Abstract
Horizontal cells mediate inhibitory feedforward and feedback lateral interactions in the outer retina at photoreceptor terminals and bipolar cell dendrites; however, the mechanisms that underlie synaptic transmission from mammalian horizontal cells are poorly understood. The localization of a vesicular γ-aminobutyric acid (GABA) transporter (VGAT) to horizontal cell processes in primate and rodent retinae suggested that mammalian horizontal cells release transmitter in a vesicular manner. Toward determining whether the molecular machinery for vesicular transmitter release is present in horizontal cells, we investigated the expression of SNAP25 (synaptosomal-associated protein of 25 kDa), a key SNARE protein, by immunocytochemistry with cell type-specific markers in the retinae of mouse, rat, rabbit, and monkey. Different commercial antibodies to SNAP25 were tested on vertical sections of retina. We report the robust expression of SNAP25 in both plexiform layers. Double labeling with SNAP25 and calbindin antibodies demonstrated that horizontal cell processes and their endings in photoreceptor triad synapses were strongly labeled for both proteins in mouse, rat, rabbit, and monkey retinae. Double labeling with parvalbumin antibodies in monkey retina verified SNAP25 immunoreactivity in all horizontal cells. Pre-embedding immunoelectron microscopy in rabbit retina confirmed expression of SNAP25 in lateral elements within photoreceptor triad synapses. The SNAP25 immunoreactivity in the plexiform layers and outer nuclear layer fell into at least three patterns depending on the antibody, suggesting a differential distribution of SNAP25 isoforms. The presence of SNAP25a and SNAP25b isoforms in mouse retina was established by reverse transcriptase-polymerase chain reaction. SNAP25 expression in mammalian horizontal cells along with other SNARE proteins is consistent with vesicular exocytosis.
INDEXING TERMS: SNARE complex, exocytosis, immunocytochemistry, retina, vision
Although there is general agreement that horizontal cells mediate inhibitory feedback in the outer retina, how these cells signal to their postsynaptic partners in the mammalian retina remains poorly understood. Horizontal cell endings invaginate the synaptic terminals of photoreceptors, along with the dendrites of rod and cone ON bipolar cells, to form the triad synapse, the initial site of information transfer in the visual system. Horizontal cells collect visual information from a spatially wide area with respect to that of photoreceptors and bipolar cells, and provide lateral inhibition in the outer retina. They play a critical role in sensing contrasts, controlling synaptic gain, and, in some cases, color opponency (Hirano et al., 2010).
The immunohistochemical localization of the vesicular γ-aminobutyric acid (GABA) transporter (VGAT) to horizontal cell processes in primate and rodent retinae (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002) suggested that mammalian horizontal cells release transmitter in a vesicular manner. More recently, we and others reported that the SNARE and synaptic vesicle-associated proteins, syntaxin-1a and –4, complexin-I/II, and synapsin 1 (Hirano et al., 2005, 2007; Sherry et al., 2006; Lee and Brecha, 2010), are expressed in horizontal cell processes, lending further support to the idea.
SNAP25 (synaptosomal-associated protein of 25 kDa) along with syntaxins and the vesicular protein synaptobrevin/VAMP (vesicle-associated membrane protein) comprise the core fusion complex in synaptic vesicle exocytosis. The t-SNAREs (target-membrane-associated soluble N-ethylmaleimide fusion protein attachment protein [SNAP] receptor) SNAP25 and syntaxin combine in an 1:1 complex and act as an acceptor for VAMP present on synaptic vesicles (Söllner et al., 1993a,b; Pobbati et al., 2006). The strands of the SNARE proteins interact to form a helical structure (Sutton et al., 1998) that drives the fusion of vesicles with the plasma membrane (Brunger, 2005). Indeed, clostridial toxins that selectively cleave these SNARE proteins block vesicular exocytosis, indicating the essential nature of these proteins for synaptic transmission (Schiavo et al., 1993, 2000). Studies of SNAP25 null mutants indicated that although it was not necessary for stimulus-independent neurotransmitter release, SNAP25 was indispensable for evoked transmitter release (Washbourne et al., 2002; Tafoya et al., 2006).
A characteristic of vesicle trafficking proteins is that they occur in many isoforms and splice variants, which lends specificity to the targeting process and confers varied functional characteristics to synaptic transmission (Söllner et al., 1993b; Morgans, 2000). In retina, syntaxin-3 is found at ribbon synapses (Morgans et al., 1996), whereas syntaxin-1 occurs at most conventional synapses (Barnstable et al., 1985; Morgans et al., 1996). Synaptotagmin (syt)-2 is found in horizontal cells and two classes of bipolar cells, whereas syt-1 appears to be localized to most, if not all, of the remaining retinal cells (Fox and Sanes, 2007; Wässle et al., 2009). Recently, four different splice variants of syntaxin-3 have been isolated, and it was shown that syntaxin-3b was expressed at all retinal ribbon synapses (Curtis et al., 2008). Furthermore, syntaxin-3b was found to have significantly lower binding affinity for SNAP25 than syntaxin-1a in a calcium-dependent manner (Curtis et al., 2008). To date, there are four members of the SNAP25 family (SNAP25, Oyler et al., 1989; SNAP23, Ravichandran et al., 1996; SNAP29, Steegmaier et al., 1998; and SNAP47, Holt et al., 2006; Gordon et al., 2010). Whereas the other members appear to be ubiquitously expressed, SNAP25 is expressed predominantly in neuronal and neuroendocrine cells (Holt et al., 2006; Prescott et al., 2009).
The SNAP25 gene has been cloned and found to give rise to two splice variants, SNAP25a and SNAP25b, which differ in the usage of exon 5 (Bark, 1993; Bark and Wilson, 1994). The two SNAP25 isoforms are differentially expressed during neural development (Bark et al., 1995; Boschert et al., 1996), indicating functional differences. Of particular interest is that exon 5b incorporates a palmitoylation site, leading to the plasma membrane association of the protein (Hess et al., 1992; Veit et al., 1996; Lane and Liu, 1997). These palmitoylated cysteines are important for vesicular release, as exocytosis is slowed when these sites are mutated (Nagy et al., 2008).
Due to conflicting earlier reports regarding SNAP25 immunoreactivity in mammalian retina and its importance for exocytosis, we reinvestigated the cellular localization of SNAP25 in mouse, rat, rabbit, and monkey retinae by using a panel of nine SNAP25 antibodies. We consistently observed strong immunolabeling of horizontal cell processes and endings in the outer retina in all species tested. This localization to endings of horizontal cells was confirmed by pre-embedding immunoelectron microscopy in rabbit retina. In addition to horizontal cell labeling, SNAP25 antibodies produced at least three distinct immunolabeling patterns in mammalian retina. Finally, we provide reverse transcriptase-polymerase chain reaction (RT-PCR) data indicating the presence of both SNAP25a and –25b isoforms in adult mouse retina.
MATERIALS AND METHODS
Adult C57BL/6 mice (20–30 g; Jackson Laboratory, Bar Habor, ME), Sprague-Dawley rats (250–300 g) and New Zealand White rabbits (3–4 kg; Charles River, Wilmington, MA) of both sexes were used for these studies. The monkey retina was from an adult macaque monkey (Macaca fascicularis) that was sacrificed after electrophysiological experiments unrelated to those in this paper. All experiments were performed in accordance with the guidelines for the welfare of experimental animals issued by the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals (2002) and the University of California-Los Angeles (UCLA) Animal Research Committee.
Following deep anesthesia with 1–3% isofluorane (rodents; IsoFlo, Abbott Laboratories, North Chicago, IL), ketamine-xylazine (30 mg/kg ketamine intraperitoneally [i.p.], Ketajet, Phoenix Scientific, Fort Dodge, IA; 3 mg/kg xylazine i.p., Xyla-jet, Phoenix Scientific) followed by pentobarbital (rabbits; 100–200 mg/kg intravenously, Nembutal, Abbott Laboratories), or a lethal dose of pentobarbitone (100 mg/kg, monkey), the eyes were enucleated, and the anterior chamber and lens were removed. The eyecups were immersion-fixed in 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4, for 15–30 minutes or in 2% PLP (2% [w/v] PFA, 1.37% [w/v] D, L-lysine, 0.214% [w/v] sodium periodate [NaIO4]) for 20 minutes, cryoprotected in graded (10–30%) or 30% sucrose, and sectioned vertically at 12–14 μm on a cryostat onto gelatin-coated slides. For sliding microtome sections, the retina was isolated, flattened, and cut parallel to the vitreal surface at 30 μm to obtain horizontal sections of retina. Free-floating sections were stored in 0.1 M PB at 4°C until antibody staining and processed similarly to vertical sections, except that free-floating sections were incubated in primary antibody for several days at 4°C with gentle agitation.
Antibodies
Primary antibodies and their dilutions were as follows: mouse monoclonal antibody against calbindin-28K (1:2,000–1:5,000, Sigma, St. Louis, MO, cat. C9848, clone cl. CB-955), rabbit polyclonal antibody to parvalbumin (Swant, Bellinzona, Switzerland, cat. no. PV-28), mouse monoclonal antibodies to SNAP25 (Synaptic Systems, Göttingen, Germany, cat. nos. 111 011 and 111 111; Sigma, S5187; Covance/Sternberger Monoclonals, Emeryville, CA, cat. no. SMI-81R), rabbit polyclonal antibodies to SNAP25 (BIOMOL/Affiniti Research Products (ARP), Plymouth Meeting, PA, cat. no. SL3730; Millipore Bioscience Research Reagents/Chemicon, Temecula, CA, cat. no. AB1762; Sigma, cat. no. S9684; StressGen Biotechnologies/Assay Designs, Plymouth Meeting, PA, cat. no. VAP-SV002), goat polyclonal antibody to SNAP25 (Millipore/Chemicon, cat. no. AB5871P), and a rabbit polyclonal antibody to VGAT (VGAT-N2, a kind gift of Dr. Robert Reimer, Stanford University School of Medicine, Palo Alto, CA). The antibodies used in this study are listed in Table 1. Chemicals used were purchased from Sigma unless otherwise noted.
TABLE 1.
List of Antibodies
| Antibody | Host | Antigen | Source | Dilution |
|---|---|---|---|---|
| Calbindin D-28k | Ms | Purified bovine kidney calbindin D-28k | Sigma (St. Louis, MO), C9848, clone CB-955 | 1:2,500–1:5,000 |
| Calbindin D-28k | Rb | Recombinant rat calbindin D-28k | Swant (Bellinzona, Switzerland), CB38 | 1:1,000 |
| Parvalbumin | Rb | Rat muscle parvalbumin | Swant, PV-28 | 1:2,000 |
| SNAP-25 | Gt | Recombinant human SNAP-25 | Millipore/Chemicon (Temecula, CA), AB5871P | 1:250–1:1,000 |
| SNAP-25 | Ms | SNAP-25 protein, human brain extracts, epitope ≈ C-terminus of SNAP-25 |
Covance/Sternberger Monoclonals (Emeryville, CA), SMI 81 | 1:5,000–1:500,000 |
| SNAP-25 | Ms | Human presynaptic protein precipitate | Sigma, S5187, clone SP12 | 1:5,000–1:10,000 |
| SNAP-25 | Ms | Recombinant full-length rat SNAP-25, epitope ≈ N-terminal amino acids 20–40 |
Synaptic Systems Göttingen, Germany), #111 011, cl. 71.1 | 1:20,000 |
| SNAP-25 | Ms | Recombinant full-length rat SNAP-25, epitope ≈ N-terminal amino acids 1–20 |
Synaptic Systems, #111 111, cl. 71.2 | 1:100–1:50,000 |
| SNAP-25 | Rb | N-terminal amino acids 9–29 human SNAP-25 | Sigma, S9684 | 1:7,000 |
| SNAP-25 | Rb | C-terminal amino acids 190–206 mouse SNAP-25 | BIOMOL/Affiniti Research Products (Plymouth Meeting, PA), SL3730 | 1:1,000–1:10,000 |
| SNAP-25 | Rb | C-terminal amino acids 190–206 mouse SNAP-25 | Millipore/Chemicon, AB1762 | 1:500 |
| SNAP-25 | Rb | C-terminal amino acids 195–206 mouse SNAP-25 | Assay Designs/StressGen Biotechnologies (Plymouth Meeting, PA), VAP-SV002 | 1:200–1:2,000 |
| VGAT-N2 | Rb | N-terminal amino acids 1–99 of VGAT, GST fusion protein | Dr. Robert Reimer, Stanford University School of Medicine (Palo Alto, CA) | 1:2,000 |
Antibody characterization
SMI 81 (Sternberger Monoclonals and ARP; Brandstätter et al., 1996; Morgans and Brandstätter, 2000): The monoclonal antibody was generated against the whole SNAP25 protein in human brain extracts (manufacturer’s datasheet; Connell et al., 2009). Specific labeling in vertical sections of rat retina was abolished when the antibody was preincubated overnight with purified SNAP25 protein on nitrocellulose (Brandstätter et al., 1996). In addition, SMI 81 recognized a 25-kDa band on Western blots of rat retina membrane proteins (Brandstätter et al., 1996), mouse neocortex (Mehta et al., 1996; Grabs et al., 1996), mouse hippocampal neurons (Tafoya et al., 2006), and rat brain (Garbelli et al., 2008; Connell et al., 2009) and hippocampus (Frassoni et al., 2005). The SNAP25 band is eliminated in immunoblots prepared from the SNAP25 null mice (Washbourne et al., 2002; Tafoya et al., 2006). Epitope mapping indicated that SMI 81 recognizes the extreme N-terminus of SNAP25, particularly when it is N-terminal acetylated (Connell et al., 2009).
Sigma S5187, clone cl. SP12, mouse monoclonal antibody (mAb) to SNAP25 (Honer et al., 1993): The mAbs were raised against crude human synaptic proteins immunoprecipitated by the SY38 antibody (Boehringer Mannheim, Indianapolis, IN) to synaptophysin (Honer et al., 1993). Cl. SP12 recognized a 26–27-kDa band on Western blots of human (Honer et al., 1993) and rat brain (Honer et al., 1997) and hippocampus (Frassoni et al., 2005), and COS cells transfected with SNAP25a and SNAP25b expression constructs (Honer et al., 1997). The higher apparent molecular weight is due to post-translational modification by palmitoylation (Hess et al., 1992; Veit et al., 1996).
Synaptic Systems, cat. no. 111 011, mouse mAb to SNAP25, cl. 71.1: This mAb was generated against recombinant full-length rat SNAP25 protein (Bruns et al., 1997). This mAb labeled a 25-kDa band on Western blots of rat brain crude synaptosomal fraction (Bruns et al., 1997), mouse retina extracts (Curtis et al., 2008), rat hippocampus (Frassoni et al., 2005), and chromaffin cells (Nagy et al., 2004). The epitope resides in a N-terminal domain (amino acids 20–40), which is conserved between the two splice forms (manufacturer’s information; Xu et al., 1999).
Synaptic Systems, cat. no. 111 111, mouse mAb to SNAP25, cl, 71.2: This monoclonal antibody, also generated against recombinant full-length rat SNAP25 protein, recognized a 25-kDa band on Western blots of rat brain crude synaptosomal fraction (manufacturer’s datasheet), bovine retinal homogenates, and synaptosomes (von Kriegstein et al., 1999). The epitope has been mapped to the conserved N-terminus of SNAP25 (amino acids 1–20, manufacturer’s datasheet; Xu et al., 1999). Thus, it recognizes both splice variants, SNAP25a and -b.
Sigma, S9684, rabbit polyclonal antibody generated against the N-terminus of human SNAP25 amino acids 9–29, which is identical in rat, mouse, and chicken. This antibody recognized SNAP25 protein on Western blots, and this binding was inhibited when the antibody was preincubated with the immunizing peptide (amino acids 9–29 with a C-terminal lysine; manufacturer’s datasheet). Thus, it recognizes both splice variants, SNAP25a and -b.
ARP/BIOMOL SL3730, rabbit polyclonal antibody generated against mouse SNAP25, KLH-conjugated C-terminal amino acids 190–206: This antibody recognized a 25-kDa band on Western blots of bovine brain and rat brain extracts (Söllner et al., 1993a). The C-terminus is conserved between the two splice variants.
Millipore/Chemicon, AB1762, rabbit polyclonal antibody generated against mouse SNAP25, amino acids 190–206 with a C-terminal Cys: AB1762 recognized a 25-kDa band on Western blots of bovine adrenal medulla and rat brain fractions (manufacturer’s datasheet; Blasi et al., 1993). It does not recognize botulinum neurotoxin (BoNT)/A- and BoNT/E-cleaved SNAP25 (Blasi et al., 1993), confirming that the epitope is in the C-terminus.
Assay Designs/StressGen, VAP-SV002, rabbit polyclonal antibody generated against mouse SNAP25, amino acids 195–206 with a C-terminal cysteine: This antibody detected a 25-kDa band on Western blots of mouse and rat brain tissue, whereas no band was observed with mouse liver or HeLa extracts. This 25-kDa band was blocked by preincubation with a GST-SNAP25 fusion protein or the antigenic peptide (manufacturer’s datasheet; Oyler et al., 1989). No band was observed when preiummune serum was used (Oyler et al., 1989).
Numerous antibodies to calbindin D-28k have been used to identify horizontal cells in mammalian retinae (Röhrenbeck et al., 1987; Chun and Wässle, 1993; Massey and Mills, 1996; Haverkamp and Wässle, 2000; Cuenca et al., 2002; Hirano et al., 2005; Gargini et al., 2007; Guo et al., 2009, 2010; Lee and Brecha, 2010). In addition, parvalbumin antibodies label both H1 and H2 horizontal cells in the monkey retina (Röhrenbeck et al., 1989), whereas calbindin antibodies only label the H2 horizontal cells (Röhrenbeck et al., 1989; Wässle et al., 2000).
Anti-calbindin mouse mAb (Sigma C9848, cl. CB-955) was raised against purified bovine kidney calbindin-D-28K. It recognized a 28-kDa band corresponding to calbindin D-28K on immunoblots (manufacturer’s datasheet). No cross-reactivity was observed with other EF-hand-containing proteins (e.g., calbindin-D-9K, calretinin, myosin light chain, parvalbumin, S-100a/b/A2/A6; manufacturer’s datasheet). Cl. CB-955 mAb has been used as a marker to identify Purkinje cells in mouse cerebellum (Soderling et al., 2003; Sadakata et al., 2007), known to express calbindin-D28K (Garcia-Segura et al., 1984). The specificity of this calbindin mAb has been documented previously and used to identify horizontal cells in rodent retina (Rossi et al., 2003; Renteria et al., 2005; Hirano et al., 2007; Gargini et al., 2007).
Anti-calbindin rabbit polyclonal antibody (Swant CB38) was raised against recombinant rat calbindin D-28K protein. This antibody detects a 28-kDa band on immunoblots and shows 10% cross-reactivity to calretinin (manufacturer’s datasheet). Finally, the rabbit polyclonal and the mouse mAbs to calbindin produced a similar pattern of labeling in mouse, rat, and rabbit retina (Haverkamp and Wässle, 2000; Hirano et al., 2005).
Anti-VGAT (VGAT-N2) antibodies, a kind gift of Dr. Robert Reimer (Stanford School of Medicine), were generated against a GST fusion protein containing the N-terminal amino acids 1–99 of the rat VGAT protein (Chaudhry et al., 1998). VGAT-N2 recognized a single band of the predicted molecular weight (57 kDa) on immunoblots of rat brain homogenates and a PC12 cell line stably expressing rat VGAT (McIntire et al., 1997), whereas homogenates of liver and wild-type PC12 cells showed no band (Chaudhry et al., 1998).
Light microscopy
Immunostaining was performed by using the indirect fluorescence method (Grünert and Wässle, 1993; Hirano et al., 2005). In brief, retinal sections were incubated in primary antibody diluted in 3% normal goat serum (Invitrogen, Carlsbad, CA), 1% bovine serum albumin, 0.5% Triton X-100, 0.05% sodium azide (NaN3), 0.1 M PB, for 12–16 hours at room temperature. The specific immunolabel was visualized by using Alexa Fluor 488-, 568- or 594-conjugated anti-rabbit, mouse, or goat IgG secondary antibodies (Invitrogen) at 1:500 dilutions for 1 hour at room temperature. As a negative control, the omission of the primary antibodies in the single or double labeling studies confirmed the elimination of specific labeling for all antibodies used. In addition, nonimmune rabbit serum was used in place of the primary antibody, and no specific labeling was observed (data not shown).
The immunostaining was examined on a Zeiss Laser Scanning Microscope 510 Meta (Carl Zeiss, Thornwood, NY) with Zeiss 40× 1.3 NA Plan-NEOFLUAR oil and C-Apochromat 40× 1.2 NA corrected water objectives. Typically, stacks of three images (n = 3–7, 2,048 × 2,048 pixels) of 1.0-μm thickness (0.9 μm green channel, 1.0–1.2-μm red channel) were collected, separated by z-steps of 0.3 μm (range = 0.3–0.5 μm). Exceptions to this are noted in the figure legends. Confocal images were analyzed by using the Zeiss LSM 510 proprietary software (version 3.2). Intensity levels and brightness/contrast were adjusted in Adobe Photoshop CS2 v.9.02 (Adobe Systems, San Jose, CA).
Pre-embedding immunoelectron microscopy
Immunolabeling was performed by using pre-embedding immunoelectron microscopy techniques, as previously described (Brandstatter et al., 2004). In brief, the eyecups were fixed for 50 minutes in 4% (w/v) PFA, and the retinae were removed, cryoprotected in graded sucrose, and frozen and thawed three times to improve antibody penetration. Vibratome sections were cut at 50 μm into cold PBS, pH 7.4. The primary antibody against SNAP25 (Synaptic Systems, cat. no. 111 011, cl. 71.1) was used at 1:20,000 and diluted in the same media used for light microscopy, except that Triton X-100 was omitted, and the sections were incubated for 4 days at 4°C. Immunolabeling was visualized by the ABC method (Vectastain Elite ABC kit, Vector, Burlingame, CA) using 3,3′-diaminobenzidine (DAB) as substrate. The DAB reaction product was silver intensified and gold toned, and the tissue was flat embedded in Epon 812 (Serva, Heidelberg, Germany). Ultrathin sections were cut and then contrasted with uranyl acetate and lead citrate. The ultrathin sections were examined and photographed with a Zeiss EM10 electron microscope (Zeiss, Oberkochen, Germany) and a GATAN BioScan digital camera (1,024 × 1,024 pixels; GATAN Munich, Germany) in combination with the software program DIGITAL MICROGRAPH 3.1 (GATAN, Pleasanton, CA).
RT-PCR
Total RNA was isolated from mouse retina by using the Absolutely RNA Purification miniprep kit (Strategene/Agilent Technologies, La Jolla, CA) following the manufacturer’s instructions. The isolated poly(A)+ RNA (300 ng) was used as template for first-strand cDNA synthesis by using oligo(dT) to prime the 20-μl-volume reaction, using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen), following the manufacturer’s instructions. The sequences for isoform-specific primers for SNAP25a and –b were obtained from Grant et al. (1999): S25e2 (sense), 5′-AGGACGCAGACATGCGTAATGAACTGGAGG-3′ and S25e5a (antisense), 5′-TTGGTTGATATGGTTCATGCCTTCTTCGACACGA-3′ or S25e5b (antisense), 5′-CTTATTGATTTGGTCCATCCCTTCCTCAATGCGT-3′, to detect the SNAP25a and SNAP25b transcripts, respectively, in mouse retina. The sense primer corresponds to bases 8–37 from the rat SNAP25 mRNA sequence, and the antisense primers to bases 174–207 of SNAP25a (AB003991) and 174–207 of SNAP25b (AB003992). The presence of “total SNAP25” was assessed using primers S25e2 (sense) and S25e8 (antisense), 5′-GTTGGAGTCAGCCTTCTCCATGATCCTGTC-3′, corresponding to bases 564–535 in exon 8 (Grant et al., 1999).
The PCRs were performed in a 50-μl reaction volume containing 0.2 μM of each primer, 200 μM deoxynucleotides, 2 U of Taq Polymerase (Roche, Indianapolis, IN, cat. no. 11418432001), and 1× supplied buffer (10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl, pH 8.3 [20°C]) with 2 μl (1/10th) of the cDNA synthesis reaction as template. The following temperature protocol was used: 5 minutes at 94°C, then 35 cycles of 45 seconds at 94°C, 1 minute at 60°C, 1 minute at 72°C, followed by 5 minutes at 72°C. Then 10 μl of the PCR was run out on a 2.5% agarose gel in 1X TAE (40 mM Tris-acetate, 1 mM EDTA buffer) and the DNA was visualized by using ethidium bromide (10 μg/ml). The identity of the SNAP25a and –b PCR amplification products was confirmed by DNA sequencing at the UCLA DNA Sequencing Core Facility.
RESULTS
SNAP25 co-localizes with VGAT in horizontal cell processes
We showed earlier that VGAT localized to the processes and endings of mammalian horizontal cells (Cueva et al., 2002). To determine whether SNAP25 occurred in the same cells as VGAT, vertical sections of mouse (Fig. 1A–C), rat (Fig. 1D–F), and rabbit (Fig. 1G–I) retinae were double labeled with antibodies to SNAP25 and VGAT. Antibodies to VGAT produced immunolabeling in the plexiform layers of both the inner and outer retina in all three species (Fig. 1A,D,G), as shown previously (Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2009, 2010). Strong VGAT immunoreactivity occurred throughout the layers of the inner plexiform layer (IPL) with less intense immunoreactivity occurring in the outer plexiform layer (OPL). The appearance of immunolabeling in the OPL was consistent with the localization of VGAT in the processes and endings of horizontal cells (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2009).
Figure 1.
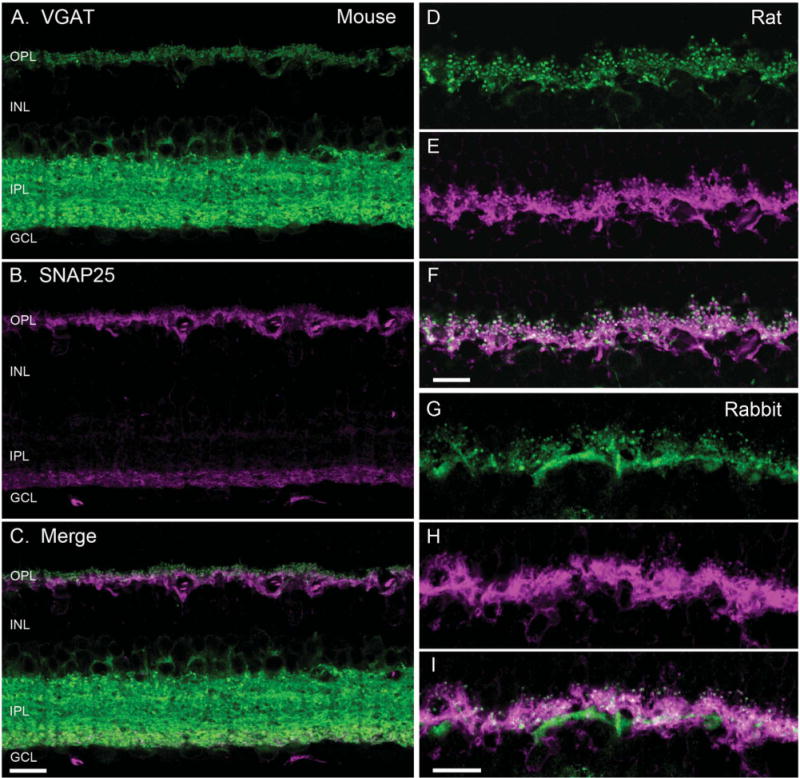
SNAP25 co-localized with VGAT in horizontal cell processes. VGAT antibody staining (green) of a vertical section of mouse (A), rat (D), and rabbit (G) retinae showed immunolabeling in the outer plexiform layer (OPL) and the inner plexiform layer (IPL), and around cell bodies of the proximal inner nuclear layer (INL). SNAP25 antibody labeling (magenta) of the same section produced immunolabeling in the OPL and the proximal IPL of mouse (B), and OPL of rat (E), and rabbit (H) retinae. Merged image of the VGAT and SNAP25 immunolabeling (white) indicated co-localization of SNAP25 with VGAT in the tips of horizontal cells in mouse (C), rat (F), and rabbit (I). GCL, ganglion cell layer. Scale bar = 10 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
Similarly, SNAP25 antibodies consistently produced immunolabeling of horizontal cell processes and endings in the OPL in all three species (Fig. 1B,E,H). The double label images showed that the SNAP25 and VGAT immunoreactivities occurred in the same processes and endings in the OPL (Fig. 1C,F,I). However, as seen in Figure 1B for mouse retina, the immunoreactivity for SNAP25 with the Synaptic Systems (SySy) cl. 71.1 antibody occurred only in the proximal portion of the IPL, in contrast to immunolabeling by other SNAP25 antibodies and VGAT of the entire IPL.
SNAP25 co-localizes with calbindin, a horizontal cell marker
To confirm that the SNAP25 immunoreactivity was localized to horizontal cell processes, double label experiments with antibodies to SNAP25 and calbindin, a well-characterized horizontal cell marker (Röhrenbeck et al., 1987; Chun and Wässle, 1993; Massey and Mills, 1996; Haverkamp and Wässle, 2000; Cuenca et al., 2002; Hirano et al., 2005; Gargini et al., 2007), were conducted. Calbindin antibodies labeled the entire horizontal cell, including the soma, processes, and endings (Fig. 2, top row) in rat, mouse, and rabbit retinae. In the middle row of panels, the immunoreactivity for SNAP25, principally in horizontal cell processes and endings, from the same vertical sections is shown. The bottom row of panels in Figure 2 depicts the merged image of the two immunoreactivities, indicating the strong co-localization in the processes and tips of horizontal cells. In rabbit OPL (Fig. 2I) in particular, the concentration of SNAP25 in horizontal cell processes adjacent to the base of cone pedicles is evident, in addition to the axonal tips invaginating into rod spherules.
Figure 2.
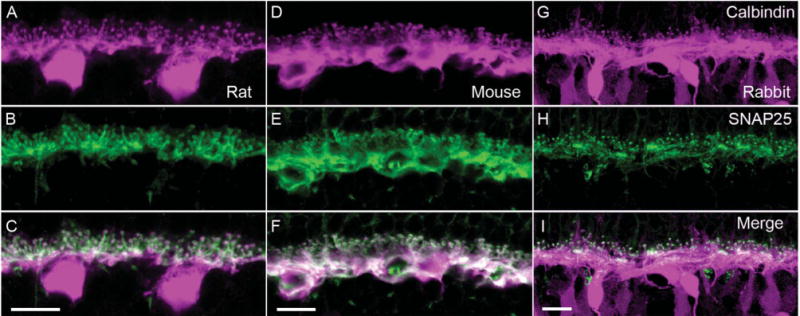
SNAP25 immunoreactivity co-localizes with that of calbindin, a horizontal cell marker. Top row shows the immunolabeling for calbindin (magenta) in (A) rat, (D) mouse, and (G) rabbit OPL. In all three species, calbindin immunoreactivity identified horizontal cell bodies, processes, and endings. In rabbit retina, calbindin immunolabeled also a subset of bipolar cells (Massey and Mills, 1996). Middle row shows the immunolabeling for SNAP25 (green) in (B) rat, (E) mouse, and (H) rabbit OPL. The SNAP25 immunoreactivity was present primarily in the tips and processes in the OPL. Bottom row shows the merged images of calbindin and SNAP25 immunreactivities (white), which demonstrated the strong expression of SNAP25 in the processes and tips of horizontal cells of all three species (C,F,I). In rabbit OPL (I) in particular, the concentration of SNAP25 in horizontal cell processes adjacent to the base of cone pedicles is evident, in addition to the tips invaginating into rod spherules. Scale bar = 10 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
SNAP25 co-localizes with parvalbumin, a horizontal cell marker in primate retina
To see whether the SNAP25 localization to horizontal cells proved true also for the primate retina, double labeling experiments using antibodies to SNAP25 and parvalbumin, which labels all horizontal cells in monkey retina (Röhrenbeck et al., 1987; Martin and Grünert, 1992; Wässle et al., 2000; Hendrickson et al., 2007), were conducted. Figure 3A depicts a vertical section of macaque retina that had been immunolabeled with SNAP25 and parvalbumin antibodies. The SNAP25 immunolabeling is primarily in the OPL and IPL, with much fainter labeling of cell bodies in the ONL and INL, and nerve fiber layer (NFL). To the right are higher magnification views of the OPL, corresponding to the boxed area in Figure 3A, with the SNAP25 (Fig. 3B) and parvalbumin (Fig. 3C) immunoreactivities. The merged image (Fig. 3D) shows the localization of SNAP25 to the processes and tips of horizontal cells in monkey retina.
Figure 3.
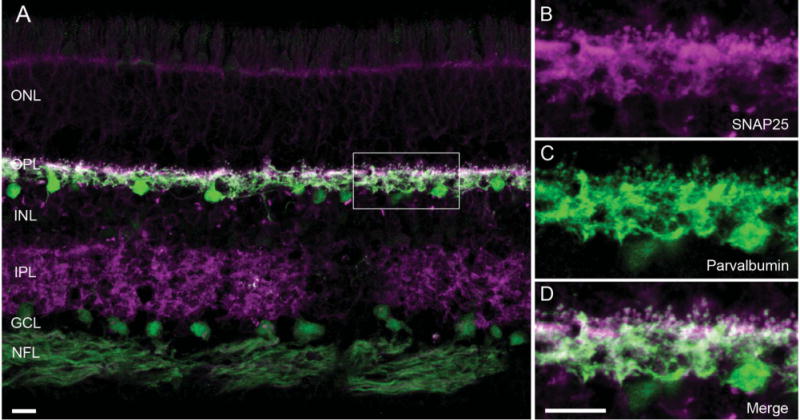
SNAP25 immunoreactivity co-localized with that of parvalbumin, a horizontal cell marker in primate retina. A: A vertical section of macaque retina was double labeled with antibodies to SNAP25 (magenta) and parvalbumin (green), showing SNAP25 (SySy cl. 71.1) immunoreactivity primarily in the two plexiform layers. B–D: Higher power view of the boxed area of the outer plexiform layer (OPL) in A. B: SNAP25 immunolabeling (magenta) in processes and tips in the OPL. C: Parvalbumin immunolabeling (green) of the same region showed the presence of horizontal cell processes and tips. D: Merged image of the two immunoreactivities (white) indicated co-localization of SNAP25 to horizontal cell processes and tips in macaque retina. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer. Scale bar = 10 μm in A and D (applies to B–D).
SNAP25 immunoreactivity occurs in horizontal cell tips at rod and cone terminals
To ascertain the localization of SNAP25 to horizontal cells at the ultrastructural level, the SNAP25 immunoreactivity at the triad synapse was evaluated in rabbit retina by pre-embedding immunoelectron microscopy using the SySy cl. 71.1 antibody. Figure 4A shows an electron micrograph of a cone pedicle containing DAB reaction product in the lateral elements at the ribbon synapse, which correspond to the horizontal cell endings from the dendrites that invaginate into the cone photoreceptor terminals. Note that the photoreceptor terminal itself and invaginating bipolar cell dendrite did not show specific immunolabeling for SNAP25. In addition, not every lateral element contained SNAP25 immunolabeling, which is likely due to the incomplete penetration of the antibody into the tissue and the necessity of having to section deep enough into the block to be confident of specific immunolabeling. In a similar fashion, examination of sections containing rod terminals indicated that the black immunoreaction product occurred in the lateral elements, consistent with SNAP25 immunolabeling in horizontal cell axonal endings that invaginate into the rod photoreceptor terminals (Fig. 4B,C). Again, the rod terminals themselves and invaginating bipolar cell dendrites never showed SNAP25 labeling.
Figure 4.

SNAP25 immunoreactivity occurs in horizontal cell tips at rod and cone terminals at the ultrastructural level. Preembedding immunoelectron micrographs of SNAP25 (SySy cl. 71.1) immunoreactivity at (A) cone and (B,C) rod photoreceptor terminals showed dark DAB reaction product in the lateral elements at these ribbon synapses, indicating localization of SNAP25 to horizontal cell tips. The DAB reaction product was silver-intensified and gold-toned, such that the appearance is of a fine granular nature. H, horizontal cell lateral element; B, bipolar cell process; arrowheads, synaptic ribbons. Scale bar = 0.2 μm in A–C.
SNAP25 immunolabeling patterns in mammalian retinae
In the course of testing SNAP25 antibodies from various commercial sources on vertical sections of retina, several patterns of SNAP25 immunolabeling emerged. For each of the labeling patterns, typically more than one antibody produced such a pattern. All of the SNAP25 antibodies screened produced immunolabeling of horizontal cells in mouse, rat, and rabbit retinae (for example, see Fig. 5A–C). Some antibodies gave strong labeling of horizontal cell processes and endings (Fig. 5A, Millipore/Chemicon AB5871P), and labeling of a band deep in the IPL in addition (Fig. 1B, SySy cl. 71.1). A second pattern (Sigma S5187, StressGen VAP-SV002, BIOMOL/ARP SL3730, SySy cl. 71.2) is depicted in Figure 5B. It shows SNAP25 labeling of horizontal cell processes in the OPL and deep IPL and less intensely throughout the rest of the IPL. Furthermore, outlines of the somata in the outer nuclear layer (ONL) and occasional somata in the inner nuclear layer (INL) and the outer segments (OS) of the photoreceptors were labeled also. The third pattern (SMI 81, BIOMOL/ARP and Sternberger Monoclonal, Millipore/Chemicon AB1762) consisted of strong immunolabeling of both plexiform layers, and outlines of somata in the ONL, INL, and ganglion cell layer (GCL), the NFL, and the photoreceptor OS (Fig. 5C).
Figure 5.
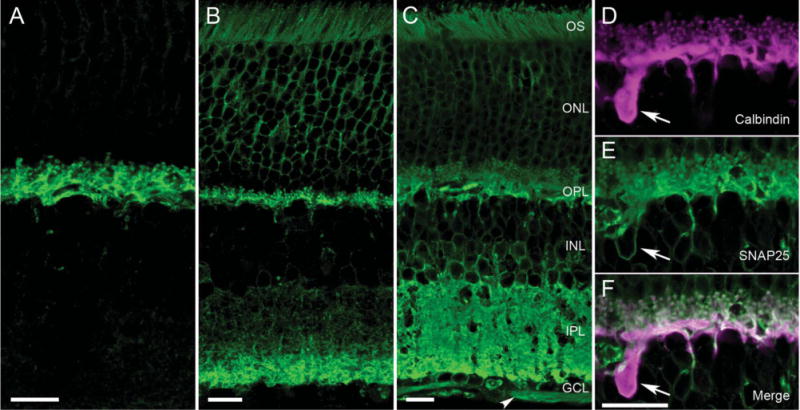
Different SNAP25 antibodies produced different immunolabeling patterns in mammalian retina. A–C: Consistently, all SNAP25 antibodies (green) appeared to immunolabel horizontal cell processes in mammalian retina (see A, mouse vertical section, Millipore/Chemicon goat Ab). Other antibodies produced labeling of the horizontal cells in the OPL and the proximal layer of the IPL (Fig. 1A). B: Still other antibodies exhibited labeling of the two plexiform layers, with the deeper layers of the IPL more strongly labeled in a vertical section of rat retina (StressGen Ab). C: SMI 81mAb produced strong labeling of both plexiform layers in mouse retina, as well as outlines of cell bodies in the ONL, INL, and GCL and the nerve fiber layer (NFL, arrowhead). D–F: These panels depict the double labeling of mouse OPL with (D, magenta) calbindin and (E, green) SMI 81 SNAP25 antibodies obtained with an aliquot of SMI 81 used in Brandstätter et al. (1996) and Morgans and Brandstätter (2000). The merged image in F indicates the co-localization of SNAP25 to horizontal cell processes, tips and soma, as well as photoreceptor terminals. OS, outer segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 10 μm in A–C; 10 μm in F (applies to D–F).
Brandstätter et al. (1996) reported strong SNAP25 immunolabeling in rat retina with a SNAP25 antibody from ARP in the OPL and IPL at the light microscopic level, with no labeling of horizontal cells. Figure 5D–5F shows the double labeling of rat retina for SNAP25 and calbindin, utilizing a sample of the SNAP25 antibody (SMI 81, ARP) used in the earlier studies (Brandstätter et al., 1996; Morgans and Brandstätter, 2000). From the merged image (Fig. 5F), there was clear SMI 81 labeling of horizontal cell processes and endings, as well as the outline of a horizontal cell body, in addition to photoreceptor terminals in the present study. Similar staining was obtained with a more recently obtained sample of SMI 81 antibody from Sternberger Monoclonals. When the antibody dilution was taken to 1:500,000 and higher, the specific immunolabeling was lost (data not shown).
Splice variants of SNAP25 are present in mouse retina
One possible explanation for the different labeling patterns produced by the various SNAP25 antibodies is the presence of different splice variants. Bark and colleagues (1993, 1995) reported the differential splicing of exon 5 to generate isoforms SNAP25a and SNAP25b, resulting in a palmitoylation site in SNAP25b. Amplification of a SNAP25 DNA product from first-strand cDNA synthesized from mouse retina poly(A)+ RNA reflecting total SNAP25 is depicted in lane 2 (Fig. 6), indicating the presence of SNAP25 mRNA in mouse retina. To test whether multiple splice variants are expressed, RT-PCR experiments were conducted by using exon 5a- or 5b-specific primers. Bands corresponding to both SNAP25a and SNAP25b were amplified (Fig. 6, lanes 3 and 4), indicating that both SNAP25 isoforms were expressed. Sequencing of the DNA amplification products confirmed the identity of the two isoforms.
Figure 6.
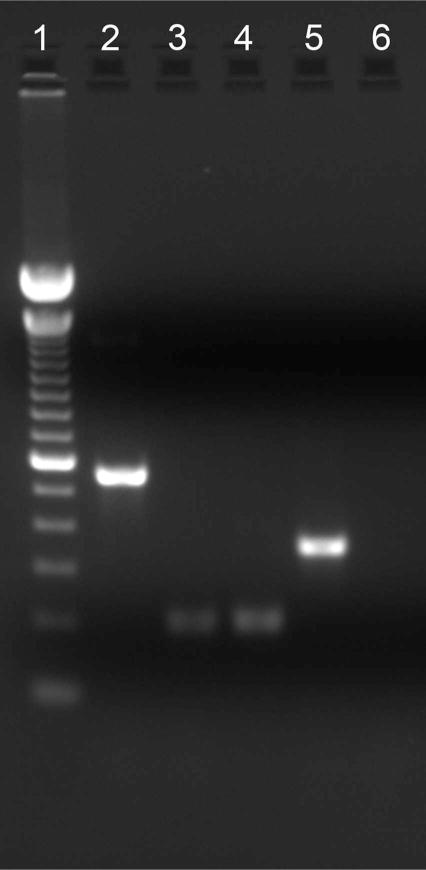
Both SNAP25a and SNAP25b isoforms are expressed in mouse retina. Amplification of a 559-bp DNA product reflected the presence of total SNAP25 message (lane 2). Amplification of a 202-bp DNA product indicated the presence of SNAP25a (lane 3) and SNAP25b (lane 4) isoforms. The amplification of a 353-bp β-actin DNA product served as a control for the successful reverse transcription of mouse retina RNA into cDNA (lane 5). No spurious products are generated in the absence of the DNA template (lane 6). Lane 1, 100-bp DNA ladder; lane 2, total SNAP25; lane 3, SNAP25a, 202 bp; lane 4, SNAP25b; lane 5, β-actin control; lane 6, no template control.
DISCUSSION
The principal finding of this report is the consistent and robust SNAP25 immunolabeling of the processes and tips of horizontal cells in mammalian retina using all nine antibodies screened in mouse, rat, and rabbit retina. In addition to the horizontal cell immunoreactivity, several SNAP25 immunolabeling patterns emerged, suggesting that different isoforms and/or conformations of the SNAP25 protein exist in retina. Indeed, our RT-PCR data (Fig. 6) demonstrated that both isoforms of SNAP25, SNAP25a and SNAP25b, are present in retina. However, the available antibodies are not known to discriminate between the two isoforms, which differ in only nine amino acids (Bark, 1993), on Western blots, as the conserved N-and C-termini proved to be most antigenic. Nevertheless, the characteristic staining pattern produced by each antibody suggested that the antigenic sites of SNAP25 fall into several categories.
The most prominent pattern was the labeling of the two synaptic layers (Figs. 1, 3), consistent with a role for SNAP25 in neurotransmitter release (Catsicas et al., 1992; Ullrich and Südhof, 1994; Brandstätter et al., 1996; Grabs et al., 1996; von Kriegstein et al., 1999; Greenlee et al., 2001). In the IPL, the most proximal layers tended to be labeled exclusively, or more strongly if the whole IPL exhibited SNAP25 immunoreactivity, similar to the pattern reported for adult rat retina (Ullrich and Südhof, 1994; Greenlee et al., 1996, 2001). Catsicas et al. (1992) reported differential expression of SNAP25 in rodent retina, with immunolabeling predominantly in processes, the inner third of the IPL, the NFL, and likely horizontal cell processes in the OPL, but not in ribbon synapses or cell bodies. Although not as pronounced as for SNAP25, synapsin I and synaptophysin also showed slightly more intense labeling in the deep IPL (Catsicas et al., 1992). The synapsin I observation suggests that there is a higher density of conventional synapses in this sublamina (Mandell et al., 1990). Functionally, it is not clear why the innermost ON sublamina of the IPL, where rod bipolar cells terminate, would exhibit more intense labeling.
The next most common pattern involved the addition of somatic labeling of photoreceptor cells in the outer nuclear layer (Brandstätter et al., 1996; von Kriegstein et al., 1999; Greenlee et al., 2001), to horizontal cell and inner IPL labeling. SNAP25 has been implicated in multiple vesicular trafficking pathways between various organelles in the cell, including the endoplasmic reticulum to the Golgi apparatus in retinal neurons (Morgans and Brandstätter, 2000), which likely accounts for this somatic labeling. Indeed, SNAP25 has been shown to be involved in transport of vesicles containing rhodopsin to the outer segments of photoreceptors (Mazelova et al., 2009), consistent with its ultrastructural localization to the Golgi apparatus in photoreceptors (Morgans and Brandstätter, 2000). The somatic photoreceptor labeling has been reported in Brazilian opossum and rat (Greenlee et al., 1996, 2001), and in some cases, the ONL labeling is more prominent during retinal development (Greenlee et al., 2001). In well-fixed adult rat retina, SNAP25 immunoreactivity was strongest in the fiber layers and throughout the ONL and the inner one-fourth of the IPL (Greenlee et al., 2001). However, SNAP25 mRNA was not observed in photoreceptors and bipolar cells, but predominantly in amacrine, ganglion, and horizontal cells of adult rat retina (Catsicas et al., 1992). Congruently, SNAP25 immunoreactivity appeared to be excluded from ribbon synapses of photoreceptor and bipolar cells in this study (Catsicas et al., 1992).
The third most common labeling pattern involved the labeling of both synaptic layers, as well as somatic labeling in the three nuclear layers and the NFL (Fig. 5C, SMI 81 mAb). This pattern indicates that the N-terminus of SNAP25, the epitope recognized by SMI 81, particularly when N-terminal acetylated (Connell et al., 2009), was quite accessible in fixed tissue. Here, both conventional and ribbon synapses showed SNAP25 expression, as would be expected if vesicular release occurs at both types of synapses. Immunoelectron microscopy data from rat retina showed localization to photoreceptor terminals in the OPL and to bipolar and amacrine cell synapses in the IPL, i.e., labeling both ribbon and conventional synapses (Brandstätter et al., 1996). This labeling of the full extent of the OPL and IPL was reported also for bovine retina using the SySy SNAP25 monoclonal antibodies (cl. 71.1 and 71.2), with cl. 71.1 showing stronger immunolabeling of the OPL compared with the IPL (von Kriegstein et al., 1999), similar to what we observed in rabbit retina with cl. 71.1. In mouse and rat retina, cl. 71.1 and cl. 71.2 produced strong labeling of horizontal cell processes and tips and the inner portion of the IPL, and much more faintly the rest of the IPL, and somata in the ONL and INL.
A similar labeling pattern was described in rat retina by using another SNAP25 antibody (I733; Ullrich and Südhof, 1994; McMahon and Südhof, 1995). Ullrich and Südhof (1994) reported strong SNAP25 immunoreactivity in the two plexiform layers and ganglion cell axons, as well as faint immunolabeling in the outer and inner nuclear layers, which contain the cell bodies of the photoreceptors, horizontal, bipolar, and amacrine cells. They also interpreted SNAP25 to be highly enriched in ribbon synapses from the strong staining of the OPL. In the adult Brazilian opossum and rat retinae, immunoreactivity produced by the StressGen (Oyler et al., 1989) SNAP25 antibody appeared throughout the entire extent of the OPL (Greenlee et al., 2001), suggesting that both photoreceptor terminals and horizontal cell processes were labeled.
SNAP25 in mammalian horizontal cells
The expression of SNAP25 in horizontal cells in combination with the previous demonstration of SNARE proteins, syntaxin-1a and –4 (Sherry et al., 2006; Hirano et al., 2005, 2007), and VAMP-1 (vesicle-associated membrane protein-1, also known as synaptobrevin-1; Bitzer and Brecha, 2006; Lee, Hirano and Brecha, in preparation) to the processes and tips indicates that there is significant vesicular trafficking to the plasma membrane in these cells. The identification of additional proteins involved in vesicular exocytosis, such as synapsin I, synaptic vesicle protein 2A (SV2A), synaptotagmin-2, and complexin I/II, to mammalian horizontal cells lends further support (Hirano et al., 2005, 2007; Fox and Sanes, 2007; Lee and Brecha, 2010). The localization of VGAT (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2010) to mammalian horizontal cell tips, furthermore, opens the possibility that these cells use this molecular machinery for the exocytosis of the inhibitory transmitter GABA. This idea is consistent with the recent demonstration of GABA and the GABA synthetic enzyme, GAD65, in guinea pig horizontal cells (Guo et al., 2010). In contrast to the lack of SNAP25 immunoreactivity in inhibitory neurons and its presence in excitatory neurons of the hippocampus (Verderio et al., 2004; Frassoni et al., 2005; Bragina et al., 2007), the inhibitory neurons of the retina appear to robustly express SNAP25, indicating that this dichotomy does not hold for the retina.
What accounts for the different patterns of labeling?
At least three patterns of immunolabeling by SNAP25 antibodies were observed, suggesting that commercially available SNAP25 antibodies recognize different epitopes and/or isoforms of the SNAP25 protein in fixed mammalian retina tissue. The intensity of labeling would affect the pattern observed, which likely reflects overall expression levels (Verderio et al., 2004; Pozzi et al., 2008; Tafoya et al., 2008; Matteoli et al., 2009). Different levels of detection may occur with different antibodies due to differences in affinities. Generally, we have found that short fixation times better preserved the immunoreactivity of synaptic proteins (Hirano et al., 2005, 2007; Guo et al., 2010). The antibodies mapping to the N-terminus, and similarly to the C-terminus, did not necessarily produce similar patterns, indicating that slightly different epitopes or conformations of these protein domains were being recognized. This observation also makes it difficult to ascertain whether certain protein-protein interactions or simple peptide conformational changes account for the differences.
Protein acylation is an important signal for targeting to lipid rafts in the plasma membrane (Melkonian et al., 1999; Salaün et al., 2004; Prescott et al., 2009). SNAP25 is known to be associated with the plasma membrane (Oyler et al., 1989) and specifically to lipid rafts via palmitoylation (Salaün et al., 2005; Greaves et al., 2010). Indeed, SNAP25 was identified as a major palmitoylation substrate in the central nervous system (CNS; Hess et al., 1992). The two SNAP25 isoforms differ at exon 5, which introduces a palmitoylation site into SNAP25b (Bark, 1993; Bark and Wilson, 1994). Variability in immunolabeling patterns may arise also from the expression of both SNAP25 splice variants in the retina and subsequent post-translational modifications, such as palmitoylation (Veit et al., 1996; Veit, 2000) and phosphorylation (Genoud et al., 1999; Iwasaki et al., 2000; Kataoka et al., 2000, 2006; Pozzi et al., 2008), which has been implicated in increasing vesicle recruitment (Nagy et al., 2004) and regulation of the primed vesicle pool size (Nagy et al., 2004).
SNAP25 levels are developmentally regulated in the mouse retina, rising during synapse formation (Catsicas et al., 1991). At P5 in mouse retina, SNAP25 immunolabeling was in presumptive horizontal cell bodies and processes, amacrine cell bodies, and ganglion cell axons, whereas, in adult, the immunolabeling was restricted mainly to processes (Catsicas et al., 1992). In the Brazilian opossum, SNAP25 is strongly expressed in photoreceptor cell bodies early in development, and this labeling disappears as the retina matures (Greenlee et al., 2001). In the CNS, the levels and the anatomical localization of SNAP25 isoforms vary, with SNAP25a being most prominent early in development and SNAP25b becoming predominant in the adult (Oyler et al., 1991; Bark et al., 1995; Boschert et al., 1996; Tafoya et al., 2006, 2008). Although the relative proportion of the two isoforms is fairly constant across different brain regions, the absolute levels differed greatly in the adult (Tafoya et al., 2008).
The increase in expression at the mRNA and protein levels corresponds with axon elongation and synaptogenesis in the IPL from E11 to E16 in chick retina (Osen-Sand et al., 1993). Indeed, the two SNAP25 isoforms have been shown to be functionally distinct (Bark et al., 1995; Verderio et al., 2004). When expressed in chromaffin cells isolated from SNAP25 null mice, the two SNAP25 isoforms produced different levels of secretion (Nagy et al., 2005). Variations in the developmental stage of the animals were correlated with differences in the SNAP25 immunolabeling patterns, but are not likely to account for the discrepancies in the literature with regards to SNAP25 localization in mature retina.
SNAP25 is involved in numerous cellular functions, such as protein trafficking, synaptic vesicle exocytosis, neuropeptide release, axon outgrowth, and photoreceptor differentiation (Söllner et al., 1993; Osen-Sand et al., 1993; Greenlee et al., 2001, 2002). Thus it forms SNARE complexes consisting of different isoforms of syntaxin and VAMP/synaptobrevin that target vesicles to distinct compartments within the cell (Chen and Scheller, 2001). These protein interactions in the varied SNARE complexes may alter epitope availability, with protein conformation changing depending on whether SNAP25 is bound to other SNARE proteins and the particular isoforms in the canonical SNARE complex. In support of this hypothesis, studies of protein-protein interactions in SNARE complexes indicate that the complexes can have multiple stable configurations that proceed to exocytosis (Takahashi et al., 2010).
Moreover, SNAP25b demonstrated a greater flexibility in its polypeptide folding as compared to SNAP25a, likely accounting for the greater sensitivity of SNAP25b to cleavage by BoNT/A than SNAP25a (Puffer et al., 2001). In chromaffin cells from SNAP25 null mice, SNAP25b isoform had a greater effect on secretion than SNAP25a due to greater availability for interaction with SNARE-associated proteins (Nagy et al., 2005). Finally, SNAP25 is also known to interact with voltage-gated calcium channels (Martin-Moutot et al., 1996; Rettig et al., 1996; Sheng et al., 1996; Tobi et al., 1998; Catterall, 1999; Wiser et al., 1999), Gβγ of G protein-coupled receptors (Gerachshenko et al., 2005), synaptotagmin-1 (Schiavo et al., 1997; Zhang et al., 2002), and other SNARE-associated proteins (Sutton et al., 1998; Veit, 2000; Matteoli et al., 2009), which may affect epitope accessibility.
The discrepancies in the retina literature regarding the expression of SNAP25 in horizontal cells probably stem also from technical differences in the preparation of the tissue, the distinct epitopes recognized by the different antibodies, and different visualization methods, resulting in varying labeling intensities (Bragina et al., 2007; Garbelli et al., 2008; Matteoli et al., 2009). The labeling of photoreceptor terminals primarily, and not horizontal cell endings, in the outer retina of rat, reported by Brandstätter et al. (1996), may reflect differences in SMI 81 monoclonal antibody preparations as well as methodological variations. In another study on developing rat retina, the SNAP25 antibody from ARP stained horizontally projecting processes resembling horizontal cell processes in the OPL, although horizontal cells were not clearly identifiable (Chen et al., 1998). In frog retina, SMI 81 produced labeling of photoreceptor inner segments, including their terminals (Mazelov et al., 2009). Similar discrepancies in staining patterns of rodent hippocampal neurons by different groups using SMI 81 (Tafoya et al., 2006, Garbelli et al., 2008) have been reported and attributed to technical differences (Matteoli et al., 2009).
We obtained definitive staining of horizontal cells, as well as the photoreceptor terminals, in mouse (Fig. 5D–F) and rat (data not shown) with an aliquot of SMI 81 used in the earlier paper (Morgans and Brandstätter, 2000). This immunolabeling of horizontal cells was also obtained with SMI 81 purchased from Sternberger Monoclonals (now Covance Research Products) as well as a rabbit polyclonal antibody to SNAP25 (cat. no. SL3730) from BIOMOL/ARP. Using a SNAP25 antibody from Sternberger on well-fixed mouse retina, Grabs et al. (1996) reported a lack of labeling of photoreceptor terminals at the EM level and surmised that the OPL labeling observed at the light level arose from horizontal cells. Our pre-embedding immunoelectron microscopic analysis of SNAP25 (SySy, cl. 71.1) immunolabeling in rabbit outer retina confirmed the subcellular localization to the lateral elements at triad synapses of cone and rod photoreceptors (Fig. 4), corresponding to horizontal cell endings, consistent with the clear labeling of horizontal cell dendritic and axonal processes and endings seen at the light level (Fig. 1G). In monkey retina (Fig. 3), the strongest immunoreactivity was in the two plexiform layers; however, faint labeling of cell bodies in the ONL and INL could be observed with longer exposures.
Although we can speculate that the different labeling patterns likely arise from the differential plasma membrane interactions of the two splice variants of SNAP25 through palmitoylation, or the interaction of SNAP25 in different combinations of SNARE protein complexes (conformation or accessibility), or even different phosphorylation states, it is difficult at present to say how these variables contribute to the SNAP25 patterns observed. Without new tools that can discriminate between SNAP25a and -b isoforms, it remains unclear whether synapses contain one or the other, or some combination in the mature retina.
When the data are taken together, SNAP25 is shown to be consistently and strongly expressed in the processes and endings of mammalian horizontal cells, suggestive of a role in exocytosis. The various labeling patterns observed in the retina using different commercially available SNAP25 antibodies are consistent with multiple roles of SNAP25 in vesicle trafficking, such as transmitter release, neurite elongation, calcium regulation, and protein trafficking, and likely arise from differential splicing and post-translational modifications, most prominently palmitoylation.
Acknowledgments
We gratefully acknowledge the excellent technical support of Anja Staab, Gong-Sun Nam, and Walter Hofer at the MPIH, Frankfurt, Germany, and Alejandro Vila and Rebecca Jones at UCLA. We thank Dr. S. Haverkamp for the monkey sections. We thank Drs. H. Wässle, I. Raymond, and S. Stella for helpful discussions during the course of this work.
Grant sponsor: National Institutes of Health; Grant number: EY15573; Grant sponsors: Veterans Administration Career Scientist Award; University of California-Los Angeles Stein-Oppenheimer Award (to N.C.B.); German Research Foundation; Grant number: BR 1643/4-1 (to J.H.B.).
LITERATURE CITED
- Bark IC. Structure of the chicken gene for SNAP-25 reveals duplicated exon encoding distinct isoforms of the protein. J Mol Biol. 1993;233:67–76. doi: 10.1006/jmbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- Bark IC, Wilson MC. Human cDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25. Gene. 1994;139:291–292. doi: 10.1016/0378-1119(94)90773-0. [DOI] [PubMed] [Google Scholar]
- Bark IC, Hahn KM, Ryabinin AE, Wilson MC. Differential expression of SNAP-25 protein isoforms during divergent vesicle fusion events of neural development. Proc Natl Acad Sci U S A. 1995;92:1510–1514. doi: 10.1073/pnas.92.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Brecha NC. Expression of different VAMP isoforms in the mouse outer retina. Invest Ophthalmol Vis Sci. 2006;47 E-Abstract 3720. [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Boschert U, O’Shaughnessy C, Dickinson R, Tessari M, Bendotti C, Catsicas S, Pich EM. Developmental and plasticity-related differential expression of two SNAP-25 isoforms in the rat brain. J Comp Neurol. 1996;367:177–193. doi: 10.1002/(SICI)1096-9861(19960401)367:2<177::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bragina L, Candiracci C, Barbaresi P, Giovedi S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience. 2007;146:1829–1840. doi: 10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Dick O, Boeckers TM. The postsynaptic scaffold proteins ProSAP1/Shank2 and Homer1 are associated with glutamate receptor complexes at rat retinal synapses. J Comp Neurol. 2004;475:551–563. doi: 10.1002/cne.20194. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Wässle H, Betz H, Morgans CW. The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. Eur J Neurosci. 1996;8:823–828. doi: 10.1111/j.1460-9568.1996.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- Bruns D, Engers S, Yang C, Ossig R, Jeromin A, Jahn R. Inhibition of transmitter release correlates with the proteolytic activity of tetanus toxin and botulinus toxin A in individual cultured synapses of Hirudo medicinalis. J Neurosci. 1997;17:1898–1910. doi: 10.1523/JNEUROSCI.17-06-01898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas S, Larhammar D, Blomqvist A, Sanna PP, Milner RJ, Wilson MC. Expression of a conserved cell-type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci U S A. 1991;88:785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas S, Catsicas M, Keyser KT, Karten HJ, Wilson MC, Milner RJ. Differential expression of the presynaptic protein SNAP-25 in mammalian retina. J Neurosci Res. 1992;33:1–9. doi: 10.1002/jnr.490330102. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann N Y Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chen ST, Garey LJ, Jen LS. Immunoreactivity to synaptosomal-associated protein-25 in developing rat retinas: effects of a glutamate agonist and retinal transplantation to a host brain. J Hirnforsch. 1998;39:253–262. [PubMed] [Google Scholar]
- Chun MH, Wässle H. Some horizontal cells of the bovine retina receive input synapses in the inner plexiform layer. Cell Tissue Res. 1993;272:447–457. doi: 10.1007/BF00318551. [DOI] [PubMed] [Google Scholar]
- Connell E, Darios F, Peak-Chew S, Soloviev M, Davletov B. N-terminal acetylation of the neuronal protein SNAP-25 is revealed by the SMI81 monoclonal antibody. Biochemistry. 2009;48:9582–9589. doi: 10.1021/bi9012403. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Lewis GP, Fisher SK, Kolb H. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J Neurocytol. 2002;31:649–666. doi: 10.1023/a:1025791512555. [DOI] [PubMed] [Google Scholar]
- Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J Comp Neurol. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Frassoni C, Inverardi F, Coco S, Ortino B, Grumelli C, Pozzi D, Verderio C, Matteoli M. Analysis of SNAP-25 immunoreactivity in hippocampal inhibitory neurons during development in culture and in situ. Neuroscience. 2005;131:813–823. doi: 10.1016/j.neuroscience.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Garbelli R, Inverardi F, Medici V, Amadeo A, Verderio C, Matteoli M, Frassoni C. Heterogeneous expression of SNAP-25 in rat and human brain. J Comp Neurol. 2008;506:373–386. doi: 10.1002/cne.21505. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Baetens D, Roth J, Norman AW, Orci L. Immunohistochemical mapping of calcium-binding protein immunoreactivity in the rat central nervous system. Brain Res. 1984;296:75–86. doi: 10.1016/0006-8993(84)90512-2. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud S, Pralong W, Riederer BM, Eder L, Catsicas S, Muller D. Activity-dependent phosphorylation of SNAP-25 in hippocampal organotypic cultures. J Neurochem. 1999;72:1699–1706. doi: 10.1046/j.1471-4159.1999.721699.x. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Gordon DE, Bond LM, Sahlender DA, Peden AA. A targeted siRNA screen to identify SNAREs required for constitutive secretion in mammalian cells. Traffic. 2010;11:1191–1204. doi: 10.1111/j.1600-0854.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Urban M, Post A, Gratzl M. Rab3 proteins and SNAP-25, essential components of the exocytosis machinery in conventional synapses, are absent from ribbon synapses of the mouse retina. Eur J Neurosci. 1996;8:162–168. doi: 10.1111/j.1460-9568.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Grant NJ, Hepp R, Krause W, Aunis D, Oehme P, Langley K. Differential expression of SNAP-25 isoforms and SNAP-23 in the adrenal gland. J Neurochem. 1999;72:363–372. doi: 10.1046/j.1471-4159.1999.0720363.x. [DOI] [PubMed] [Google Scholar]
- Greaves J, Prescott GR, Gorleku OA, Chamberlain LH. Regulation of SNAP-25 trafficking and function by palmitoylation. Biochem Soc Trans. 2010;38:163–166. doi: 10.1042/BST0380163. [DOI] [PubMed] [Google Scholar]
- Greenlee MH, Roosevelt CB, Sakaguchi DS. Differential localization of SNARE complex proteins SNAP-25, syntaxin, and VAMP during development of the mammalian retina. J Comp Neurol. 2001;430:306–320. doi: 10.1002/1096-9861(20010212)430:3<306::aid-cne1032>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Greenlee MH, Swanson JJ, Simon JJ, Elmquist JK, Jacobson CD, Sakaguchi DS. Postnatal development and the differential expression of presynaptic terminal-associated proteins in the developing retina of the Brazilian opossum, Monodelphis domestica. Brain Res Dev Brain Res. 1996;96:159–172. [PubMed] [Google Scholar]
- Greenlee MH, Wilson MC, Sakaguchi DS. Expression of SNAP-25 during mammalian retinal development: thinking outside the synapse. Semin Cell Dev Biol. 2002;13:99–106. doi: 10.1016/s1084-9521(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. doi: 10.1002/cne.903350405. [DOI] [PubMed] [Google Scholar]
- Guo C, Stella SL, Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol. 2009;512:6–26. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–1669. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Yan YH, Erickson A, Possin D, Pow D. Expression patterns of calretinin, calbindin and parvalbumin and their colocalization in neurons during development of Macaca monkey retina. Exp Eye Res. 2007;85:587–601. doi: 10.1016/j.exer.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hess DT, Slater TM, Wilson MC, Skene JH. The 25 kDa synaptosomal-associated p nsport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12:4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Brecha NC. Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J Comp Neurol. 2005;488:70–81. doi: 10.1002/cne.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Barnes S, Stella SL, Jr, Brecha NC. Information processing: horizontal cells. In: Dartt DA, editor. Encyclopedia of the eye. Vol. 2. Oxford: Academic Press; 2010. pp. 363–371. [Google Scholar]
- Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, Jahn R. Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem. 2006;281:17076–17083. doi: 10.1074/jbc.M513838200. [DOI] [PubMed] [Google Scholar]
- Honer WG, Hu L, Davies P. Human synaptic proteins with a heterogeneous distribution in cerebellum and visual cortex. Brain Res. 1993;609:9–20. doi: 10.1016/0006-8993(93)90848-h. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Young C, Wang T, Xie J, Bonner J, Hu L, Boulianne GL, Luo Z, Trimble WS. Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience. 1997;78:99–110. doi: 10.1016/s0306-4522(96)00489-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kataoka M, Sekiguchi M, Shimazaki Y, Sato K, Takahashi M. Two distinct mechanisms underlie the stimulation of neurotransmitter release by phorbol esters in clonal rat pheochromocytoma PC12 cells. J Biochem. 2000;128:407–414. doi: 10.1093/oxfordjournals.jbchem.a022768. [DOI] [PubMed] [Google Scholar]
- Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J Comp Neurol. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- Kataoka M, Kuwahara R, Iwasaki S, Shoji-Kasai Y, Takahashi M. Nerve growth factor-induced phosphorylation of SNAP-25 in PC12 cells: a possible involvement in the regulation of SNAP-25 localization. J Neurochem. 2000;74:2058–2066. doi: 10.1046/j.1471-4159.2000.0742058.x. [DOI] [PubMed] [Google Scholar]
- Kataoka M, Kuwahara R, Matsuo R, Sekiguchi M, Inokuchi K, Takahashi M. Development- and activity-dependent regulation of SNAP-25 phosphorylation in rat brain. Neurosci Lett. 2006;407:258–262. doi: 10.1016/j.neulet.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Lane SR, Liu Y. Characterization of the palmitoylation domain of SNAP-25. J Neurochem. 1997;69:1864–1869. doi: 10.1046/j.1471-4159.1997.69051864.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur J Neurosci. 2010;31:1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J Biol Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Pozzi D, Grumelli C, Condliffe SB, Frassoni C, Harkany T, Verderio C. The synaptic split of SNAP-25: different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158:223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122:2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Battenberg E, Wilson MC. SNAP-25 and synaptotagmin involvement in the final Ca(2+)-dependent triggering of neurotransmitter exocytosis. Proc Natl Acad Sci U S A. 1996;93:10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Presynaptic proteins of ribbon synapses in the retina. Microsc Res Tech. 2000;50:141–150. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Morgans C, Brandstätter JH. SNAP-25 is present on the Golgi apparatus of retinal neurons. Neuroreport. 2000;11:85–88. doi: 10.1097/00001756-200001170-00017. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brandstätter JH, Kellerman J, Betz H, Wässle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J Neurosci. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sørensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Nagy G, Milosevic I, Fasshauer D, Muller EM, de Groot BL, Lang T, Wilson MC, Sørensen JB. Alternative splicing of SNAP-25 regulates secretion through nonconservative substitutions in the SNARE domain. Mol Biol Cell. 2005;16:5675–5685. doi: 10.1091/mbc.E05-07-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Milosevic I, Mohrmann R, Wiederhold K, Walter AM, Sørensen JB. The SNAP-25 linker as an adaptation toward fast exocytosis. Mol Biol Cell. 2008;19:3769–3781. doi: 10.1091/mbc.E07-12-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler GA, Polli JW, Wilson MC, Billingsley ML. Proc Natl Acad Sci USA. 1991;88:5247–5251. doi: 10.1073/pnas.88.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- Pozzi D, Condliffe S, Bozzi Y, Chikhladze M, Grumelli C, Proux-Gillardeaux V, Takahashi M, Franceschetti S, Verderio C, Matteoli M. Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci U S A. 2008;105:323–328. doi: 10.1073/pnas.0706211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott GR, Gorleku OA, Greaves J, Chamberlin LH. Palmitoylation of the synaptic vesicle machinery. J Neurochem. 2009;110:1135–1149. doi: 10.1111/j.1471-4159.2009.06205.x. [DOI] [PubMed] [Google Scholar]
- Puffer EB, Lomneth RB, Sarkar HK, Singh BR. Differential roles of developmentally distinct SNAP-25 isoforms in the neurotransmitter release process. Biochemistry. 2001;40:9374–9378. doi: 10.1021/bi010362z. [DOI] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Renteria RC, Strehler EE, Copenhagen DR, Krizaj D. Ontogeny of plasma membrane Ca2+ ATPase isoforms in the neural retina of the postnatal rat. Vis Neurosci. 2005;22:263–274. doi: 10.1017/S0952523805223027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci U S A. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Heizmann CW. Immunocytochemical labeling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Boycott BB. Horizontal cells in the monkey retina: immunocytochemical staining with antibodies against calcium binding proteins. Eur J Neurosci. 1989;1:407–420. doi: 10.1111/j.1460-9568.1989.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Rossi C, Strettoi E, Galli-Resta L. The spatial order of horizontal cells is not affected by massive alterations in the organization of other retinal cells. J Neurosci. 2003;23:9924–9928. doi: 10.1523/JNEUROSCI.23-30-09924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem. 2005;280:19449–19453. doi: 10.1074/jbc.M501923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335:99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Stenbeck G, Rothman JE, Söllner TH. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci U S A. 1997;94:997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci U S A. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH. Three novel proteins of the syntaxin/SNAP-25 family. J Biol Chem. 1998;273:34171–34179. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Noguchi J, Ohno M, Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafoya LC, Shuttleworth CW, Yanagawa Y, Obata K, Wilson MC. The role of the t-SNARE SNAP-25 in action potential-dependent calcium signaling and expression in GABAergic and glutamatergic neurons. BMC Neurosci. 2008;9:105. doi: 10.1186/1471-2202-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi D, Wiser O, Trus M, Atlas D. N-type voltage-sensitive calcium channel interacts with syntaxin, synaptotagmin and SNAP-25 in a multiprotein complex. Receptors Channels. 1998;6:89–98. [PubMed] [Google Scholar]
- Ullrich B, Südhof TC. Distribution of synaptic markers in the retina: implications for synaptic vesicle traffic in ribbon synapses. J Physiol Paris. 1994;88:249–257. doi: 10.1016/0928-4257(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Veit M. Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem J. 2000;3451:145–151. [PMC free article] [PubMed] [Google Scholar]
- Veit M, Söllner TH, Rothman JE. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 1996;385:119–123. doi: 10.1016/0014-5793(96)00362-6. [DOI] [PubMed] [Google Scholar]
- Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, Proux-Gillardeaux V, Galli T, Rossetto O, Frassoni C, Matteoli M. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F, Link E, Südhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur J Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wässle H, Dacey DM, Haun T, Haverkamp S, Grünert U, Boycott BB. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Vis Neurosci. 2000;17:591–608. doi: 10.1017/s0952523800174097. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rammner B, Margittai M, Artalejo AR, Neher E, Jahn R. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]


