Key Points
CBFβ is not required for the emergence of nascent HSCs but is essential for a subsequent step before their release from the AGM.
RUNX1 is able to drive the emergence of nascent HSCs in the AGM in the absence of its cofactor CBFβ.
Abstract
CBFβ and RUNX1 form a DNA-binding heterodimer and are both required for hematopoietic stem cell (HSC) generation in mice. However, the exact role of CBFβ in the production of HSCs remains unclear. Here, we generated and characterized 2 zebrafish cbfb null mutants. The cbfb−/− embryos underwent primitive hematopoiesis and developed transient erythromyeloid progenitors, but they lacked definitive hematopoiesis. Unlike runx1 mutants, in which HSCs are not formed, nascent, runx1+/c-myb+ HSCs were formed in cbfb−/− embryos. However, the nascent HSCs were not released from the aorta-gonad-mesonephros (AGM) region, as evidenced by the accumulation of runx1+ cells in the AGM that could not enter circulation. Moreover, wild-type embryos treated with an inhibitor of RUNX1-CBFβ interaction, Ro5-3335, phenocopied the hematopoietic defects in cbfb−/− mutants, rather than those in runx1−/− mutants. Finally, we found that cbfb was downstream of the Notch pathway during HSC development. Our data suggest that runx1 and cbfb are required at 2 different steps during early HSC development. CBFβ is not required for nascent HSC emergence but is required for the release of HSCs from AGM into circulation. Our results also indicate that RUNX1 can drive the emergence of nascent HSCs in the AGM without its heterodimeric partner CBFβ.
Introduction
Hematopoietic development is evolutionarily conserved among vertebrates. Similar to mammals, zebrafish embryos undertake sequential waves of hematopoiesis at distinct locations during embryonic development. The first wave is primitive hematopoiesis, in which erythroid progenitors arise from the posterior lateral mesoderm and form at later stages the intermediate cell mass, where erythroblasts are produced.1 In parallel, primitive myeloid progenitors originate from the anterior lateral mesoderm and later differentiate into macrophages.2 The second wave is definitive hematopoiesis with the generation of hematopoietic stem cells (HSCs), which can differentiate to all definitive blood lineages.3,4 Starting at 30 hours postfertilization (hpf), HSCs emerge from the hemogenic endothelium of the ventral wall of the dorsal aorta (DA) in the zebrafish equivalent of the aorta-gonad-mesonephros (AGM) region. High-resolution imaging revealed a stereotyped cell behavior during which endothelial cells from the ventral DA bend into the subaortic space and transdifferentiate into HSCs.5,6 This dynamic process has been termed endothelial hematopoietic transition (EHT).6 The HSCs in the subaortic mesenchyme enter the circulation through the axial vein and colonize the caudal hematopoietic tissue (CHT), which is considered functionally analogous to the mammalian fetal liver.7,8 HSCs in the CHT give rise to erythroid and myeloid progenitors5,6,8 and then migrate toward the definitive hematopoietic organs in adult fish, thymus, and kidney.8 Similar to the mouse, a transient population of erythromyeloid progenitors (EMPs) originates within the posterior blood island and sustains the initiation of the definitive hematopoietic wave in zebrafish.9
Core binding factor (CBF) is a heterodimeric DNA-binding complex that consists of a DNA-binding α-subunit (encoded in mammals by RUNX1, RUNX2, or RUNX3) and a non-DNA-binding β-subunit, encoded by CBFB.10,11 RUNX1 and CBFβ, encoded by RUNX1 and CBFB, respectively, are both required for the development of definitive hematopoiesis. Mice with targeted disruption of either Runx1 or Cbfb show essentially identical phenotypes with complete lack of definitive hematopoiesis and lethality between embryonic days 11.5 and 13.5.12-15 The observations suggest that RUNX1 and CBFβ function together in vivo, which is consistent with biochemical studies that RUNX1 and CBFβ form a heterodimer for binding DNA and regulating the expression of downstream target genes.16,17 The absence of all definitive hematopoietic lineages in both Runx1−/− and Cbfb−/− embryos also suggests that both genes are required at the stage of HSC specification. Subsequent studies demonstrated that Runx1 is required for the emergence of HSCs from the hemogenic endothelium within the AGM region in the mouse.18,19
Our group previously generated a zebrafish runx1 mutant with a nonsense mutation (W84X) within the RUNT domain, resulting in a prematurely truncated RUNX1 protein. Homozygous runx1W84X/W84X mutants lack expression of the HSC marker c-myb and do not develop definitive blood lineages in the CHT and thymus.20,21 Further studies from other groups demonstrated that, in zebrafish, runx1 is also required for the emergence of HSCs from the hemogenic endothelium in the AGM.6
On the other hand, relatively little is known about the exact role of Cbfb during the early stages of HSC development in the mouse,22 although it is assumed that Cbfb plays a similar role as Runx1 does. Even though a highly conserved cbfb gene (the encoded protein is 87% identical to the mammalian CBFβ proteins) has been identified in the zebrafish,23 the role of the zebrafish cbfb during HSC production in definitive hematopoiesis remains to be investigated.
In this study, we generated and characterized 2 independent zebrafish cbfb knockout mutant lines (cbfb−/−), which revealed a previously unknown role of cbfb during definitive hematopoiesis, and showed that the function of RUNX1 and CBFβ during HSC development could be uncoupled.
Methods
Zebrafish lines and maintenance
Zebrafish were maintained and used following approved National Human Genome Research Institute Animal Care and Use Committee protocols. Zebrafish handling and breedings were performed as described previously.24 The following strains were used: wild-type (WT) EK (Ekkwill), runx1W84X,21 tg(c-myb:eGFP),25 tg(cd41:GFP),26 and tg(flk1:moesin1-eGFP).27 The mind bombta52b mutant line,28 the transgenic line tg(uas:NICD), and tg(hsp70:gal4)29 were kindly provided by Ajay Chitnis.
Generation of cbfb mutants and genotyping
Sixteen pairs of CompoZr zinc-finger nucleases (ZFNs) targeting the first half of the open reading frame of the cbfb gene were designed and evaluated for in vitro activity by Sigma-Aldrich (St. Louis, MO), and messenger RNA (mRNA) from the pair with the highest in vitro activity was chosen for subsequent targeted mutagenesis. Injection of mRNA, founder screening, and identification of cbfb heterozygous adult fish has been described in detail previously.30 For each experiment, cbfb-Del4 and cbfb-Ins4 were genotyped by fluorescent polymerase chain reaction (PCR) using a mixture of M13F-tailed (5′-TGTAAAACGACGGCCAGT-3′) cbfb-specific forward primer (5′-ATGCTCGGGCCTGGCTTTCT-3′), 6-FAM–labeled M13F primer, and PIG-tailed (5′-GTGTCTT-3′) cbfb-specific reverse primer (5′-AGGGGCGTGAGTTAGAGT-3′) in the PCR mix.30 Genotyping of fixed samples has been performed as above but using a different PCR mix (Sigma RED Extract-N-Amp PCR Ready Mix R4775 REDExtract-N-Amp PCR Ready Mix, R4775; Sigma-Aldrich).
WISH, o-dianisidine staining, and imaging
Whole-mount in situ hybridization (WISH) was carried out essentially as described by Thisse and Thisse.31 The cbfb antisense mRNA probe has been generated as described by Blake et al.23 The following DIG-labeled antisense mRNA probes were generated by using UTP-digoxigenin (Roche): cbfb, gata1, ae1-globin (hbae1), l-plastin, mpx, ikaros, rag1, runx1, and c-myb. Zebrafish embryos were stained in o-dianisidine staining solution for 15 minutes in the dark, as previously described.1 The embryos were observed with a Leica MZ16F stereomicroscope, and the pictures were taken with a Leica DC500 camera using Leica FireCam (version 1.7.1).
Time-lapse experiments and statistical analysis
Dechorionated embryos obtained from cbfb+/del4/tg(c-myb:eGFP) incrosses were anesthetized with tricaine, mounted in 0.8% low-melting agarose, and imaged from 48 to 63 hpf. Z stacks were collected every 5 minutes for 15 hours. The embryos were then recovered and genotyped. Tg(flk1:moesin1-eGFP) embryos were treated with dimethylsulfoxide (DMSO) (0.1%) or Ro5-3335 at 5 μM from 24 hpf and at 48 hpf were mounted for imaging as described above and covered with 4 mL of DMSO (0.1%) or Ro5-3335 at 5 μM. Treated embryos were imaged every 5 minutes for 10 hours. Details about the imaging systems used are available in the supplemental Methods on the Blood Web site.
Significance and standard deviation between samples were calculated using Excel (Microsoft).
Ro5-3335 treatment
Two zebrafish embryos at 22 hpf were placed into each well of 96-well plates (Costar #3635). These embryos were then incubated with Ro5-3335 at 5 μM, 2.5 μM, 0.5 μM, and 0.25 μM in E3 embryo medium (32-48 embryos per treatment in a final volume of 300 μL per well). As a control, embryos were treated with 0.1% DMSO in E3 embryo medium. Embryos were treated from 24 hpf to 36 hpf, or from 24 hpf to 3 days postfertilization (dpf), and then fixed with 4% paraformaldehyde and processed for in situ hybridization for c-myb or runx1 expression.
Heat-shock treatment of NICD transgenic embryos
The transgenic line tg(uas:NICD) was crossed to tg(hsp70:gal4). Embryos between 8- and 12-somite stages were collected in 50-mL Falcon tubes and were heat shocked for 30 minutes in a 37°C water bath. After the heat shock, the embryos were placed in Petri dishes, allowed to develop until 36 to 40 hpf, and then fixed in 4% paraformaldehyde. Fixed embryos were then processed for in situ hybridization for c-myb, runx1, or cbfb expression.
Results
Generation of zebrafish cbfb−/− mutants
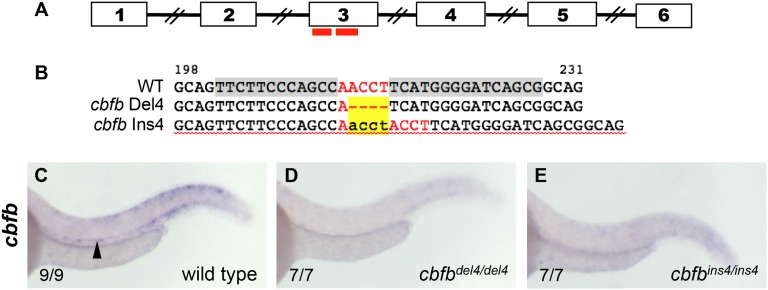
To determine the role of CBFβ in the formation of HSCs, we generated 2 independent cbfb mutant lines by ZFN-mediated targeted mutagenesis.32,33 The selected CompoZr ZFN pair targeted a specific region within cbfb exon 3 (Figure 1A).30 Among 9 mutations identified30 from 6 germline-transmitting founders, we selected 2 mutations predicted to cause frameshifts with premature terminations, cbfbhg10 (c.215delACCT, p.N72IfsX25) and cbfbhg11 (c.215insACCT, p.H74PfsX43), denoted here as cbfbdel4 and cbfbins4 (Figure 1B). In order to test whether the mutations lead to loss of cbfb expression, we evaluated the presence of cbfb transcripts in cbfbdel4/del4 and cbfbins4/ins4 mutants by WISH. At 36 hpf, cbfb expression in the ventral DA was detectable in WT embryos (Figure 1C), but not in cbfbdel4/del4 (Figure 1D) or cbfbins4/ins4 (Figure 1E) embryos, suggesting that the mutant mRNA was degraded in both cbfbdel4/del4 and cbfbins4/ins4 mutants.
Figure 1.
ZFN-mediated targeted mutagenesis of cbfb. (A) A schematic of genomic organization of cbfb with numbered boxes depicting exons, with connecting lines depicting introns and red bars marking the location of the ZFN pair used to generate knockout mutants. (B) Alignment of nucleotide sequences from nt198-231 of the cbfb open reading frame to show the ZFN target site (gray highlight in WT), spacer sequence (red letters), and exact sequences of the mutant alleles, del4 and ins4, which are characterized in this study. Yellow highlighted area marks the deleted or inserted nucleotides. (C) At 36 hpf, expression of cbfb in WT sibling was detectable in the ventral DA, where HSCs originate (black arrowhead). cbfb expression in the ventral DA was abrogated in both cbfbdel4/del4 (D) and cbfbins4/ins4 mutants (E).
Embryos heterozygous for the mutations in cbfb were indistinguishable from their WT clutchmates and presented normal hematopoiesis (data not shown). cbfbdel4/del4 and cbfbins4/ins4 embryos did not show any obvious morphologic or developmental defects and were indistinguishable in appearance from their WT or heterozygous clutchmates. cbfbdel4/del4 and cbfbins4/ins4 embryos presented identical hematopoietic phenotypes and died around 14 dpf; therefore, they are frequently referred collectively as cbfb−/− mutants in this report.
Loss of cbfb does not affect primitive hematopoiesis and EMP formation
In a previous report, we showed that in WT embryos cbfb expression is detectable in the posterior lateral mesoderm, where primitive hematopoietic progenitors are formed, and in the intermediate cell mass, where primitive erythroid cells arise.23 To determine if cbfb−/− mutants had any primitive hematopoietic defects, we tested the expression of several hematopoietic markers in cbfb−/− mutants and WT siblings by WISH. The expression of the erythroid marker gata1 appeared unaltered in cbfbdel4/del4 and cbfbins4/ins4 mutants at 16 somites and 24 hpf (supplemental Figure 1A-F), suggesting that the development of primitive erythroid cells was unaffected in cbfb−/− mutants. Primitive erythroblasts differentiated into erythrocytes in cbfbdel4/del4 and cbfbins4/ins4 embryos as whole-embryo o-dianisidine staining appeared normal at 48 hpf (supplemental Figure 1J-L), and the expression of the hemoglobin gene ae1-globin (hbae1) was unaffected (supplemental Figure 1M-O). The expression of the myeloid marker l-plastin was also maintained in both cbfb null mutants at 24 hpf (supplemental Figure 1G-I), suggesting that primitive myeloid cells were unaffected. In addition, cbfb null embryos had normal expression of gata1, l-plastin, and mpx in the posterior blood island at 36 hpf, indicating that the EMP progenitors were correctly specified (supplemental Figure 2A-I).
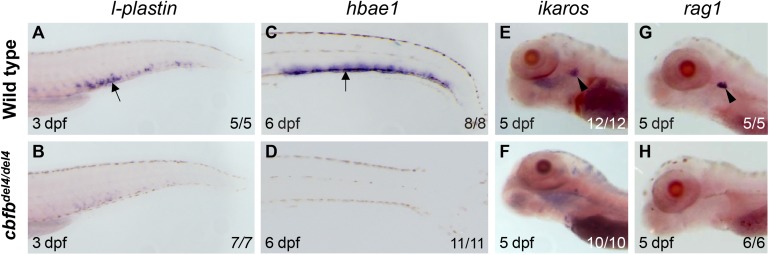
The zebrafish cbfb−/− embryos lack definitive hematopoiesis
It is known that loss of either Runx1 or Cbfb abolishes the onset and the development of definitive hematopoietic cells in mouse embryos.12,13 Because the zebrafish runx1W84X/W84X mutant embryos also lack definitive hematopoiesis,21 we evaluated the presence of definitive blood lineages in the cbfb−/− mutants (Figure 2 and supplemental Figure 3). At 3 dpf, the expression of l-plastin in definitive myeloid progenitors within the CHT was almost undetectable in cbfbdel4/del4 (Figure 2B) and cbfbins4/ins4 (supplemental Figure 3B) mutants when compared with WT siblings (Figure 2A and supplemental Figure 3A). The expression of hbae1 in erythroid precursors within the CHT was also abrogated in cbfbdel4/del4 (Figure 2C-D) and cbfbins4/ins4 (supplemental Figure 3C-D) embryos at 6 dpf. The lymphoid markers ikaros and rag1 in the developing thymus were absent in cbfbdel4/del4 (Figure 2E-H) and cbfbins4/ins4 (supplemental Figure 3E-H) mutants at 5 dpf as well. Moreover, circulating thrombocytes were almost undetectable in cbfbdel4/del4/tg(cd41:GFP) embryos, in which the expression of green fluorescent protein is driven by the promoter of a thrombocyte-specific gene, cd4126 (supplemental Movies 1 and 2). Therefore, our results are consistent with a complete failure of definitive blood lineages in the cbfb−/− zebrafish mutants.
Figure 2.
Definitive blood lineages are absent in cbfbdel4/del4 embryos. Expression of markers for definitive hematopoietic lineages in WT control siblings (A,C,E,G) and cbfbdel4/del4 embryos (B,D,F,H) by WISH. At 3 dpf, the myeloid marker l-plastin was expressed in the CHT of WT embryos (A, black arrow), but not in cbfbdel4/del4 embryos (B). hbae1 expression in the erythroid progenitors within the CHT (C, black arrow) was completely abrogated in cbfbdel4/del4 embryos (D) at 6 dpf. Expression of the T-lymphocyte markers ikaros and rag1 within the thymus (E,G black arrowheads) was also abrogated in cbfbdel4/del4 embryos (F,H) at 5 dpf.
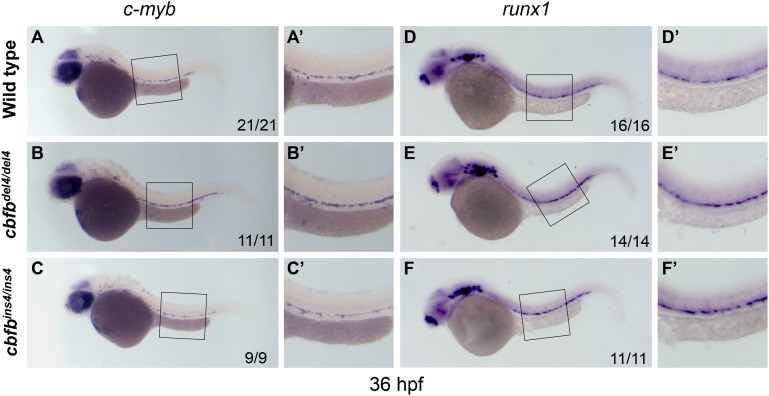
The emergence of nascent HSCs is unaffected in cbfb−/− embryos
To investigate whether loss of cbfb affected the onset of definitive hematopoiesis, we analyzed HSC development by testing the expression of runx1 and c-myb by WISH. Nascent runx1+/c-myb+ HSCs emerge from the hemogenic endothelium in the ventral wall of the DA around 30 hpf.34,35 In runx1W84X/W84X mutants, c-myb+ HSCs were absent.21 However, at 36 hpf, c-myb expression was observed along the ventral wall of the DA in both cbfbdel4/del4 (Figure 3B-B′) and cbfbins4/ins4 (Figure 3C-C′) embryos at similar levels to their WT clutchmates (Figure 3A-A′). Similarly, expression of the early HSC marker, runx1, was intact or even slightly increased in the ventral DA region of the cbfbdel4/del4 (Figure 3E-E′) and cbfbins4/ins4 (Figure 3F-F′) embryos at 36 hpf, as compared with the controls (Figure 3D-D′). Given the normal expression of c-myb and the strong expression of runx1 in cbfb−/− mutants at 36 hpf, we evaluated the presence of a compensatory mechanism involving other runx family members. However, in cbfb−/− embryos between 36 hpf and 3dpf, the expression pattern and level of runx2a, runx2b, and runx3 were normal (in the pharyngeal arches and cartilage) and no ectopic expression was detectable, especially in the hematopoietic tissues (data not shown).
Figure 3.
HSCs emerge from hemogenic endothelium in cbfb−/− embryos. Expression of the HSC markers c-myb and runx1 in cbfb−/− and WT embryos at 36 hpf by WISH. Compared with WT siblings (A-A′), the HSC marker c-myb was normally expressed in the hemogenic endothelium of the ventral DA of cbfbdel4/del4 (B-B′) and cbfbins4/ins4 (C-C′) embryos at 36 hpf. Similarly, the expression of runx1, another HSC marker, was also unaffected in cbfbdel4/del4 (E-E′) and cbfbins4/ins4 (F-F′) embryos, as compared with WT embryos (D-D′). Panels A′-F′ depict the boxed regions in panels A-F.
Overall, the presence of runx1+ and c-myb+ cells within the hemogenic endothelium of cbfbdel4/del4 and cbfbins4/ins4 mutants indicates that the emergence of nascent HSCs does occur in cbfb−/− embryos.
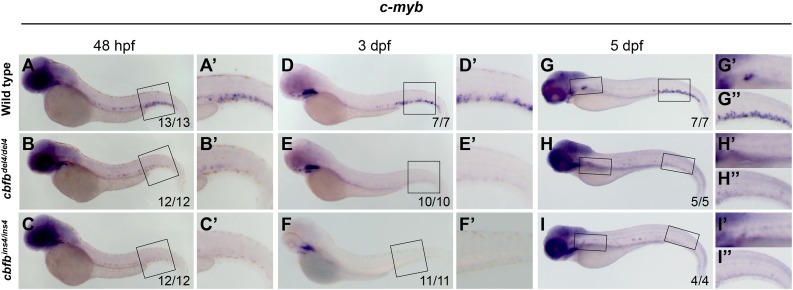
HSCs do not reach the CHT and kidney in the cbfb−/− embryos
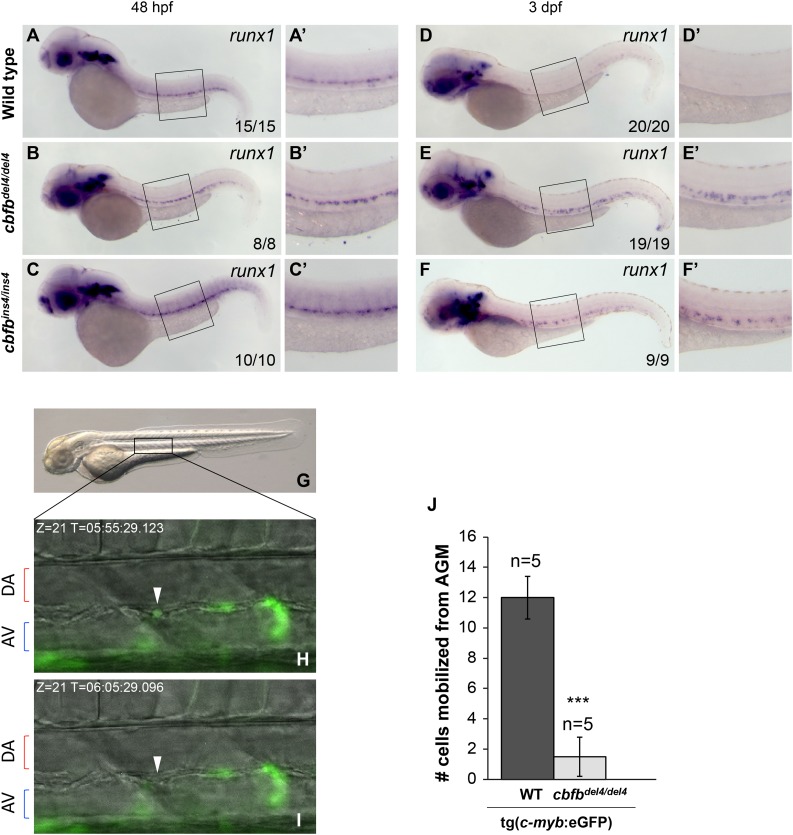
Starting from 30 hpf, HSCs asynchronously egress from the ventral DA into the subaortic space and intravasate into the axial vein to seed the CHT.7 Consistent with the translocation of HSCs, at 2 dpf, c-myb expression is observed in both AGM and CHT regions of WT embryos (Figure 4A-A′). However, c-myb expression in cbfb−/− mutants appeared strongly reduced and was detectable only in the AGM (Figure 4B-C′). At 3 dpf, when c-myb+ cells were found only in the CHT of WT embryos (Figure 4D-D′), no c-myb expression was detected in any hematopoietic region of cbfbdel4/del4 (Figure 4E-E′) and cbfbins4/ins4 (Figure 4F-F′) mutants. At 5 dpf, c-myb expression in CHT and kidney was detectable in WT (Figure 4G-G′′), but not in cbfb−/− (Figure 4H-4I′′), embryos.
Figure 4.
HSCs do not translocate from the AGM to the CHT and kidney in cbfb−/− embryos. Expression of the HSC marker c-myb in the CHT and kidney between 48 hpf and 5 dpf by WISH. At 48 hpf, c-myb+ HSCs had started to populate the CHT in WT embryos (A-A′), whereas they did not in cbfbdel4/del4 (B-B′) or cbfbins4/ins4 (C-C′) embryos. At 3 dpf, c-myb+ hematopoietic cells could readily be detected in the CHT in WT embryos (D-D′), whereas no c-myb expression was detectable in the CHT in cbfbdel4/del4 (E-E′) or cbfbins4/ins4 (F-F′) embryos. At 5 dpf, c-myb expression in the CHT and kidney was detectable in WT (G-G′′), but not in cbfbdel4/del4 (H-H′′) or cbfbins4/ins4 (I-I′′), embryos. Panels A′-F′ depict the boxed regions in panels A-F. Panels G′, H′, and I′ depict the regions in the left boxes in panels G-I. Panels G′′- I′′ depict the regions in the right boxes in panels G-I.
Apoptosis was not likely the reason for the reduction in c-myb expression, because terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining was not increased in cbfb−/− embryos between 36 and 48 hpf (supplemental Figure 4A). Moreover, the expression of the proliferating cell nuclear antigen (PCNA)36 in hematopoietic progenitors within the AGM of cbfb−/− mutants between 36 and 48 hpf appeared comparable to WT controls (supplemental Figure 4B). Similarly, anti-phosphohistone H3 staining of cbfbdel4/del4/tg(c-myb:eGFP) embryos and WT siblings at 36 and 48 hpf did not show differences in proliferation of the hematopoietic progenitors (eGFP+ cells) within the AGM (supplemental Figure 4C-F).
On the other hand, the expression of the early HSC marker, runx1, was maintained in the AGM of cbfb−/− mutants (Figure 5B-C′) at 48 hpf as compared with the WT embryos (Figure 5A-A′). In WT embryos at 3 dpf, HSCs were located in the CHT and runx1 expression was no longer detectable in the AGM (Figure 5D-D′). At the same stage of development, however, strong runx1 expression was still detectable in the AGM region in cbfbdel4/del4 and cbfbins4/ins4 mutants (Figure 5E-F′). Because cbfbdel4/del4 and cbfbins4/ins4 mutants presented normal blood circulation (supplemental Movies 3-5), these findings suggest that HSCs could not leave the AGM in cbfb−/− mutant embryos.
Figure 5.
HSCs are not released from the AGM in cbfb−/− embryos. (A-F′) Expression of the HSC marker runx1 in embryos at 48 hpf and 3 dpf, detected by WISH. At 48 hpf, the expression of runx1 was maintained in the AGM of cbfbdel4/del4 (B-B′) and cbfbins4/ins4 (C-C ′) embryos than in the WT embryos (A-A′). runx1 expression in hematopoietic regions, including both the AGM and the CHT regions, was downregulated in WT embryos at 3 dpf (D-D′). In cbfbdel4/del4 and cbfbins4/ins4 embryos, however, runx1 remained strongly expressed in the AGM (E-F′). Panels A′-F′ depict boxed regions in panels A-F. (G-J) Time-lapse imaging analysis of WT tg(c-myb:eGFP) and cbfbdel4/del4/tg(c-myb:eGFP) embryos between 48 and 63 hpf (5-minute intervals for 15 hours) to record the numbers of eGFP+ HSCs released from the AGM. Panel G is a lateral view of a 2-dpf embryo, with the boxed area indicating the region that was imaged by time lapse. Panels H and I show merged video captures of fluorescence and bright-field images (Z = 21) of the same region at different time points, displaying the egression of an eGFP+ cell (white arrowhead) in a WT tg(c-myb:eGFP) embryo (present in H but disappeared in I). AV, axial vein. (J) Bar graphs depicting average numbers of eGFP+ HSCs leaving the AGM in 5 embryos of each genotype. On average, 12 eGFP+ cells per embryo left the AGM and entered the circulation through the axial vein in WT tg(c-myb:eGFP) embryos during the recording period. In cbfbdel4/del4/tg(c-myb:eGFP) embryos, an average of 1 cell per embryo was released into circulation during the same recording period. ***P < .001 vs WT.
HSCs are not released from the AGM in cbfb−/− embryos
In order to demonstrate directly the behavior of HSCs in live embryos, we incrossed cbfb+/del4/tg(c-myb:eGFP) mutants and counted the number of eGFP+ cells that were released from the AGM into the circulation by performing time-lapse imaging analysis of multiple cbfbdel4/del4/tg(c-myb:eGFP) and WT tg(c-myb:eGFP) siblings between 48 and 63 hpf (Figure 5G-I and supplemental Movies 6 and 7). We observed an average of 12 eGFP+ cells leaving the AGM and entering the circulation through the axial vein per tg(c-myb:eGFP) embryo (n = 5) during the recorded period (15 hours; Figure 5J). On the other hand, on average we observed only 1 eGFP+ cell leaving the AGM per cbfbdel4/del4/tg(c-myb:eGFP) embryo (n = 5) in the same recorded period (Figure 5J), which was significantly lower than the control (P < .001).
Thus, unlike runx1W84X/W84X mutants, where HSC formation was completely abrogated, nascent HSCs were formed in the cbfb−/− embryos, but they could not leave the AGM. Taken together, our results indicate that CBFβ is dispensable for the emergence of nascent HSCs but is necessary for their release from the AGM.
Pharmacologic inhibition of RUNX1-CBFβ interaction phenocopies the hematopoietic defects in cbfb−/− embryos
Recently, we identified a specific inhibitor of the RUNX1-CBFβ interaction, Ro5-3335.37 Zebrafish embryos treated with Ro5-3335 from 24 hpf to 6 dpf showed defects in the development of definitive hematopoiesis as demonstrated by the reduction of circulating thrombocytes in the transgenic line tg(cd41:GFP). Moreover tg(cd41:GFP) embryos carrying 1 allele of the runx1 truncation mutation (runx1+/W84X) are more sensitive to Ro5-3335 treatment (for developing hematopoietic defects) than WT transgenic embryos.37 Because Ro5-3335 induces defects in definitive hematopoiesis by blocking the RUNX1-CBFβ interaction, we reasoned that its inhibition would reproduce the early HSC phenotype observed in cbfb−/− mutants, but not the one in the runx1 mutants.
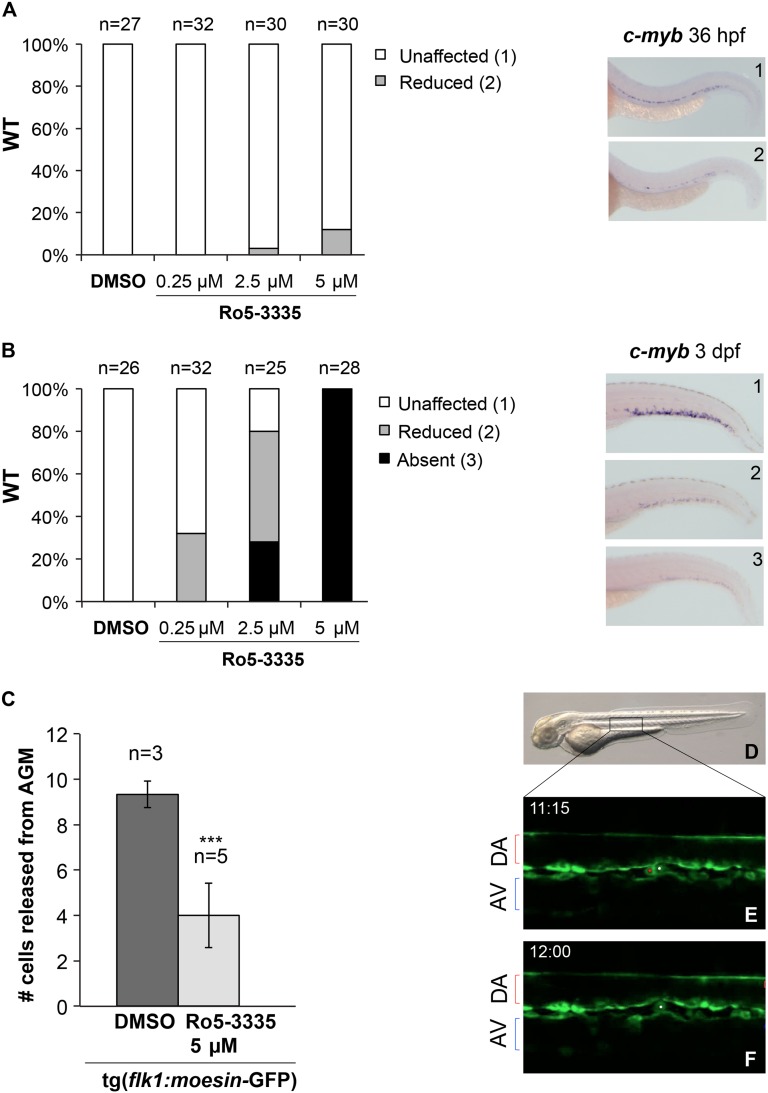
Therefore, we treated WT embryos with different concentrations of Ro5-3335 from 24 hpf to 36 hpf, or from 24 hpf to 3 dpf, and then evaluated the effect of Ro5-3335 treatment on HSC markers by WISH. WT embryos treated with Ro5-3335 at 5 μM, 2.5 μM, and 0.25 μM from 24 hpf to 36 hpf showed normal expression of c-myb and runx1 within the ventral DA (Figure 6A and supplemental Figure 5A-D). At a higher concentration (5 μM), we observed only a slight reduction in c-myb expression in 12% of the embryos when compared with their DMSO controls (Figure 6A). Neither cbfb+/del4 nor runx1+/W84X embryos showed more reduction in c-myb expression than WT embryos at 36 hpf after Ro5-3335 treatment (data not shown). On the other hand, WT embryos treated with Ro5-3335 at these same concentrations showed a dose-dependent reduction of c-myb expression in the CHT region at 3 dpf, similar to the phenotype in the cbfb−/− embryos (Figure 6B). In addition, cbfb+/del4 mutants were more sensitive to Ro5-3335 treatment than WT embryos for the absence of c-myb expression in CHT, as more cbfb+/del4 embryos developed the phenotype than WT embryos at a given concentration (compare Figure 6B with supplemental Figure 5E).
Figure 6.
Treatments with Ro5-3335 phenocopy cbfb−/− hematopoietic defects. Bar graphs showing the effect of Ro5-3335 treatment on c-myb expression in WT embryos from 24 hpf to 36 hpf (A) and from 24 hpf to 3 dpf (B). Percentages of embryos with unaffected (white bars), reduced (gray bars), and absence of (black bars) c-myb expression are depicted on the y-axis. Right panels show representative images of different categories of c-myb expression (1, unaffected; 2, reduced; 3, absent). (C-F) Confocal time-lapse imaging of the AGM region of tg(flk1:moesin1-eGFP) embryos between 48 and 58 hpf (5-minute intervals for 10 hours), which were treated with DMSO (0.1%) or Ro5-3335 (5 μM) from 24 hpf. Panel C shows bar graphs representing average numbers of eGFP+ HSCs leaving the AGM in DMSO-treated (n = 3) and Ro5-3335–treated (n = 5) embryos. ***P < .001 vs DMSO. Panel D shows a lateral view of a 2-dpf embryo, and the box indicates the region that was imaged by time lapse. Panels E and F show 2 representative video frames of fluorescence images (Z = 6-8) of the same AGM region of a DMSO-treated tg(flk1:moesin1-eGFP) embryo at 2 time points (45 minutes apart). The red and the white dots in panel E mark 2 eGFP+ cells within the AGM. The eGFP+ cell marked in red in panel E is no longer present in panel F, indicating that it had been released into the axial vein. AV, axial vein.
To confirm that Ro5-3335 fully recapitulated the cbfb−/− mutant hematopoietic phenotype, we used the transgenic line tg(flk1:moesin1-eGFP), which expresses the Moesin1-eGFP fusion protein from the promoter of flk1, a gene specifically expressed in endothelial cells. The tg(flk1:moesin1-eGFP) embryos were treated with DMSO or Ro5-3335 at 5 μM from 24 hpf, and their AGM regions were monitored between 48 and 58 hpf with time-lapse imaging (Figure 6D-F and supplemental Movies 8 and 9). The inhibition of RUNX1-CBFβ interaction by Ro5-3335 resulted in a significant impairment of HSC release from the AGM into the circulation in the recorded period (10 hours; P < .001; Figure 6C).
Taken together, our results showed that treatments with the RUNX1-CBFβ inhibitor Ro5-3335 phenocopied the phenotype observed in cbfb−/− mutants and confirmed that the function of RUNX1 and CBFβ during HSC development could be uncoupled.
cbfb acts downstream of the Notch pathway
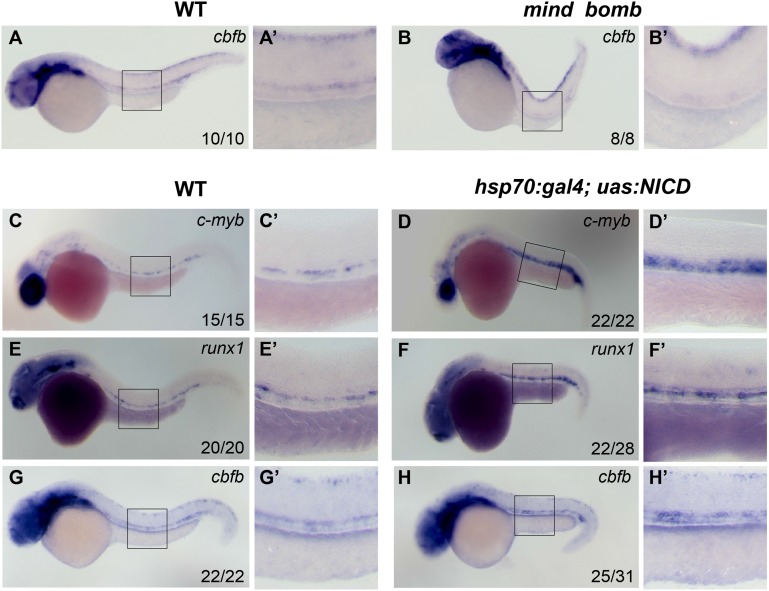
The Notch-Runx1 pathway is critical for the initial specification of HSCs during definitive hematopoiesis.34,38 Transient overexpression of an activated form of notch (NICD) in zebrafish embryos has been shown to induce ectopic expression of runx1 and expand definitive HSCs.34 Conversely, runx1 expression in HSCs is abrogated in the mind bomb mutant, where an E3 ubiquitin ligase essential for Notch signaling is mutated.34 Based on these observations, we evaluated whether cbfb was also controlled by Notch activity. We confirmed that 36-hpf mind bomb mutants lacked the expression of c-myb and runx1 in the artery (data not shown). Interestingly, we observed that cbfb expression within the hematopoietic progenitors in the ventral wall of the DA was also abolished in 36-hpf mind bomb mutants (Figure 7A-B′). We then examined the expression of c-myb, runx1, and cbfb by WISH in 36-hpf hsp70:gal4;uas:NICD embryos, which were heat shocked between 8 and 12 somites. We confirmed that the expression of c-myb and runx1 was expanded in the heat-shocked embryos (Figure 7C-F′). We observed that cbfb expression was also expanded in the aorta and ectopically expressed in the vein (Figure 7G-H′), similar to both c-myb and runx1 (Figure 7C-F′). These results suggest that cbfb expression is regulated by Notch activity.
Figure 7.
cbfb acts downstream of the Notch pathway. cbfb expression in the hematopoietic progenitors in the ventral DA was detectable in control siblings (A-A′), but not in the mind bomb mutant (B-B′), at 36 hpf. The expression of c-myb and runx1 was expanded in the DA and the axial vein of heat-shocked hsp70:gal4;uas:NICD embryos at 36 hpf (C-F′). Similarly, cbfb expression was expanded in the heat-shocked hsp70:gal4;uas:NICD embryos when compared with WT embryos (G-H′) at 36 hpf. Panels A′-H′ depict the boxed regions in panels A-H.
Discussion
The Cbfb gene has been demonstrated as a key regulator of definitive hematopoiesis during embryogenesis in mice.12,15 Cbfb−/− embryos lacked definitive hematopoiesis, whereas some EMPs remained.15 In a recent study, lineage specific expression of a Cbfb transgene in Cbfb knockout mice showed that EMPs and HSCs differentiate from distinct populations of hemogenic endothelial cells.22 However, there have been no reported studies on the exact roles of Cbfb for the emergence of HSCs from hemogenic endothelium.
In this study, we generated 2 independent zebrafish cbfb knockout mutants (cbfb−/−), which presented identical hematopoietic phenotypes. cbfb−/− embryos retained primitive hematopoiesis and EMPs but completely lacked all definitive blood lineages. Studies in both mouse and zebrafish clearly demonstrated that Runx1 is required for the EHT of the hemogenic endothelium into HSC during the early phases of definitive hematopoiesis.6,18,19 Therefore, because CBFβ is considered the obligate partner of RUNX1, the impairment of all definitive hematopoietic lineages in both Runx1−/− and Cbfb−/− mice suggested that the CBF heterodimer is required for HSC formation. Our present data, however, suggest that runx1 and cbfb are required at different steps during the early formation of HSCs. Indeed, the emergence of the nascent, runx1+/c-myb+ HSCs from the hemogenic endothelium along the ventral wall of the DA was unaffected in the cbfb−/− mutants. Further support for this finding comes from our data with pharmacologic inhibition of the RUNX1-CBFβ interaction in WT zebrafish embryos with a specific inhibitor, Ro5-3335.37 Similar to the cbfb−/− mutants, the emergence of nascent HSCs within the ventral DA was not affected by Ro5-3335 treatments, even at relatively high doses. Moreover, neither cbfb+/del4 nor runx1+/W84X embryos showed a reduction in c-myb expression within the DA after Ro5-3335 treatment. The presence of nascent runx1+/c-myb+ HSCs does not appear to be due to compensatory mechanism driven by other runx family members, as their expression in cbfb−/− mutants was normal. We can also exclude any contribution from maternal cbfb mRNA as cbfb expression is only zygotic.23 Overall, the emergence of nascent HSCs from the hemogenic endothelium in the absence of cbfb or a functional CBF complex indicates that CBFβ is not necessary for the EHT and strongly suggests that the function of RUNX1 and CBFβ during HSC development can be uncoupled. In the future, the temporal requirement of CBFβ during HSC development can be defined more precisely by treating the embryos with Ro5-3335 within different time windows.
Interestingly, we found that c-myb expression in the HSCs was progressively lost and no c-myb+ cell colonizes the CHT region of the cbfb−/− embryos at 3 dpf. Similarly, treatments of WT embryos with Ro5-3335 resulted in a dose-dependent reduction of c-myb expression in the CHT region. We confirmed that this phenotype resulted from the specific inhibition of the RUNX1-CBFβ interaction by showing that cbfb+/del4 mutants were more sensitive to Ro5-3335 treatment than WT embryos.
The loss of function of cbfb did not affect the expression of the early HSC marker runx1. Strikingly, runx1+ cells persisted in the AGM of cbfb homozygous embryos and never translocated to the CHT region. However, this phenotype did not appear to be related to any circulatory defect, as blood circulation and heart development in the cbfb−/− mutants were normal. Similar to the phenotype reported for the cmybhkz3 mutants,39 quantitative time-lapse observations of cbfbdel4/del4/tg(c-myb:eGFP) embryos demonstrated a strong impairment in the intravasation of c-myb:eGFP+ cells to the axial vein from the subaortic mesenchyme. The same phenotype was also recapitulated in tg(flk1:moesin1-eGFP) embryos treated with the RUNX1-CBFβ inhibitor Ro5-3335. Our study, therefore, demonstrates a novel function of cbfb in the release of HSCs from the AGM region during definitive hematopoiesis.
In order to gain insight into the genetic mechanisms that regulate cbfb expression, we investigated the Notch pathway, because the Notch-Runx1 pathway is critical for the initial specification of HSCs during definitive hematopoiesis.34 We found that transient Notch activation enhanced cbfb expression and expanded it ectopically. On the other hand, in the Notch-signaling mutant mind bomb, cbfb expression in hematopoietic regions was abrogated. Thus, our results suggest that cbfb is also downstream of the Notch pathway during hematopoiesis.
Overall, our results indicate that a functional CBF complex is important for the onset of definitive hematopoiesis, but runx1 and cbfb functions appear to be required at 2 different steps during HSCs development. Our study strongly suggests a novel role for CBFβ and the CBFβ-RUNX1 heterodimer in the release of HSCs from the AGM during early definitive hematopoiesis. The presence of nascent, runx1+/c-myb+ HSCs in cbfb−/− embryos indicates that cbfb is dispensable for the emergence of HSCs but also implies that RUNX1 is able to drive HSC formation in the absence of its known obligate cofactor CBFβ. The mechanism for this functional separation of RUNX1 and CBFβ during early definitive hematopoiesis is unclear. It is possible, however, that a certain level of RUNX1 is adequate to turn on hematopoietic markers, but a higher functional level, achieved by increased binding in the presence of CBFβ, is necessary to get through the later process.
Acknowledgments
The authors thank Ajay Chitnis for the tg(uas:NICD), tg(hsp70:gal4), and mind bombta52b fish, Leonard Zon for the tg(c-myb:eGFP) fish, and Jeff Essner for the tg(flk1:moesin1-eGFP) fish line. They also thank Liu laboratory members and Alberto Rissone for helpful discussions and advice.
The work described in this paper was supported by the Intramural Research Programs at the National Human Genome Research Institute, National Institutes of Health, and the National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.B., B.C., S.W., M.P.J., and A.V.G. designed and performed the experiments and analyzed the data; B.M.W., R.S., and P.P.L. designed and organized the experiments and analyzed the data; and E.B. and P.P.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: P. Paul Liu, 49 Convent Dr, Building 49, Room 3A26, Bethesda, MD 20892; e-mail: pliu@mail.nih.gov.
References
- 1.Detrich HW, III, Kieran MW, Chan FY, et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92(23):10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126(17):3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 3.Sood R, Liu P. Novel insights into the genetic controls of primitive and definitive hematopoiesis from zebrafish models. Adv Hematol. 2012;2012:830703. [DOI] [PMC free article] [PubMed]
- 4.Paik EJ, Zon LI. Hematopoietic development in the zebrafish. Int J Dev Biol. 2010;54(6-7):1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 7.Kissa K, Murayama E, Zapata A, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111(3):1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 8.Murayama E, Kissa K, Zapata A, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25(6):963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134(23):4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa E, Inuzuka M, Maruyama M, et al. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Wang Q, Crute BE, Melnikova IN, Keller SR, Speck NA. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki K, Yagi H, Bronson RT, et al. Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proc Natl Acad Sci USA. 1996;93(22):12359–12363. doi: 10.1073/pnas.93.22.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Stacy T, Miller JD, et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell. 1996;87(4):697–708. doi: 10.1016/s0092-8674(00)81389-6. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- 17.Mangan JK, Speck NA. RUNX1 mutations in clonal myeloid disorders: from conventional cytogenetics to next generation sequencing, a story 40 years in the making. Crit Rev Oncog. 2011;16(1-2):77–91. doi: 10.1615/critrevoncog.v16.i1-2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North T, Gu TL, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 19.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin H, Sood R, Xu J, et al. Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development. 2009;136(4):647–654. doi: 10.1242/dev.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood R, English MA, Belele CL, et al. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood. 2010;115(14):2806–2809. doi: 10.1182/blood-2009-08-236729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blake T, Adya N, Kim CH, et al. Zebrafish homolog of the leukemia gene CBFB: its expression during embryogenesis and its relationship to scl and gata-1 in hematopoiesis. Blood. 2000;96(13):4178–4184. [PubMed] [Google Scholar]
- 24.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; 1995.
- 25.Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135(10):1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HF, Traver D, Zhu H, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Kaiser MS, Larson JD, et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137(18):3119–3128. doi: 10.1242/dev.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang YJ, Brand M, Heisenberg CP, et al. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- 29.Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80(2):153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 30.Sood R, Carrington B, Bishop K, et al. Efficient methods for targeted mutagenesis in zebrafish using zinc-finger nucleases: data from targeting of nine genes using CompoZr or CoDA ZFNs. PLoS ONE. 2013;8(2):e57239. doi: 10.1371/journal.pone.0057239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3(1):59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 32.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26(6):695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyon Y, McCammon JM, Miller JC, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26(6):702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19(19):2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8(3):389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Koudijs MJ, den Broeder MJ, Keijser A, et al. The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS Genet. 2005;1(2):e19. doi: 10.1371/journal.pgen.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham L, Finckbeiner S, Hyde RK, et al. Identification of benzodiazepine Ro5-3335 as an inhibitor of CBF leukemia through quantitative high throughput screen against RUNX1-CBFβ interaction. Proc Natl Acad Sci USA. 2012;109(36):14592–14597. doi: 10.1073/pnas.1200037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa M, Ichikawa M, Kumano K, et al. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108(10):3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Jin H, Li L, Qin FX, Wen Z. cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood. 2011;118(15):4093–4101. doi: 10.1182/blood-2011-03-342501. [DOI] [PubMed] [Google Scholar]