IQR}) |
75 [0–100] {40–90} |
| AMT (median [range] {IQR}) (n = 268) | 7 [0–10] {4–9} |
| Total number of medications (median [range] {IQR}) | 6 [0–16] {4–8} |
| ARS (median [range] {IQR}) | 0.0 [0–7] {0–1} |
| DBAC (median [range] {IQR} | 0.00 [0.00–2.42] {0–0.5} |
| Number of anticholinergic medications (median [range] {IQR}) |
0 [0–4] {0–1} |
| Use of anticholinergic drug (%) | 47% |
AMT, Abbreviated Mental Test; ARS, Anticholinergic Risk Scale; DBAC, anticholinergic drug burden; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease.
Figure 1.
Frequency distributions of: (a) Abbreviated Mental Test (AMT) score; (b) Barthel Index; (c) Anticholinergic Risk Scale (ARS); (d) Anticholinergic Drug Burden (DBAC); (e) number of anticholinergic medications.
Main results
The results of univariate analysis for qualitative variables are displayed in Table 3. Categorical variables dementia, delirium and institutionalization were associated in nonparametric analyses (data not shown). There was an inverse correlation (p < 0.2) of the primary outcome measure, BI, with ARS, DBAC, number of anticholinergic medications and use of anticholinergic medication. There was an inverse correlation (p < 0.2) of the secondary outcome measure, AMT, with DBAC, number of anticholinergic medications and use of anticholinergic medication.
Table 3.
Univariate analysis.
| Barthel Index Correlation coefficient, ρ (p value) |
AMT Correlation coefficient, ρ (p value) |
|
|---|---|---|
| Age | −0.058 (0.344) |
−0.009 (0.882) |
| Charlson Comorbidity Index | −0.244 (<0.01)* |
−0.079 (0.200) |
| MDRD eGFR | 0.024 (0.706) |
0.052 (0.425) |
| Serum albumin | 0.245 (<0.01)* |
−0.013 (0.832) |
| Serum sodium | −0.092 (0.130) |
−0.129 (0.035)* |
| Total number of medications | −0.103 (0.092) |
0.116 (0.058) |
| ARS | −0.107 (0.078) |
−0.049 (0.423) |
| DBAC | −0.176 (<0.01)* |
−0.106 (0.084) |
| Number of anticholinergic medications | −0.188 (<0.01)* |
−0.107 (0.081) |
| Anticholinergic use | −0.179 (<0.01)* |
−0.105 (0.085) |
Statistically significant p < 0.05.
AMT, Abbreviated Mental Test; ARS, Anticholinergic Risk Scale; DBAC, anticholinergic drug burden; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Variables that correlated with the BI (p < 0.2) were included in multinomial logistic regression analysis (see Table 4). Multinomial logistic regression showed that higher DBAC was associated with a greater risk of being in the lower BI quartiles (Q2 and Q3) versus the highest BI quartile (Q4). A similar result was observed with the number of anticholinergic drugs (Q2 versus Q4) and with use of anticholinergic medication (Q3 versus Q4). By contrast, no independent associations were observed between ARS and BI quartiles.
Table 4.
Multinomial logistic regression of the effect of anticholinergic measures and other factors on risk of lower (1-3) versus highest (4) Barthel quartiles.
| Barthel quartile | RR | p value | 95% CI | |
|---|---|---|---|---|
| ARS | ||||
| 1 (0–25) | Living in institution | 2.50 | 0.11 | 0.81–7.71 |
| Charlson Comorbidity Index | 1.20 | 0.05 | 1.00–1.46 | |
| Presenting with delirium | 1.44 | 0.45 | 0.56–3.71 | |
| History of dementia | 1.09 | 0.86 | 0.41–2.90 | |
| Serum albumin | 0.83 | <0.01 | 0.75–0.92 | |
| AMT | 0.73 | <0.01 | 0.63–0.85 | |
| Nonanticholinergic medications | 1.03 | 0.69 | 0.88–1.20 | |
| ARS | 1.16 | 0.32 | 0.86–1.59 | |
| 2 (26-50) | Living in institution | 0.38 | 0.28 | 0.07–2.18 |
| Charlson Comorbidity Index | 1.10 | 0.37 | 0.89–1.38 | |
| Presenting with delirium | 1.21 | 0.72 | 0.43–3.38 | |
| History of dementia | 1.13 | 0.82 | 0.41–3.08 | |
| Serum albumin | 0.93 | 0.20 | 0.83–1.04 | |
| AMT | 0.74 | <0.01 | 0.63–0.88 | |
| Nonanticholinergic medications | 0.98 | 0.83 | 0.83–1.16 | |
| ARS | 1.19 | 0.29 | 0.86–1.65 | |
| 3 (51-75) | Living in institution | 0.52 | 0.39 | 0.12–2.29 |
| Charlson Comorbidity Index | 1.08 | 0.36 | 0.91–1.29 | |
| Presenting with delirium | 0.60 | 0.31 | 0.23–1.59 | |
| History of dementia | 1.78 | 0.18 | 0.77–4.14 | |
| Serum albumin | 0.96 | 0.40 | 0.88-1.05 | |
| AMT | 0.84 | 0.02 | 0.73–0.97 | |
| Nonanticholinergic medications | 1.18 | 0.01 | 1.01–1.34 | |
| ARS | 1.19 | 0.17 | 0.93–1.53 | |
| DBac | ||||
| 1 (0-25) | Living in institution | 2.37 | 0.14 | 0.76–7.34 |
| Charlson Comorbidity Index | 1.23 | 0.04 | 1.01–1.49 | |
| Presenting with delirium | 1.39 | 0.49 | 0.54–3.61 | |
| History of dementia | 0.99 | 0.98 | 0.37–2.64 | |
| Serum albumin | 0.84 | <0.01 | 0.76–0.93 | |
| AMT | 0.73 | <0.01 | 0.63-0.85 | |
| Nonanticholinergic medications | 1.02 | 0.78 | 0.87–1.20 | |
| DBac | 2.25 | 0.07 | 0.95–5.31 | |
| 2 (26-50) | Living in institution | 0.35 | 0.24 | 0.06–2.03 |
| Charlson Comorbidity Index | 1.13 | 0.27 | 0.91–1.42 | |
| Presenting with delirium | 1.14 | 0.81 | 0.40–3.20 | |
| History of dementia | 0.96 | 0.97 | 0.35–2.67 | |
| Serum albumin | 0.93 | 0.22 | 0.83–1.04 | |
| AMT | 0.74 | <0.01 | 0.63–0.87 | |
| Nonanticholinergic medications | 0.96 | 0.68 | 0.81–1.15 | |
| DBac | 2.96 | 0.02 | 1.21–7.27 | |
| 3 (51-75) | Living in institution | 0.50 | 0.36 | 0.11–2.20 |
| Charlson Comorbidity Index | 1.10 | 0.30 | 0.92–1.31 | |
| Presenting with delirium | 0.58 | 0.28 | 0.22–1.55 | |
| History of dementia | 1.61 | 0.27 | 0.69–3.80 | |
| Serum albumin | 0.96 | 0.40 | 0.88–1.05 | |
| AMT | 0.84 | 0.02 | 0.73–0.97 | |
| Nonanticholinergic medications | 1.17 | 0.02 | 1.03–1.33 | |
| DBac | 2.15 | 0.04 | 1.02–4.51 | |
| Number of anticholinergic medications | ||||
| 1 (0-25) | Living in institution | 2.23 | 0.14 | 0.75–7.20 |
| Charlson Comorbidity Index | 1.23 | 0.04 | 1.01–1.49 | |
| Presenting with delirium | 1.47 | 0.43 | 0.57–3.80 | |
| History of dementia | 1.00 | 0.99 | 0.38–2.68 | |
| Serum albumin | 0.84 | <0.01 | 0.76–0.93 | |
| AMT | 0.73 | <0.01 | 0.63-0.85 | |
| Nonanticholinergic medications | 1.02 | 0.79 | 0.87–1.19 | |
| Number of anticholinergic medications | 1.54 | 0.06 | 0.99–2.41 | |
| 2 (26-50) | Living in institution | 0.34 | 0.23 | 0.06–1.98 |
| Charlson Comorbidity Index | 1.13 | 0.29 | 0.90–1.41 | |
| Presenting with delirium | 1.19 | 0.74 | 0.42–3.34 | |
| History of dementia | 0.98 | 0.97 | 0.35–2.72 | |
| Serum albumin | 0.93 | 0.22 | 0.84–1.04 | |
| AMT | 0.74 | <0.01 | 0.63–0.88 | |
| Nonanticholinergic medications | 0.96 | 0.65 | 0.81–1.14 | |
| Number of anticholinergic medications | 1.74 | 0.02 | 1.08–2.80 | |
| 3 (51-75) | Living in institution | 0.50 | 0.36 | 0.11–2.22 |
| Charlson Comorbidity Index | 1.09 | 0.34 | 0.91–1.30 | |
| Presenting with delirium | 0.61 | 0.32 | 0.23–1.62 | |
| History of dementia | 1.67 | 0.24 | 0.72–3.91 | |
| Serum albumin | 0.96 | 0.43 | 0.88–1.05 | |
| AMT | 0.84 | 0.02 | 0.73–0.97 | |
| Nonanticholinergic medications | 1.17 | 0.02 | 1.03–1.33 | |
| Number of anticholinergic medications | 1.37 | 0.11 | 0.94–2.02 | |
| Anticholinergic use | ||||
| 1 (0-25) | Living in institution | 2.34 | 0.14 | 0.75–7.25 |
| Charlson Comorbidity Index | 1.21 | 0.048 | 1.002–1.47 | |
| Presenting with delirium | 1.41 | 0.47 | 0.55–3.63 | |
| History of dementia | 1.00 | 1.00 | 0.37–2.67 | |
| Serum albumin | 0.84 | <0.01 | 0.76–0.93 | |
| AMT | 0.73 | <0.01 | 0.63–0.85 | |
| Nonanticholinergic medications | 1.03 | 0.67 | 0.89–1.21 | |
| Anticholinergic use | 1.78 | 0.15 | 0.82–3.87 | |
| 2 (26-50) | Living in institution | 0.36 | 0.25 | 0.06–2.04 |
| Charlson Comorbidity Index | 1.11 | 0.35 | 0.89–1.38 | |
| Presenting with delirium | 1.18 | 0.76 | 0.42–3.29 | |
| History of dementia | 1.01 | 0.98 | 0.37–2.81 | |
| Serum albumin | 0.93 | 0.23 | 0.84–1.04 | |
| AMT | 0.74 | <0.01 | 0.63–0.88 | |
| Nonanticholinergic medications | 0.98 | 0.83 | 0.83–1.16 | |
| Anticholinergic use | 1.93 | 0.13 | 0.82–4.52 | |
| 3 (51-75) | Living in institution | 0.48 | 0.33 | 0.11–2.11 |
| Charlson Comorbidity Index | 1.09 | 0.35 | 0.91–1.30 | |
| Presenting with delirium | 0.58 | 0.27 | 0.22–1.54 | |
| History of dementia | 1.60 | 0.28 | 0.68–3.78 | |
| Serum albumin | 0.97 | 0.44 | 0.88–1.06 | |
| AMT | 0.84 | 0.02 | 0.73–0.97 | |
| Nonanticholinergic medications | 1.18 | 0.01 | 1.04–1.34 | |
| Anticholinergic use | 2.12 | 0.03 | 1.08–4.15 | |
AMT, Abbreviated Mental Test; ARS, Anticholinergic Risk Scale; CI, confidence interval; DBAC, anticholinergic drug burden; RR, relative risk.
Variables that correlated with AMT (p < 0.2) were included in multinomial logistic regression analysis. However, there was no independent association between AMT and anticholinergic use (data not shown).
Discussion
Interpretation
DBAC and the number of anticholinergic medications showed some independent associations with BI. The DBI was designed as a tool to define the functional burden of medications in older people [Hilmer et al. 2007] and our study confirms an inverse functional association with DBAC. The DBI has been well validated in various countries and settings, with community-dwelling participants and those in institutions [Hilmer et al. 2007; Cao et al. 2008; Gnjidic et al. 2009, 2012a, 2012b; Lowry et al. 2012], and studies have analyzed the separate components DBAC and DBS [Hilmer et al. 2007; Cao et al. 2008; Gnjidic et al. 2009; Lowry et al. 2012]. In our study, simple count measures of anticholinergic drug use were comparable to DBAC in terms of association with functional status. Whilst DBAC is a simple equation, the mental arithmetic to calculate DBAC at the bedside is often not trivial. DBAC lends itself to computer-based applications, whereas for busy clinicians the number of anticholinergic drugs on a prescription list is simpler.
We did not show any significant association between ARS and functional status. The ARS was derived to predict risk of anticholinergic adverse effect [Rudolph et al. 2008] (not measured in this study), rather than function per se. A limitation of the ARS is that it relies on a predetermined list of medications, which does not allow for the inclusion of newer anticholinergic drugs, or those more frequently prescribed in the USA (e.g. cyclizine, cinnarizine, duloxetine, trospium). In contrast to our findings, a study of older hospitalized patients in the UK did find that a higher ARS score was associated with decreased BI [Lowry et al. 2011a].
In this study, measures of anticholinergic drug exposure showed some associations with global function (BI), but not mental function (AMT). Although BI is a measure of independence in physical activities (e.g. bathing, dressing), the ability to perform these activities relies on cognitive function. Whilst recognizing that AMT is a screening tool, its inclusion as a secondary outcome measure was justified by its widespread clinical use. Multinomial regression showed no significant association between AMT and any of the four measures of anticholinergic exposure. An area of further study would be to assess the association of an alternative measure of mental function, for example, the Mini Mental State Examination [Folstein et al. 1975] with different measures of anticholinergic exposure.
Study strengths and limitations
The strength of this study is the comparison of different measures of anticholinergic drug exposure within the same population. Bias was limited by studying a series of consecutive admissions with no exclusions. All data were collected by a single trained research nurse, using consistent sources from the medical and nursing notes. This is a relatively small sample size compared with landmark DBI studies, however it is the same order of magnitude as the ARS derivation study (149 subjects) [Rudolph et al. 2008].
The information on drug history was obtained after medicine reconciliation from the medical notes; however, this only includes medications used at the time of admission. There was no account of medications taken, and potentially discontinued, in the weeks leading up to admission, which may have had a residual effect on functional status. For purposes of consistency, medications taken on an as-required basis were excluded. Some as-required medications did have an anticholinergic effect and the extent of use of these medications may have influenced the results.
Attempts were made to address potential confounding factors, but the CCI accounts more for physical than mental comorbidities. Whilst dementia is included in CCI and clinical data were collected on history of dementia, no data were collected on history of depression. Many of the anticholinergic drugs taken by this population are psychotropic drugs, including antidepressants. Future studies could adjust for depression as a potential confounder.
The presence of an inverse correlation between anticholinergic drug use and functional status does not imply causation. There have been longitudinal studies which have shown that anticholinergic medications are associated with function decline [Han et al. 2008; Hilmer et al. 2009]. In contrast, a longitudinal study of frailer, functionally limited individuals from residential aged care facilities showed no statistical association between cumulative anticholinergic use, as measured by DBI, and measures of physical function [Wilson et al. 2011].
Generalizability
This is a study of older patients, admitted acutely to a care of the elderly hospital in Scotland, UK. Previous studies using DBI and ARS in different settings have not shown consistent results across all settings. It would be prudent to compare measures of anticholinergic use in other settings and in other countries.
Conclusion
In this population of older hospitalized patients, DBAC and crude measures of anticholinergic exposure, but not ARS, showed some independent associations with lower BI. Our results highlight differences between various measures of anticholinergic drug exposure when studying their associations with functional status.
Acknowledgments
The authors thank Research Nurse Hazel Clark for data collection.
Footnotes
Funding: This work was supported by an Endowment Grant from NHS Grampian and sponsored by NHS Grampian Research and Development. Dr Soiza’s contribution is funded by an NHS Research Scotland (NRS) Career Research Fellowship. The funder/sponsor had no role in study design, conduct or data-analysis.
Conflict of interest statement: All authors declare no conflict of interest.
Contributor Information
Clare V Bostock, NHS Grampian, Department of Medicine for the Elderly, Woodend Hospital, Eday Road, Aberdeen AB15 6XS, UK.
Roy L Soiza, Division of Applied Medicine, University of Aberdeen, Aberdeen, UK.
Arduino A Mangoni, Department of Clinical Pharmacology, Flinders University, Adelaide, SA, Australia.
References
- Bostock C., Soiza R., Mangoni A. (2010) Association between prescribing of antimuscarinic drugs and antimuscarinic adverse effects in older people. Expert Rev Clin Pharmacol 3: 441–452 [DOI] [PubMed] [Google Scholar]
- British Medical Association and Royal Pharmaceutical Society of Great Britain (2011) British National Formulary, 62nd edition. London: RPS Publishing [Google Scholar]
- Cao Y., Mager D., Simonsick E., Hilmer S., Ling S., Windham B., et al. (2008) Physical and cognitive performances and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther 83: 422–429 [DOI] [PubMed] [Google Scholar]
- Casarosa P., Keichle T., Sieger P., Pieper M., Ganter F. (2010) The constitutive activity of the human muscarinic M3 receptor unmasks differences in the pharmacology of anticholinergics. J Pharmacol Exp Ther 333: 201–209 [DOI] [PubMed] [Google Scholar]
- de Groot V., Beckerman H., Lankhorst G., Bouter L. (2003) How to measure comorbidity: a critical review of available methods. J Clin Epidemiol 56: 221–229 [DOI] [PubMed] [Google Scholar]
- Folstein M., Folstein S., McHugh P. (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198 [DOI] [PubMed] [Google Scholar]
- Gnjidic D., Bell J., Hilmer S., Lönnroos E., Sulkava R., Hartikainen S. (2012a) Drug Burden Index associated with function in community-dwelling older people in Finland: A cross sectional study. Ann Med 44: 458–467 [DOI] [PubMed] [Google Scholar]
- Gnjidic D., Cumming R., Le Couteur D., Handelsman D., Naganathan V., Abernethy D., et al. (2009) Drug Burden Index and physical function in older Australian men. Br J Clin Pharmacol 68: 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjidic D., Le Couter D., Abernethy D., Hilmer S. (2012b) Drug Burden Index and Beers Criteria: impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol 52: 258–265 [DOI] [PubMed] [Google Scholar]
- Hall W., Ramachandran R., Narayan S., Jani A., Vijayakumer S. (2004) An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 4: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Agostini J., Allore H. (2008) Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc 56: 2203–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmer S., Mager D., Simonsick E., Cao Y., Ling S., Windham G., et al. (2007) A Drug Burden Index to define the functional burden of medications in older people. Arch Intern Med 167: 781–787 [DOI] [PubMed] [Google Scholar]
- Hilmer S., Mager D.E., Simonsick E., Ling S., Windham B., Harris T., et al. (2009) Drug Burden Index score and functional decline in older people. Am J Med 122: 1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson H. (1972) Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1: 233–238 [DOI] [PubMed] [Google Scholar]
- Lechevallier-Michel N., Molimard M., Dartidues J., Fabrigoule C., Fourrier-Reglat A. (2005) Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID study. Br J Clin Pharmacol 59: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A., Coresh J., Greene T., Stevens L., Zhang Y., Hendriksen S., et al. (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254 [DOI] [PubMed] [Google Scholar]
- Lowry E., Woodman R., Soiza R., Mangoni A. (2011a) Associations between the Anticholinergic Risk Scale score and physical function: potential implications for adverse outcomes in older hospitalized patients. J Am Med Dir Assoc 12: 565–572 [DOI] [PubMed] [Google Scholar]
- Lowry E., Woodman R., Soiza R., Mangoni A. (2011b) Clinical and demographic factors associated with antimuscarinic medication use in older hospitalised patients. Hosp Pract 39: 30–36 [DOI] [PubMed] [Google Scholar]
- Lowry E., Woodman R., Soiza R., Hilmer S., Mangoni A. (2012) Drug Burden Index, physical function, and adverse outcomes in older hospitalized patients. J Clin Pharmacol 52: 1584–1591 [DOI] [PubMed] [Google Scholar]
- Mahoney F., Barthel D. (1965) Functional evaluation: the Barthel Index. Md State Med J 14: 61–65 [PubMed] [Google Scholar]
- Mangoni A. (2011) Assessing the adverse effects of antimuscarinic drugs in older patients: which way forward? Exp Rev Clin Pharmacol 4: 531–533 [DOI] [PubMed] [Google Scholar]
- Rudolph J., Salow M., Angelini M., McGlinchey R. (2008) The Anticholinergic Risk Scale and anticholinergic adverse effects in older persons. Arch Intern Med 168: 508–513 [DOI] [PubMed] [Google Scholar]
- Wilson N., Hilmer S., March L., Cameron I., Lord S., Seibel M., et al. (2011) Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc 59: 875–880 [DOI] [PubMed] [Google Scholar]