Abstract
BACKGROUND
S100B is supposed to be a peripheral biomarker of central nervous system (CNS) injury. The purpose of this study was to compare levels of S100B in women with preeclampsia with levels in healthy pregnant control subjects and furthermore to analyze levels of S100B in relation to possible CNS effects.
METHODS
A cross-sectional case–control study in antenatal care centers in Uppsala, Sweden, was performed. Fifty-three women with preeclampsia and 58 healthy pregnant women were recruited at similar gestational length; women with preeclampsia were recruited at time of diagnosis, and control subjects were recruited during their routine visit to an antenatal clinic. Plasma samples were collected, and levels of S100B were analyzed with an enzyme-linked immunosorbent assay. Information about demographic and clinical characteristics, including symptoms related to CNS affection, was collected from the medical records. The main outcome measures were plasma levels of S100B and possible CNS effects.
RESULTS
Levels of S100B were significantly higher among women with preeclampsia than among control subjects (0.12 µg/L vs. 0.07 µg/L; P < 0.001). In preeclampsia, there was a significant association between high levels of S100B and visual disturbances (P < 0.05).
CONCLUSIONS
S100B is increased among women with preeclampsia, and high levels of S100B associate with visual disturbances, which might reflect CNS affection in women with preeclampsia.
Keywords: blood pressure, hypertension, neurological dysfunction, preeclampsia, S100B
Preeclampsia, characterized by new-onset of hypertension and proteinuria after gestational week 20, is a multiorgan, pregnancy-specific disorder that is one of the most common causes of maternal, as well as perinatal, morbidity and mortality worldwide.1 Sixty-five thousand women die each year because of complications related to preeclampsia. Seventy-five percent of these are deaths caused by acute cerebral complications such as eclampsia, brain hemorrhage, and brain edema.1,2 Eclampsia is known as the occurrence of tonic-clonic seizures during pregnancy or in the early postpartum period.3
The pathogenesis of preeclampsia is not fully understood. The main hypothesis is based on endothelial cell dysfunction, which affects placental microvasculature and the vasculature of the woman in general.4 Women with preeclampsia have been shown to display cerebral hemodynamic changes, which appear before clinical symptoms of the disorder.2 Eclampsia is associated with vasogenic edema.2 There are 3 main different theories for the occurrence of this edema: one is that it is caused by severe hypertension, leading to inadequate autoregulation and hyperperfusion in the brain; a second possibility is vasoconstriction and hypoperfusion leading to brain ischemia; a third theory is related to a possible endothelial dysfunction due to circulating toxins that may affect the blood–brain barrier and lead to subsequent extravasation.5 This increased permeability through the blood-brain barrier has been described in recent animal studies, where it is claimed that increased permeability can also occur in preeclampsia without seizures.6 In eclampsia and severe preeclampsia, there is a risk for acute cerebral complications, but it has also been shown that women who have had preeclampsia or eclampsia without other acute complications have an increased risk for persistent cognitive impairment for months or even years after the pregnancy.2
There is no treatment for preeclampsia, except for delivery. It is not always easy to decide when to deliver, and the risk of maternal morbidity and mortality, as well as the risk of severe perinatal outcomes, must be taken into consideration. A reliable biochemical marker that reflects affection of the maternal brain during preeclampsia could be useful in clinical work when a decision about whether to deliver or not has to be taken.
S100B is a protein produced and released primarily by glial cells in the central nervous system (CNS).7 The protein is released during a trauma to the head, and serum levels correlate positively with the degree of cerebral insult induced and negatively with outcome.8,9 S100B is also used as a marker for damaged blood–brain barrier,10 and it has been postulated that levels of S100B are increased in patients with neurodegenerative diseases, psychiatric disorders, and stroke.11–13 We have recently shown that levels of S100B increase during pregnancy in women who develop preeclampsia.14
The hypotheses of this cross-sectional case–control study were that women with preeclampsia have higher levels of S100B compared with healthy pregnant control subjects and that S100B levels reflect the degree of possible CNS effects in women with preeclampsia.
METHODS
The study was approved by the regional Ethics Committee of the Medical Faculty of Uppsala University, and informed consent was obtained from each patient included in the study.
Study population
This study was designed as a case–control study. The women were recruited between 2007 and 2010 at the Department of Obstetrics and Gynaecology, Uppsala University Hospital, Uppsala, Sweden. Case patients (n = 53) were pregnant women with preeclampsia. They were included at the time when the diagnosis was confirmed. The definition of preeclampsia was new-onset hypertension (≥140/90mm Hg, observed on at least 2 separate measurements ≥6 hours apart), combined with proteinuria (≥2 units on a dipstick or a 24-hour urine sampling showing ≥300mg albumin/24 hours) after gestational week 20. The control subjects (n = 58) were frequency matched according to gestational weeks ± 2 weeks and consisted of women with normal pregnancies defined as an uncomplicated progress throughout the whole pregnancy, with delivery after 37 weeks of gestation of an infant not small for gestational age (defined as birth weight >2 SDs below mean birth weight for gestational age of the reference population).15 Most of the women in the control group were recruited during their routine visit to 2 of the antenatal clinics in Uppsala Municipality. A few women were recruited during a visit to the obstetrics emergency outpatient department at the University Hospital, Uppsala. Both nullipara and parous women were included, but only singleton pregnancies were accepted. Women were not included if they were suffering from chronic hypertension, renal disease, or diabetes mellitus or if their plasma levels of S100B had not been measured. None of the women in the study suffered from heart disease according to the medical records.
At inclusion, maternal height, weight, and blood pressure were registered. The routine for measuring blood pressure was standardized and measured after approximately 15 minutes of rest, in supine position on the right upper arm, with a manual blood pressure apparatus (Umedico, Rosersberg, Sweden). Information about reproductive history and smoking habits, as well as pregnancy-related complications, gestational age at delivery, and birth weight of the infant, was collected from the medical records. Gestational age was defined as completed weeks of gestation calculated from the estimated date of delivery after the second trimester ultrasound screening.
Symptoms and signs related to possible CNS effects
Symptoms and signs related to possible CNS effects were defined as visual disturbances, altered tendon reflexes, and headache. Data were collected from the medical records, and each symptom was categorized into present or nonpresent depending on whether the symptom had been reported as present within 2 days of blood sampling. A variety of visual disturbances and headaches were recorded as symptoms. No women were, when examined, found to have altered tendon reflexes within this time period, and therefore this sign was excluded in the analysis.
Sample collection
A plasma sample was collected in lithium/heparin–containing tubes from each woman at inclusion. After collection, the blood samples were kept at room temperature for no longer than half an hour before being centrifuged for 10 minutes at 2,000g. The plasma samples were separated and stored in new tubes at −70 °C until levels of S100B were analyzed. The mean storage time was <3 years.
Enzyme-linked immunosorbent assay
Plasma samples were analyzed for levels of S100B by an enzyme-linked immunosorbent assay (ELISA). A commercially available kit (Sangtec 100 ELISA; Diasorin, MN) was used, and the tests were performed according to the manufacturer’s recommendations. The inter- and intra-assay coefficients of variation were 3.3% and 7.7%, respectively. The limit of detection was 0.015 µg/L.
Statistics
Demographic and clinical characteristics were compared between case patients and control subjects by use of Student t test and χ2 tests. Levels of S100B were compared between case patients and control subjects by use of Mann–Whitney U test. S100B levels less than the detection limits of the ELISA were recorded as 0.015 µg/L. Values are presented as mean ± SD and median with range, respectively. All significance tests were 2-tailed. A receiver operator characteristics curve was constructed to determine the best cutoff value for levels of S100B and association with preeclampsia (0.14 µg/L). Logistic regression analyses were used to calculate unadjusted and adjusted odds ratios (ORs) for the association between preeclampsia and levels of S100B. The following maternal factors were analyzed: maternal age as completed years in the first trimester, parity as nullipara or not, and body mass index (kg/m2) calculated by first trimester height and weight. Smoking was not included in the statistical model because of a too-low number of smokers in the population to allow calculations. Factors that were significantly (P < 0.05) associated with the outcome in univariable analyses were included in the final multivariable model. Internal validation in means of a 2-fold cross-validation was performed to avoid overoptimism in the OR estimates. In women with preeclampsia, we estimated whether symptoms related to CNS affection were more common in women with high S100B levels according to the cutoff value from the receiver operator characteristics curve through logistic regression analysis, adjusting for headache. The level of significance was P < 0.05. Statistical analysis was performed using SPSS version 20.0 (IBM SPSS statistics, Armank, NY) for MAC software package.
RESULTS
Background characteristics
Maternal characteristics and pregnancy outcome of case patients and control subjects are presented in Table 1. At first antenatal visit, women who developed preeclampsia did not differ from control subjects in terms of maternal age or smoking habit, but they were more often nulliparous and had higher body mass index and blood pressure. All women in the group of preeclampsia had ≥2 units on a urine dipstick at inclusion; no one among the control subjects had any sign of proteinuria. At partus, 85% of the women with preeclampsia were treated with antihypertensive medication. Case patients and control subjects were matched for gestational age at time of inclusion, but women with preeclampsia had, as expected, a shorter gestational length at delivery and delivered infants with lower birth weight than control subjects.
Table 1.
Background characteristics of the study population
| Background characteristics | Normal pregnancy (n = 58) | Preeclampsia (n = 53) | P value |
|---|---|---|---|
| Maternal age, y | 30±4 | 30±5 | NS |
| Nulliparous, no. (%) | 29 (50) | 37 (70) | <0.05 |
| S100B, µg/L, median (range) | 0.07 (0.02–0.31) | 0.12 (0.02–0.77) | <0.001 |
| At first antenatal visit | |||
| Smokers, no. (%) | 2 (3) | 0 (0) | NS |
| BMI, kg/m2 | 23±3 | 27±6 | <0.001 |
| Systolic BP, mm Hg | 114±9 | 124±11 | <0.001 |
| Diastolic BP, mm Hg | 65±7 | 79±8 | <0.001 |
| MAP, mm Hg | 81±7 | 94±8 | <0.001 |
| At inclusion | |||
| Gestational age, d | 242±34 | 247±31 | NS |
| BMI, kg/m2 | 27±4 | 33±7 | <0.001 |
| Systolic BP, mm Hg | 114±9 | 146±12 | <0.001 |
| Diastolic BP, mm Hg | 69±7 | 91±11 | <0.001 |
| MAP, mm Hg | 84±7 | 109±10 | <0.001 |
| Proteinuria,a no. (%) | 0 (0) | 53 (100) | <0.001 |
| At partus | |||
| Gestational age, d | 280±9 | 250±29 | <0.001 |
| Antihypertensive drugs, no. (%) | 0 (0) | 45 (85) | <0.001 |
| Infant birth weight, g | 3,658±434 | 2,554±988 | <0.001 |
Data are presented as mean ± SD, unless otherwise noted. Student t test was used for all variables except S100B, which used the Mann–Whitney test.
Abbreviations: BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; NS, not significant.
a >2 units on a dipstick.
Plasma levels of S100B
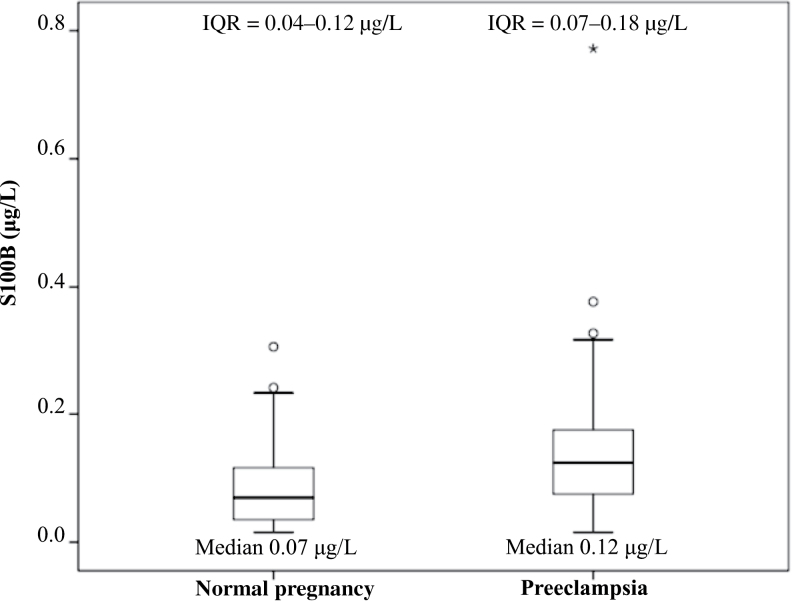
Levels of S100B in plasma for women with preeclampsia and healthy control subjects are presented as a boxplot in Figure 1. Women with preeclampsia had significantly higher median levels of S100B compared with control subjects (0.12 µg/L, 0.02–0.77 µg/L; vs. 0.07 µg/L, 0.02–0.31 µg/L; P < 0.001). Postpartum samples were available from some (n = 12) of the women with preeclampsia and the postpartum median levels were significantly higher when compared with samples collected before partus (n = 41) in that group of women (0.16 µg/L, 0.03–0.77 µg/L; vs. 0.11 µg/L, 0.02–0.33 µg/L; P < 0.05).
Figure 1.
Boxplot showing levels of S100B among case patients and control subjects. The top and bottom of the box represent the third and first quartiles. The horizontal line within the box represents the median value. The bars on the sides of the box represent the highest and the lowest value. The circles represent extreme values >1.5 × interquartile range (IQR), and the asterisks represent extreme values >3 × IQR. P < 0.001.
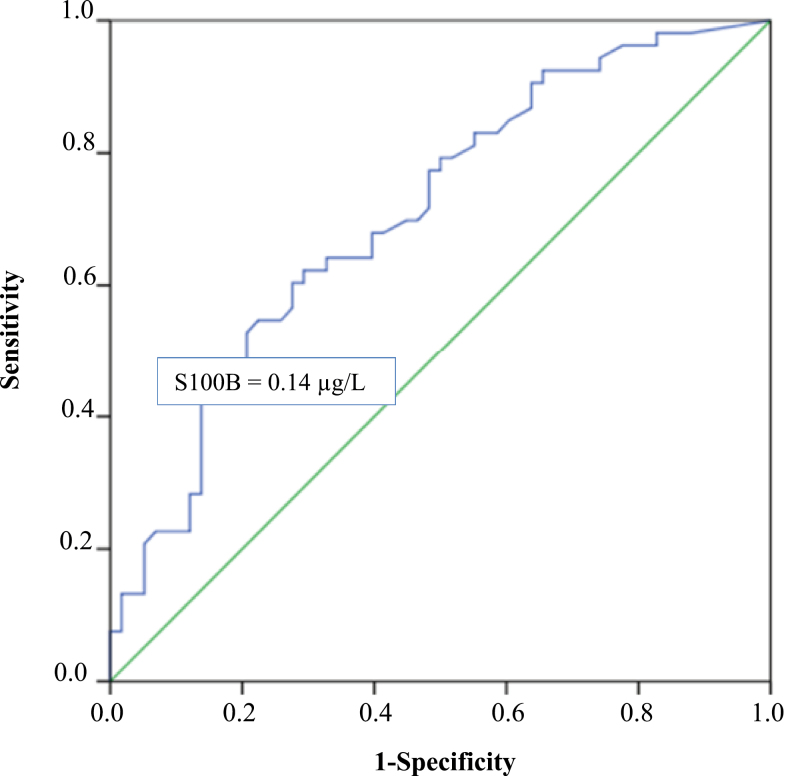
The association between preeclampsia and high levels of S100B was analyzed further. A receiver operator characteristics curve for levels of S100B and risk of preeclampsia was constructed, and a cutoff value of 0.14 µg/L for levels of S100B was chosen to optimize sensitivity (44%) and specificity (86%). The area under the curve was 0.71 (Figure 2). When levels of S100B were divided into 2 groups based on the cutoff value and used in a bivariable regression analysis, the results confirmed an association between preeclampsia and high levels of S100B (OR = 4.71; 95% confidence interval (CI) = 1.79–12.37). In a multivariable logistic regression analysis adjusted for parity and body mass index, high levels of S100B still remained as an independent factor associated with preeclampsia (OR = 5.56; 95% CI = 1.91–16.19) (Table 2). The results were confirmed in a 2-fold cross validation where levels of S100B remained as an independent factor associated with preeclampsia (data not shown).
Figure 2.
Receiver operator characteristics (ROC) curve. The ROC curve shows optimal cutoff level of S100B (µg/L) to discriminate between women with preeclampsia and women with normal pregnancies. The area under curve (AUC) is 0.706.
Table 2.
Logistic regression analysis with odds ratios (ORs) and 95% confidence intervals (CIs) for likelihood of preeclampsia based on background factors that differed significantly in Table 1
| Background factors that differed significantly in Table 1 | Adjusted ORa | (95% CI) | |
|---|---|---|---|
| Parity | Parous | NS | |
| Nullipara | |||
| BMI, first antenatal visit | <25 | 1.00 | (referent) |
| ≥25 | 5.32 | (2.11–13.38) | |
| Plasma levels of S100B | <0.14 µg/L | 1.00 | (referent) |
| ≥0.14 µg/L | 5.56 | (1.91–16.19) |
Abbreviations: BMI, body mass index; NS, not significant.
aAdjusted for parity, BMI, and levels of S100B.
Symptoms related to possible CNS effects and levels of S100B
Based on the knowledge that S100B might reflect degree of possible CNS effects, a multivariable regression analysis was performed where clinical markers of neurological dysfunction were compared with high levels of S100B in women with preeclampsia. The most common symptom of suspected CNS effects was headache, but women with high levels of S100B did not have headache more often (OR = 0.63; 95% CI = 0.20–2.01) (Table 3). Six women had symptoms of visual disturbance, mostly blurred vision, and women with high levels of S100B tended to have visual disturbances more often (OR = 8.58; 95% CI = 0.90–81.72) (Table 3). A Pearson phi coefficient correlation test was also performed, and a significant correlation between visual disturbances and high levels of S100B was found (P < 0.05; data not shown)
Table 3.
Logistic regression analysis with odds ratios (ORs) and 95% confidence intervals (CIs) for likelihood of possible central nervous system effects in relation to levels of S100B among case patients with preeclampsia (n = 53)
| Possible CNS effects | Adjusted ORa | (95% CI) | |
|---|---|---|---|
| Headache | No | 1.00 | (referent) |
| Yes | 0.63 | (0.20–2.01) | |
| Visual disturbances | No | 1.00 | (referent) |
| Yes | 8.58 | (0.90–81.72) |
Abbreviation: CNS, central nervous system.
aAdjusted for headache and visual disturbances.
DISCUSSION
Based on the knowledge that women with preeclampsia have been shown to have cerebral hemodynamic changes2 and that women who have had preeclampsia or eclampsia have an increased risk for persistent cognitive impairment for months or even years after the pregnancy,2 it was of interest to investigate whether it is possible to measure a potential CNS affection during pregnancy. Because S100B is supposed to be a peripheral biomarker of CNS injury with increased blood–brain barrier permeability, we investigated the association between plasma levels of S100B and preeclampsia as well as S100B’s association to CNS affection.
We showed that plasma levels of S100B are elevated among women with preeclampsia compared with control subjecs, which is in agreement with previous results.14,16 The results from our study indicated, furthermore, that levels of S100B increase irrespective of blood pressure level and that increased levels of S100B during pregnancy had a tendency to associate with visual disturbances, which might reflect possible CNS effects.
Preeclampsia and eclampsia seem to influence the CNS,2,5 but little is known about the actual importance of this, both in the short and long term. According to previous results from others, and us, serum levels of S100B are elevated in women with preeclampsia.14,16 In a study by Vettorazzi et al.,16 a correlation between severe preeclampsia and high levels of S100B was shown, although there was no significant elevation in S100B levels among women with preeclampsia who developed eclampsia, compared with women with preeclampsia only. However, S100B was analyzed in the blood sample collected at time of diagnosis, and the time elapsed to occurrence of seizures was not specified. It would have been of interest to investigate changes in S100B levels among these women in the period between time of diagnosis and occurrence of seizures. Vettorazzi et al.,16 furthermore, did not identify a correlation between levels of S100B and CNS-related symptoms. In their study, relatively few women with severe preeclampsia were included, and the absence of a correlation with CNS-related symptoms might have been because of loss of statistical power. Also, the symptoms were not subdivided into specific neurologic symptoms, which could have diluted their results.
In patients with preeclampsia or eclampsia who have neurological symptoms, areas with cortical and subcortical vasogenic edema are often observed on computed tomography17 and magnetic resonance imagingstudies.18 The most commonly affected regions are in the parietal and occipital lobes, supplied by the posterior cerebral artery. The combined syndrome of neurological symptoms and imaging findings has been described as the posterior reversible leucoencephalopathy syndrome, which is not exclusively observed in preeclampsia or eclampsia. Other etiologies include, but are not restricted to, hypertension from other causes,18 immunosuppressive19 or cytotoxic drugs,20 and autoimmune conditions.21 The predilection in imaging studies for pathological changes to the parieto-occiptal region, including the visual cortex, corresponds well to the fact that visual disturbances are one of the most common symptoms in posterior reversible leucoencephalopathy syndrome.22–25 Therefore, visual disturbances might have a high specificity as a symptom reflecting possible CNS effects of preeclampsia, and our finding of visual disturbance as more common in women with high S100B levels indicates S100B as a peripheral biomarker of possible CNS effects.
It is known that women with preeclampsia have cerebral hemodynamic changes, even predating the preeclamptic syndrome2 and also that preeclampsia and eclampsia are associated with a blood–brain barrier disruption6 that makes it possible for the high cerebral S100B content to leak over to the maternal circulation. This supports the theory that it is S100B produced by the glial cells, predominantly in the astrocytes, of the CNS that is the source of increased S100B levels in women with preeclampsia. Another hypothesis about the reason for increased levels of S100B in preeclampsia is that S100B originates from extracerebral sources affected by the disease. This issue has been discussed in the literature in relation to other diseases7,26 but there is evidence that S100B is mainly expressed in astrocytes and that its low expression in other tissues is of less importance clinically when evaluating brain damage.10 Pham and collegues27 have, in a recent publication, shown that changes in serum levels of S100B primarily reflect extravasation across a disrupted blood–brain barrier.27 They show furthermore that the main molecular species of total S100B related to blood–brain barrier disruption is the S100B homodimer. Levels of S100B in that study were evaluated with the same kind of ELISA kit as in our study. There have also been studies investigating the levels of S100B in cord blood, amniotic fluid, and urine samples from newborns in relation to fetal well-being.28 It would have been of interest in our study to put the S100B levels in maternal blood in relation to these parameters, but this material was not available. In future prospective studies, this can be taken into consideration to further correct for confounders.
In some of the women in our study, blood samples were also collected postpartum. When women within the preeclampsia group were compared, there was a significant increase in S100B among women with samples collected postpartum compared with before delivery. For women with normal pregnancies, there was only 1 instance where the blood sample collection had been performed postpartum and because of that it was not possible to perform a statistical analysis. The results suggest, accordingly, that S100B continues to rise postpartum, at least in women with preeclampsia, which is in line with the fact that preeclampsia and eclampsia also occur postpartum.3,29 One could speculate about the importance of also evaluating women with preeclampsia postpartum to estimate the risk of future cerebral complications. Because one-third of all eclampsia cases occur postpartum, we believe that this would be of great interest.3,30
There are also other potential markers for cell damage in CNS in relation to preeclampsia. Among the factors that would be of interest to analyze are neuron-specific enolase and glial fibrillary acidic protein, which both seem to be of major importance when evaluating brain damage caused by traumatic events.8,31,32 Perhaps measurement of S100B in combination with 1 of these, or some other factor, could be helpful in clinical decisions about whether and when to deliver.
One limitation with our study is the relatively low number of patients included. In spite of this, we achieved significant results in many of our outcomes, indicating a strong association. Concerning the association between visual disturbances and higher levels of S100B, a Pearson phi coefficient showed a significant correlation, whereas a logistic regression analysis resulted in a P value of 0.06; this is probably because of the size of the study population, and the outcome needs to be confirmed in a larger study. Another limitation is the retrospective design of the study; the information about CNS-related symptoms was collected from the medical records instead of asking the women individually for symptoms at the time of sampling according to a predefined protocol. The major strengths of this study, on the other hand, are that the study population is well characterized in aspects of preeclampsia diagnosis and it is also, to our knowledge, the first study investigating levels of S100B in relation to specific symptoms that correspond to possible CNS effects. CNS-related symptoms were collected from the medical records without knowledge of S100B levels, minimizing information bias. The ELISA analysis of levels of S100B is, furthermore, a well-defined and often-used method with highly accurate results.
That S100B might reflect possible CNS effects in relation to preeclampsia needs to be confirmed in larger studies, but one can speculate about its importance as a clinical marker of relevance to take into consideration when decisions about treatment and/or delivery are needed during pregnancy. To be able to estimate the degree of CNS effects in the individual woman would be of great relevance and help for the obstetrician.
Our findings indicate that plasma levels of S100B are elevated among women with preeclampsia compared with control subjects. The results indicate, furthermore, that levels of S100B increase and that women with high levels of S100B during pregnancy more often have visual disturbances, which might reflect possible CNS effects in women with preeclampsia.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants provided by the Swedish Society of Medicine; the Uppsala-Örebro Regional Research Council, Sweden; and the Centre for Clinical Research, Dalarna, Sweden. We would like to thank Malin Olsson, Department of Women’s and Children’s Health, Uppsala University, for valuable technical assistance.
REFERENCES
- 1. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010; 376:631–644. [DOI] [PubMed] [Google Scholar]
- 2. Zeeman GG. Neurologic complications of pre-eclampsia. Semin Perinatol 2009; 33:166–172. [DOI] [PubMed] [Google Scholar]
- 3. Kullberg G, Lindeberg S, Hanson U. Eclampsia in Sweden. Hypertens Pregnancy 2002; 21:13–21. [DOI] [PubMed] [Google Scholar]
- 4. Heilmann L, Rath W, Pollow K. Hemostatic abnormalities in patients with severe preeclampsia. Clin Appl Thromb Hemost 2007; 13:285–91. [DOI] [PubMed] [Google Scholar]
- 5. Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension 2007; 50:14–24. [DOI] [PubMed] [Google Scholar]
- 6. Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension 2010; 56:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med 2013; 13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 8. Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JL. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 2010; 75:1786–1793. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez-Rodriguez A, Egea-Guerrero JJ, Leon-Justel A, Gordillo-Escobar E, Revuelto-Rey J, Vilches-Arenas A, Vico AC, Dominguez-Roldan JM, Murillo- Cabezas F, Montavez JM. Role of S100B protein in urine and serum as an early predictor of mortality after severe traumatic brain injury in adults. Clin Chim Acta 2012; 414:228–233. [DOI] [PubMed] [Google Scholar]
- 10. Gradisek P, Osredkar J, Korsic M, Kremzar B. Multiple indicators model of long-term mortality in traumatic brain injury. Brain Injury 2012; 26:1472–1481. [DOI] [PubMed] [Google Scholar]
- 11. Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB. Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J Neuroinflammation 2010; 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech 2003; 60:614–632. [DOI] [PubMed] [Google Scholar]
- 13. Machado-Vieira R, Lara DR, Portela LV, Goncalves CA, Soares JC, Kapczinski F, Souza DO. Elevated serum S100B protein in drug-free bipolar patients during first manic episode: a pilot study. Eur Neuropsychopharmacol 2002; 12:269–272. [DOI] [PubMed] [Google Scholar]
- 14. Wikström AK, Ekegren L, Karlsson M, Wikström J, Bergenheim M, Ǻkerud H. Plasma levels of S100B during pregnancy in women developing pre-eclampsia. Pregnancy Hypertens 2012; 2:398–402. [DOI] [PubMed] [Google Scholar]
- 15. Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996; 85:843–848. [DOI] [PubMed] [Google Scholar]
- 16. Vettorazzi J, Torres FV, De Ávila TT, Martins-Costa SH, Souza DO, Portela LV, Ramos JG. Serum S100B in pregnancy complicated by preeclampsia: a case–control study. Pregnancy Hypertens 2012; 2:101–105. [DOI] [PubMed] [Google Scholar]
- 17. Colosimo C, Jr, Fileni A, Moschini M, Guerrini P. CT findings in eclampsia. Neuroradiology 1985; 27:313–317. [DOI] [PubMed] [Google Scholar]
- 18. Weingarten K, Barbut D, Filippi C, Zimmerman RD. Acute hypertensive encephalopathy: findings on spin-echo and gradient-echo MR imaging. Am J Roentgenol 1994; 162:665–670. [DOI] [PubMed] [Google Scholar]
- 19. Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW. Posterior reversible encephalopathy syndrome after solid organ transplantation. Am J Neuroradiol 2008; 29:924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanchez-Carpintero R, Narbona J, Lopez de Mesa R, Arbizu J, Sierrasesumaga L. Transient posterior encephalopathy induced by chemotherapy in children. Ped Neurol 2001; 24:145–148. [DOI] [PubMed] [Google Scholar]
- 21. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome—an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol 2006; 33:2178–2183. [PubMed] [Google Scholar]
- 22. Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Practical Neurol 2011; 11:136–144. [DOI] [PubMed] [Google Scholar]
- 23. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol 2008; 29:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hugonnet E, Da Ines D, Boby H, Claise B, Petitcolin V, Lannareix V, Garcier JM. Posterior reversible encephalopathy syndrome (PRES): features on CT and MR imaging. Diagn Interv Imaging 2013; 94:45–52. [DOI] [PubMed] [Google Scholar]
- 25. Roos NM, Wiegman MJ, Jansonius NM, Zeeman GG. Visual disturbances in (pre)eclampsia. Obstet Gynecol Surv 2012; 67:242–250. [DOI] [PubMed] [Google Scholar]
- 26. Missler U, Orlowski N, Notzold A, Dibbelt L, Steinmeier E, Wiesmann M. Early elevation of S-100B protein in blood after cardiac surgery is not a predictor of ischemic cerebral injury. Clin Chim Acta 2002; 321:29–33. [DOI] [PubMed] [Google Scholar]
- 27. Pham N, Fazio V, Cucullo L, Teng Q, Biberthaler P, Bazarian JJ, Janigro D. Extracranial sources of S100B do not affect serum levels. PLos One 2010; 5:e12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sannia A, Risso FM, Zimmermann LJ, Gavilanes AW, Vles HJ, Gazzolo D. S100B urine concentrations in late preterm infants are gestational age and gender dependent. Clin Chim Acta 2013; 417:31–34. [DOI] [PubMed] [Google Scholar]
- 29. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012; 206:470–475. [DOI] [PubMed] [Google Scholar]
- 30. Knight M. Eclampsia in the United Kingdom 2005. BJOG 2007; 114:1072–1078. [DOI] [PubMed] [Google Scholar]
- 31. Streitburger DP, Arelin K, Kratzsch J, Thiery J, Steiner J, Villringer A, Mueller K, Schroeter ML. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain - a combined serum, gene expression and MRI study. PLoS One 2012; 7:e43284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stein DM, Lindell AL, Murdock KR, Kufera JA, Menaker J, Bochicchio GV, Aarabi B, Scalea TM. Use of serum biomarkers to predict cerebral hypoxia after severe traumatic brain injury. J Neurotrauma 2012; 29:1140–1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.