Abstract
Gastrointestinal symptoms are a common manifestation of adverse drug effects. Non-steroid anti-inflammatory drugs (NSAIDs) are widely prescribed drugs that induce the serious side effect of gastric mucosal ulceration. Biomarkers for these side effects have not been identified and ulcers are now only detectable by endoscopy. We previously identified five metabolites as biomarker candidates for NSAID-induced gastric ulcer using capillary electrophoresis–mass spectrometry (CE–MS)-based metabolomic analysis of serum and stomach from rats. Here, to clarify mechanism of changes and limitations of indications of biomarker candidates, we performed CE–MS-based metabolomic profiling in stomach and serum from rats with gastric ulcers induced by ethanol, stress, and aspirin. The results suggest that a decrease in hydroxyproline reflects the induction of gastric injury and may be useful in identifying gastric ulcer induced by multiple causes. While extrapolation to humans requires further study, hydroxyproline can be a new serum biomarker of gastric injury regardless of cause.
Keywords: metabolomics, capillary electrophoresis–mass spectrometry (CE–MS), gastric injury, diagnostic marker candidate
Introduction
Gastrointestinal complaints are common manifestations of adverse drug effects.1 Non-steroid anti-inflammatory drugs (NSAIDs) are useful drugs for a range of conditions, including rheumatoid arthritis, osteoarthritis, and others.2 However, they have serious adverse effects on gastric mucosa, and an association between use of NSAIDs and admission to hospital for upper gastrointestinal hemorrhage and perforation and other upper gastrointestinal events has been established.3 Presently, however, gastric ulceration and hemorrhage are only detectable by endoscopy,4 and no serum biomarkers for NSAID-induced gastric ulcer have yet been identified.
We previously reported five biomarker candidates in serum that predict gastric injury induced by NSAIDs in rats.5 We also reported that the decrease in hydroxyproline in stomach induced by aspirin recovered to the control level on co-administration with omeprazole and famotidine. In contrast, the levels of the other metabolites in stomach did not change on co-administration with these agents.6 With regard to the mechanism of change, we speculated that the changes in hydroxyproline were due to a lesion-induced decrease in the amount of collagen in stomach tissue, whereas those in the other metabolites were due to NSAID-induced depression of mitochondrial function.6
Gastric ulcer is thought to result from an imbalance between damaging factors within the lumen and protective mechanisms in the gastric mucosa. Ethanol, stress, and NSAIDs are known to cause severe gastric irritation, but the mechanism is still poorly understood. Non-NSAID experimental models of gastric ulcer induction include those based on ethanol and stress.7 Ethanol is known to cause gastric damage via its alteration of protective factors, including decreasing mucus production and blood circulation within the mucosa. In addition, ethanol may also cause gastric damage via the generation of reactive species, decreased cell proliferation, and an exacerbated inflammatory response.8 Long-term psychological stress also leads to ulceration by stimulating the secretion of acid and pepsin and/or by decreasing mucosal defense.9 Several mechanisms to explain the gastric ulceration associated with NSAID use have been suggested, including increased permeability of the stomach,10 inhibition of prostaglandin synthesis, mitochondria dysfunction,11 or a combination of these. While details of the mechanism of gastric ulceration remain to be elucidated, the common point of these models is that all induce gastric ulcer as an endpoint. Comparison of the metabolic profile of these three models should cast light on the mechanism of changes and limitations of indications of biomarker candidates for NSAIDs-induced gastric injury identified in the previous studies.5
Metabolomics is a rapidly evolving technology that identifies metabolites or metabolic pathways that are changed by the pathogenesis of a disease or injury by comparison with concentrations of endogenous metabolites between patients and controls. Several analytical methods have been used to analyze concentrations of endogenous metabolites,12–16 including CE–MS. This method provides rapid analysis and efficient resolution with high selectivity and sensitivity, and is a powerful tool for metabolomics analysis.17 The efficacy of this approach is demonstrated by the various biomarker identification studies that have used it.18–20
Here, to clarify the mechanism of changes and limitations of identified biomarker candidates in NSAIDs-induced gastric injury, we applied a shotgun metabolomics approach based on CE-time of flight (TOF)–MS profiles of endogenous metabolites to rat models of gastric ulcer induced by ethanol, stress, and aspirin. We then determined the serum concentrations of these biomarkers.
Materials and Methods
Study design
Male Sprague-Dawley rats were obtained from Charles River Japan (Yokohama, Japan) and maintained under controlled environmental conditions, with free access to a pellet diet (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and filtered tap water (containing 2 ± 1 ppm-free chlorine adjusted with sodium hypochlorite). After acclimatization for three days, the animals were used in the study at six weeks old and weighed 140–180 g.
A total of 20 animals (n = 8 animals for the control group, n = 4 animals per treatment group) were fasted for 24 hours in wire mesh cages but allowed water ad libitum and assigned to a control group (0.5% methylcellulose, p.o.) or one of the three treatment groups: (1) single-dose ethanol (Nacalai Tesque, Kyoto, Japan) at 5 mL/kg, p.o. (ethanol-induced model), (2) single-dose 0.5% methylcellulose, p.o. (stress-induced model), or (3) single-dose aspirin (Nacalai Tesque, Kyoto, Japan) at 300 mg/kg, p.o. (aspirin-induced model). The animals assigned to the stress-induced model were kept in a cold room at 6 °C for five hours after administration.
All experiments were carried out at the Kashima facilities of Astellas Pharma Inc., which have been awarded accreditation status by the AAALAC International. This study was approved by the Animal Ethical Committee of Astellas Pharma Inc. (Tokyo, Japan).
Sample collection
Rats were anesthetized with isoflurane and exsanguinated via the abdominal aorta at five hours after dosing. Serum and stomach were collected in the same way as in our previous study.5 The interior of the stomach was macroscopically assessed, and the dimensions of observed gastric ulcers were determined from photographs using WinROOF image analysis software (Mitani Corporation, Tokyo, Japan). All samples were stored at −70 °C until sample preparation.
Analysis of metabolite concentration
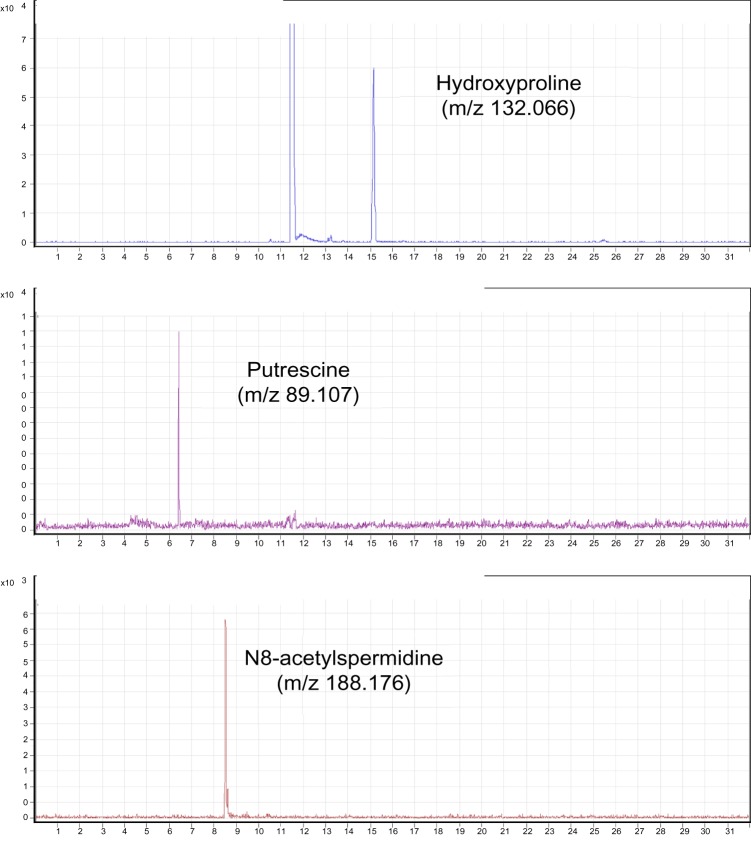
Measurement of metabolite concentrations in stomach and serum was done in the same way as described in our previous article.6 Stomach samples were homogenized in methanol containing internal standards (10 μM each of methionine sulfone [Wako, Osaka, Japan], D-camphor-10-sulfonic acid [CAS; Wako], and 2-(n-morpholino)ethanesulfonic acid [MES; Dojindo, Kumamoto, Japan]), and the supernatant was collected. Serum samples were added to methanol containing internal standards and mixed well. Deionized water and chloroform were then added, and the upper aqueous layer was collected. Following centrifugal filtration through a Millipore 5-kDa cutoff filter, the filtrate was lyophilized and dissolved in 50 μL of Milli-Q water containing reference compounds (200 μM 3-aminopyrrolidine and 200 μM trimesate). Metabolite standards for identification of metabolites and CE-TOF–MS conditions for metabolite analysis were as used in our previous study.6 All CE-TOF–MS experiments were performed using an Agilent 1600 CE capillary electrophoresis system (Agilent Technologies, Waldbronn, Germany), Agilent G3250AA LC/MSD TOF–MS system (Agilent Technologies, Palo Alto, CA, USA), Agilent 1100 isocratic HPLC pump, Agilent G1603A CE–MS adapter kit, and Agilent G1607A CE-electro spray ionization (ESI)–MS sprayer kit. For anion analysis, the Agilent G7100–60041 platinum needle was replaced with the original Agilent stainless steel ESI needle.21 System control and data acquisition were done using the Agilent G2201AA ChemStation software for CE and Analyst QS software for TOF–MS. Annotation tables were generated by the same method used in our previous study.5,6
Statistical analysis
To identify the biomarker candidates, the unpaired t-test (comparison of means of two samples with equal variance) was performed for control and individual groups to find metabolites that showed changes common to all three models. Metabolite levels between groups were compared using ANOVA and Dunnett’s test using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA, USA).
Results
Gastric ulceration
The severity of gastric ulceration is presented as ulcerative area for each group (Table 1). All treated groups showed gastric ulceration. In contrast, no gastric ulceration was noted in control animals.
Table 1.
Severity of gastric ulceration evaluated as area of ulceration.
| MODEL | CONTROL | ETHANOL | STRESS | ASPIRIN |
|---|---|---|---|---|
| Test article | Vehicle | Ethanol 5 mL/kg | Vehicle | Aspirin 300 mg/kg |
| Room temperature (for 5 hr after dosing) | 19 to 25 °C | 19 to 25 °C | 6 °C | 19 to 25 °C |
| Number of animals | 8 | 4 | 4 | 4 |
| Severity of gastric ulceration | ND (0/8) | 12.42 ± 5.90 (4/4) | 2.30 ± 1.47 (4/4) | 1.58 ± 1.60 (4/4) |
Notes: Data are expressed as mean + standard deviation of the area of ulceration (mm2). Values in parentheses are the incidence rate of animals with gastric ulceration.
Abbreviation: ND, not detected.
Metabolomic analysis of stomach tissue extracts
A total of 576 peaks (Supplementary Table S1) were identified and quantified with metabolite standards matching the closest m/z value and normalized migration time for further statistical comparison and interpretations using the CE-TOF–MS system. Although additional unnamed analytes were observed, we discuss only identified metabolites in the present study.
Metabolites that were changed in all models are summarized in Table 2 and those that were changed in each model are listed in Supplementary Table S2. In all, 57, 39, and 80 metabolites were changed in the ethanol-, stress-, and aspirin-induced models, respectively. A decrease in the level of hydroxyproline and increase in those of putrescine and N8-acetylspermidine were commonly observed in all models. The levels of these metabolites in stomach are shown in Figure 1, and their selected CE-TOF–MS ion electropherograms in Figure 2. Levels of spermine, spermidine, and N1-acetylspermidine, which are related metabolites of putrescine and N8-acetylspermidine, are shown in Figure 3. These polyamines did not increase except aspirin-induced model. Other biomarker candidates reported in our previous study5 did not show a similar change in the ethanol- and stress-induced gastric ulcer models.
Table 2.
Metabolites whose levels changed in all models.
| METABOLITE | MODEL | ||
|---|---|---|---|
| ETHANOL | STRESS | ASPIRIN | |
| Hydroxyproline | Decrease (P < 0.01) | Decrease (P < 0.05) | Decrease (P < 0.01) |
| N8-Acetylspermidine | Increase (P < 0.05) | Increase (P < 0.05) | Increase (P < 0.01) |
| Putrescine | Increase (P < 0.01) | Increase (P < 0.01) | Increase (P < 0.01) |
| Creatinine | Decrease (P < 0.05) | Decrease (P < 0.05) | Increase (P < 0.01) |
| Xanthine | Decrease (P < 0.05) | Increase (P < 0.01) | Decrease (P < 0.01) |
Notes: Values in parentheses are statistically significant differences (t-test between model and control). All metabolites that changed in each model are listed in Supplementary Table S2.
Figure 1.
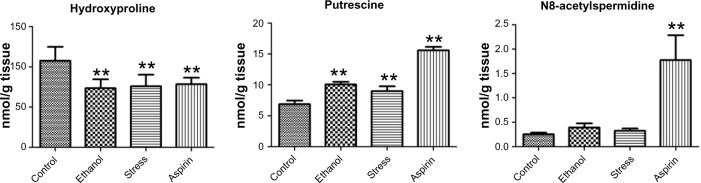
Levels of hydroxyproline, putrescine, and N8-acetylspermidine in stomach.
Notes: Data are reported as means ± standard deviation of four or eight animals per group. Asterisks indicate statistically significant differences: **P < 0.01, *P < 0.05.
Figure 2.
Selected CE-TOF–MS ion electropherograms for metabolites in stomach.
Figure 3.
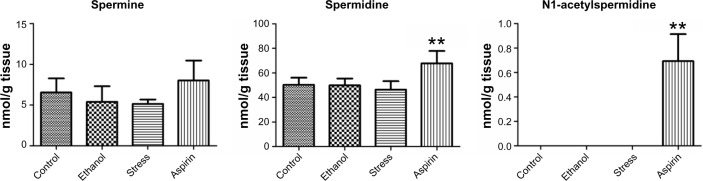
Levels of spermine, spermidine, and N1-acetylspermidine in stomach.
Notes: Data are reported as means ± standard deviation of four or eight animals per group. Asterisks indicate statistically significant differences: **P < 0.01, *P < 0.05.
Metabolomic analysis of serum
Serum concentrations of hydroxyproline and putrescine are shown in Figure 4. Statistical analysis of quantitative differences between groups showed decreases in the levels of hydroxyproline in all models. In contrast, changes in the levels of putrescine were not observed in all models. Further, the serum concentration of N8-acetylspermidine was not determined because it was below detection limits.
Figure 4.
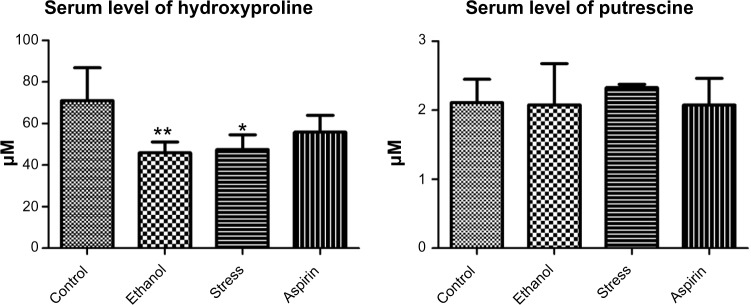
Levels of hydroxyproline, putrescine, and N8-acetylspermidine in serum.
Notes: Data are reported as means ± standard deviation of four or eight animals per group. Asterisks indicate statistically significant differences: **P < 0.01, *P < 0.05.
Discussion
Here, we investigated limitations of indications of previously reported biomarker candidates of NSAID-induced gastric injury using a shotgun metabolomics approach based on CE-TOF–MS profiles of endogenous metabolites in ethanol-, stress-, and aspirin-induced models of gastric ulcer in rats.5 We identified a decrease in hydroxyproline as a common and useful biomarker candidate in all three models. Given that this decrease reflects the induction of gastric ulcer and is monitorable in serum, monitoring of serum concentrations of hydroxyproline should prove useful in identifying the induction of gastric ulcer regardless of cause.
Gastric ulcer is thought to result from an imbalance between damaging factors within the lumen and protective mechanisms in the gastric mucosa, but the mechanism of gastric ulcer formation is not precisely known. Ethanol- and stress-induced gastric ulcer models are often used as non-NSAID experimental models of gastric ulcer.7 While details of the mechanism of gastric ulceration remain to be elucidated, the common point of these models is that they all induce gastric ulcer as an endpoint. Comparison of the metabolic profiles of different experimental models of gastric ulcer should therefore be useful in grasping the mechanism of change and limitations of indications of biomarkers identified in the previous study.5 In the present study, a decrease in the level of hydroxyproline was commonly observed in all models. In the previous study,5 we considered that similar changes induced by NSAIDs are indicative of decreased collagen levels in the stomach, because hydroxyproline is a modified amino acid specifically found in collagen and increased collagenase activity and decreased collagen levels in stomach tissue aӼicted with NSAID-induced ulcers have been reported in the previous study.22 Given that increased collagenase activity and decreased collagen levels in stomach tissue were also observed in many experimental gastric ulcer models,23–25 we considered that the decrease in the hydroxyproline level in all models was also indicative of the decrease in the amount of collagen in stomach tissue. Given the findings that the number of collagen fibers also decreases with the gastric ulcer induced by Helicobacter pylori (H. pylori) infection26 a major cause of human gastric ulcer, we also speculate that a decrease in hydroxyproline levels will also be seen with H. pylori infection-induced gastric ulcer.
Increases in the level of putrescine and N8-acetylspermidine were also commonly observed in all models. Putrescine is the precursor of spermidine and spermine. They and their acetylated derivatives (N1- and N8-acetylspermidine) are considered to be universally distributed in living cells and have been postulated to play important roles in the control of cellular growth.27 Elevated spermine, spermidine, and N1- and N8-acetylspermidine production has been reported at inflammatory sites.28,29 As shown in Figure 3, levels of spermine and spermidine were not increased in the ethanol- or stress-induced models, and N1-acetylspermidine was detected only in the aspirin-induced model. Given that an increase in putrescine and N8-acetylspermidine was also clearly observed in the aspirin-induced model and that all these metabolites changed in the same manner, these changes are thought to correlate with each other and to arise from the same cause, gastric inflammation.
Although the decrease in hydroxyproline was also observed in serum in all three models, an increase in putrescine in serum was not observed in all models. Further, the serum concentration of N8-acetylspermidine could not be determined because it was below detection limits in all three models. This indicates that although hydroxyproline can be a serum biomarker for gastric ulcer, putrescine and N8-acetylspermidine cannot be used or are not sensitive in serum.
In conclusion, this study aimed to clarify the mechanism of change and limitation of indications of biomarker candidates for NSAID-induced gastric injury reported in our previous study5 by comparing metabolic profiles of stomach and serum obtained from ethanol-, stress-, and aspirin-induced gastric ulcer models, which induced gastric ulcer by different mechanisms. This approach revealed a decrease in levels of hydroxyproline and increase in levels of putrescine and N8-acetylspermidine in stomach extracts in all experimental gastric ulcer models. Apart from hydroxyproline, however, none of the other biomarker candidates previously identified in the aspirin-induced model changed in the same manner in the ethanol- and stress-induced models. Moreover, hydroxyproline was the only candidate to show a decrease in serum in all the three models. We therefore conclude that the serum level of hydroxyproline reflects the induction of gastric injury regardless of the cause of the injury.
Supplementary Data
Supplementary Table 1. The list of analyzed metabolites.
Supplementary Table 2. The list of metabolites changed in each model.
Acknowledgments
We are grateful to Eisuke Kobayashi and Yutaka Nakahara for their technical assistance in caring for the rats, preparing samples, and estimating the dimensions of observed gastric ulcers.
Footnotes
Author Contributions
All authors conceived and designed the experiments. KT, MO, KE, KS, and TS analyzed the data. KT, KS, and TS wrote the first draft of the manuscript. All authors contributed to the writing of the manuscript, agreed with manuscript results and conclusions, and jointly developed the structure and arguments for the paper. KT, HN, KS, TO, JS, YM, MH, and TS made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
FUNDING: This work was supported by a grant from the Ministry of Health, Labour and Welfare, Drug Discovery Platform Research (H20-bio-ippan-011).
ACADEMIC EDITOR: Karen Pulford, Associate Editor
COMPETING INTERESTS: MH discloses grants from the Japan Society for the Promotion of Science, outside the submitted work. Other authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Devi DP, Sushma M, Guido S. Drug-induced upper gastrointestinal disorders requiring hospitalization: a five-year study in a South Indian hospital. Pharmacoepidemiol Drug Saf. 2004;13:859–62. doi: 10.1002/pds.988. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Fehring RA. Trends in the utilization of non-steroidal anti-inflammatory drugs in the United States, 1986–1990. J Clin Epidemiol. 1992;45:1041–4. doi: 10.1016/0895-4356(92)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald TM, Morant SV, Robinson GC, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997;315:1333–7. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmeyer J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000;2:379–85. doi: 10.1186/ar116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Ohishi M, Ota S, et al. Metabolic profiling to identify potential serum biomarkers for gastric ulceration induced by nonsteroid anti-inflammatory drugs. J Proteome Res. 2013;12:1399–407. doi: 10.1021/pr3010452. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi K, Ohishi M, Endo K, et al. Metabolomic analysis of the effects of omeprazole and famotidine on aspirin-induced gastric injury. Metabolomics. In press. [Google Scholar]
- 7.Glavin GB, Szabo S. Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J. 1992;6:825–31. doi: 10.1096/fasebj.6.3.1740232. [DOI] [PubMed] [Google Scholar]
- 8.Amaral GP, de Carvalho NR, Barcelos RP, et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 2013;55:48–55. doi: 10.1016/j.fct.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Chandranath SI, Bastaki SM, D’Souza A, Adem A, Singh J. Attenuation of stress-induced gastric lesions by lansoprazole, PD-136450 and ranitidine in rats. Mol Cell Biochem. 2011;349:205–12. doi: 10.1007/s11010-010-0675-3. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland LR, Verhoef M, Wallace JL, Van Rosendaal G, Crutcher R, Meddings JB. A simple, non-invasive marker of gastric damage: sucrose permeability. Lancet. 1994;343:998–1000. doi: 10.1016/s0140-6736(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 11.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54:101–27. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nat Biotechnol. 2000;18:1157–61. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- 13.Schauer N, Semel Y, Roessner U, et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat Biotechnol. 2006;24:447–54. doi: 10.1038/nbt1192. [DOI] [PubMed] [Google Scholar]
- 14.Monton MR, Soga T. Metabolome analysis by capillary electrophoresis–mass spectrometry. J Chromatogr A. 2007;1168:237–46. doi: 10.1016/j.chroma.2007.02.065. discussion 236. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–61. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 16.Plumb R, Granger J, Stumpf C, Wilson ID, Evans JA, Lenz EM. Metabonomic analysis of mouse urine by liquid-chromatography-time of flight mass spectrometry (LC-TOFMS): detection of strain, diurnal and gender differences. Analyst. 2003;128:819–23. doi: 10.1039/b304296k. [DOI] [PubMed] [Google Scholar]
- 17.Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res. 2003;2:488–94. doi: 10.1021/pr034020m. [DOI] [PubMed] [Google Scholar]
- 18.Soga T, Baran R, Suematsu M, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–76. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 19.Soga T, Sugimoto M, Honma M, et al. Serum metabolomics reveals gammaglutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55:896–905. doi: 10.1016/j.jhep.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama A, Nakashima E, Sugimoto M, et al. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem. 2012;404:3101–9. doi: 10.1007/s00216-012-6412-x. [DOI] [PubMed] [Google Scholar]
- 21.Soga T, Igarashi K, Ito C, Mizobuchi K, Zimmermann HP, Tomita M. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem. 2009;81:6165–74. doi: 10.1021/ac900675k. [DOI] [PubMed] [Google Scholar]
- 22.Hasebe T, Harasawa S, Miwa T, Shibata T, Inayama S. Collagen and collagenase in ulcer tissue – 1. The healing process of acetic acid ulcers in rats. Tokai J Exp Clin Med. 1987;12:147–58. [PubMed] [Google Scholar]
- 23.Hasebe T. Collagen and collagenase in ulcer tissue – 2. Restraint and water immersion induced gastric lesions and effects of cimetidine and misoprostol. Tokai J Exp Clin Med. 1987;12:181–90. [PubMed] [Google Scholar]
- 24.Swarnakar S, Mishra A, Ganguly K, Sharma AV. Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res. 2007;43:56–64. doi: 10.1111/j.1600-079X.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 25.Pradeepkumar Singh L, Vivek Sharma A, Swarnakar S. Upregulation of collagenase-1 and -3 in indomethacin-induced gastric ulcer in diabetic rats: role of melatonin. J Pineal Res. 2011;51:61–74. doi: 10.1111/j.1600-079X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa C, Ihara T, Sugamata M. Ultrastructural evaluation of apoptosis induced by Helicobacter pylori infection in human gastric mucosa: novel remarks on lamina propria mucosae. Med Electron Microsc. 2000;33:82–8. doi: 10.1007/s007950070006. [DOI] [PubMed] [Google Scholar]
- 27.Raza M, Al-Shabanah OA. Simultaneous determination of different polyamines and their mono-acetylated derivatives in gastric tissue by HPLC with post-column derivatization. Sci Pharm. 2010;78:249–58. doi: 10.3797/scipharm.1001-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motawi TK, Abd Elgawad HM, Shahin NN. Modulation of indomethacin-induced gastric injury by spermine and taurine in rats. J Biochem Mol Toxicol. 2007;21:280–8. doi: 10.1002/jbt.20194. [DOI] [PubMed] [Google Scholar]
- 29.Weiss TS, Herfarth H, Obermeier F, et al. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:529–35. doi: 10.1097/00054725-200409000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. The list of analyzed metabolites.
Supplementary Table 2. The list of metabolites changed in each model.