Abstract
Standard cancer treatments trigger immune responses that may influence tumor control. The nature of these responses varies depending on the tumor and the treatment modality. We previously reported that radiation and androgen-deprivation therapy (ADT) induce tumor-associated autoantibody responses in prostate cancer patients. This follow-up analysis was conducted to assess the relationship between autoantibody responses and clinical outcome. Patients with non-metastatic prostate cancer received external beam radiation therapy (EBRT) plus neoadjuvant and concurrent androgen deprivation. Treatment-induced autoantibodies were detected in almost a third of patients receiving combinatorial ADT and EBRT. Unexpectedly, patients that developed autoantibody responses to tumor antigens had a significantly lower 5-year biochemical failure-free survival (BFFS) than patients that did not develop an autoantibody response. Thus, tumor-reactive autoantibodies may be associated with increased risk of biochemical failure and immunomodulation to prevent autoantibody development may improve BFFS for select, high-risk prostate cancer patients receiving both ADT and EBRT.
Keywords: prostate cancer, tumor-associated autoantibody, androgen deprivation therapy, external beam radiation therapy, biochemical failure
Introduction
Prostate cancer is the most common cancer in North American men. For patients with clinically localized disease, the primary treatment options are surgery or radiation therapy in the form of external beam radiation (EBRT) or brachytherapy. Patients with high-risk disease (e.g., clinical stage ≥ T3, Gleason score ≥ 8 or prostate-specific antigen (PSA) > 20) are typically offered radiation therapy in combination with androgen deprivation therapy (ADT).1,2 In these patients, treatment with ADT and EBRT is associated with a 20% rate of biochemical failure (BF) at 5 y, increasing to 50% at 10 y.3,4 Thus, improved treatments for patients with high-risk prostate cancer are required.
Intriguingly, ADT and EBRT appear to work synergistically to improve patient outcomes, whereas ADT does not improve outcomes when combined with radical prostatectomy.5 ADT is thought to increase tumor cytoreduction and promote cancer cell apoptosis while decreasing tumor hypoxia, which may enhance the tumoricidal effects of radiation.6 ADT also has immunologic effects, such as promoting thymopoiesis, which may in turn facilitate infiltration of T cells into the prostate to exert antitumor effects.7,8 Radiation treatment can improve the ability of the immune system to recognize tumors through several mechanisms, including increased expression of the death receptor Fas and enhanced antigen presentation through upregulation of major histocompatibility complex class I molecules on tumor cells.9 Additionally, there is increasing evidence that the response to radiation treatment involves a form of immunogenic cell death incurred through a mechanism that depends on recognition of necrotic tumor cells via toll-like receptor 4 (TLR4).10 This may, in part, also explain the abscopal effect, in which tumor regression occurs outside the radiation field.11 Although the precise mechanisms underlying the abscopal effect have yet to be determined, it has been proposed that inflammation and tumor cell death at the radiation site promotes anticancer immune responses that can spread to nonirradiated lesions. The combination of immunogenic tumor cell death, inflammation, and enhanced antigen presentation at the tumor site may provide the priming and activation signals necessary for sustained T-cell–mediated antitumor responses. Consequently, ADT and EBRT may act synergistically to activate immune responses against prostate cancer12,13 such that the concept of treatment-induced immune responses remains an important research topic meriting further investigation.
A central component of the humoral immune response is antibody production by B cells, which is dependent on T helper type 2 (Th2) cytokines produced by CD4+ T cells. Studies have shown that tumor-specific humoral immune responses are induced by ADT, radiation, or vaccine approaches in some prostate cancer patients.12-14 We have previously reported that the incidence of tumor-associated autoantibodies in patients receiving ADT and EBRT is increased compared with those receiving other treatment modalities, such as brachytherapy or radical prostatectomy.15 As the development of new seroreactivities suggests antigen spread, we anticipated that the presence of treatment-induced autoantibodies would correlate with improved clinical outcomes. In this study, we assessed the relationship between treatment-associated autoantibody responses and biochemical failure in a cohort of patients receiving ADT and EBRT with median follow-up of more than 5 y. Unexpectedly, the results of our study indicate that treatment-associated autoantibody responses might be associated with unfavorable patient outcomes. While striking, these observations need to be validated in a larger cohort of patients. Nonetheless, these data raise the possibility that standard treatments for prostate cancer could have important consequences regarding anticancer immunity, particularly as we move into an era when combinatorial radiation and immunotherapeutic regimens are being actively deployed in the clinic.
Results
Detection of tumor-associated autoantibodies
We collected sera samples from a total of 56 patients with non-metastatic prostate cancer who were treated with ADT and EBRT. Since it was crucial to examine treatment-induced responses, patients were excluded if they received ADT prior to their first blood collection. In addition, some patients were lost to follow-up, leaving a small but well-controlled sample of 23 patients for further analysis (Table 1). To assess treatment-associated immune responses, patient sera were immunoblotted against whole cell lysates from the human prostate cancer cell line LNCaP as previously described.15 All results were confirmed by at least 2 independent immunoblots. Sera were scored as positive if they showed the appearance of 1 or more bands by western blotting either during treatment or within 1 y following. For all cases, we tested all available time points wherever possible. Seven of the 23 (30.4%) patients developed one or more seroreactivities. Of the 16 high-risk patients, 6 (37.5%) developed autoantibody responses, as did 1 (16%) of the intermediate-risk patients. The low-risk patient that was treated with ADT and EBRT did not develop an autoantibody response. In 4 of these patients (57.1%), new seroreactivity was observed after ADT but before EBRT. Figure 1 shows western blot results for 2 healthy donor controls (Fig. 1A), 1 representative patient who did not develop autoantibodies (Fig. 1B), and 2 representative patients who scored positive for treatment-associated autoantibodies (Fig. 1C and 1D).
Table 1. Clinicopathologic characteristics of subjects included in the study.
| Number of subjects | 23 |
|---|---|
| Age, years; median (range) | 69.7 (51.3–81.2) |
|
Gleason Score, n (%) 6 7 8–10 |
7 (30.4) 11 (47.9) 5 (21.7) |
|
Stage, n (%) T1 T2 T3 |
8 (34.8) 12 (52.2) 3 (13.0) |
|
Prostate-specific antigen at diagnosis; median, ng/mL (range) |
11.0 (3.1–100) |
|
Risk group, n (%) Low Intermediate High |
1 (4.3) 6 (26.1) 16 (69.6) |
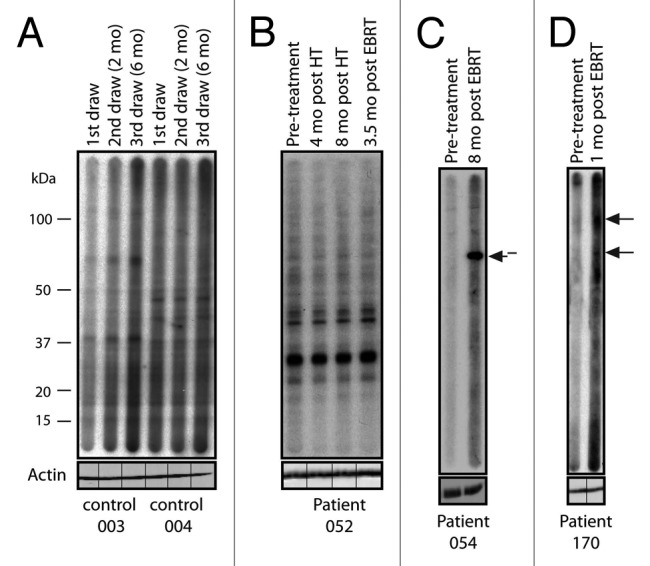
Figure 1. Treatment-associated responses to prostate tumor antigens. Western blot analysis of serum from 2 healthy donor controls and 3 patients with prostate cancer probed against LNCaP cell lysate. The timing of sample collection for each patient is indicated. New seroreactivities are indicated with arrows. (A) Two healthy donor controls showing no seroreactivity. (B) Patient 052, who did not develop an autoantibody response throughout treatment. (C) Patient 054, who was treated with androgen-deprivation therapy (ADT) plus external beam radiation therapy (EBRT) and developed a new response 8 mo post-EBRT. (D) Patient 170, who developed two new responses 1 mo post-EBRT. Each blot was re-probed with actin without the multichannel device to ensure equal protein loading across each lane. The lines indicate the original slot-blot lane for each sample.
Autoantibody responses are associated with poor prognosis following ADT and EBRT
The median follow-up time (the time from the end of treatment to the last available PSA value or death) was 73 mo (range 18–108 mo). Nine patients (39.1%) experienced biochemical failure. Specifically, 5 of 7 autoantibody-positive patients (71.4%) experienced BF compared with 4 of 16 autoantibody-negative patients (25%). By Kaplan-Meier analysis, patients who developed autoantibody responses during treatment showed a significantly higher rate of BF (Fig. 2; P = 0.025, hazard ratio [HR] = 5.99, 95% confidence interval [CI] 1.25–28.75). Multivariate analysis with risk group and autoantibody status indicated that neither risk group (P = 0.704, HR 1.342, 95% CI 0.294–6.126) nor the development of autoantibodies (P = 0.058, HR 4.283, 95% CI 0.954–19.224) was a significant independent predictor of BF (Table 2), although this is most likely due to the small number of patients in this study cohort.
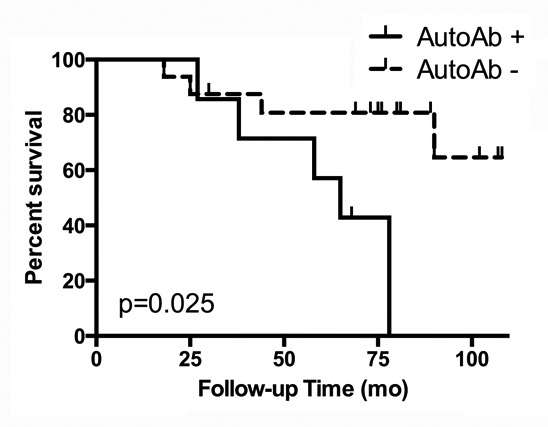
Figure 2. Treatment-associated autoantibody responses correlate with increased likelihood of biochemical failure. Kaplan-Meier analysis of biochemical failure-free survival according to development of an autoantibody response. Sera from prostate cancer patients (n = 23) receiving androgen-deprivation and external beam radiation bimodal therapy were evaluated for the presence of autoreactive antibodies and plotted according to biochemical failure, as indicated by elevated prostate specific antigen (PSA). Autoantibody-positive subjects are indicated with a solid line and autoantibody-negative subjects are indicated with a dashed line. Check marks indicate censored subjects. A log-rank test was performed to determine the P value.
Table 2. Cox multivariate analysis of biochemical failure according to risk-related patient parameters and autoantibody status.
| Variable | Hazard Ratio | 95% confidence interval | P value |
|---|---|---|---|
| Risk Group | 1.342 | 0.294–6.126 | 0.704 |
| Autoantibody Status | 4.283 | 0.954–19.224 | 0.058 |
Discussion
Tumor-associated autoantibodies are detectable in patients with a variety of cancer types, including prostate cancer, and may have prognostic value.16 The current study was undertaken to investigate whether standard therapies for prostate cancer resulted in tumor-associated humoral responses and whether these changes correlate with treatment outcomes in patients receiving ADT and EBRT. For high-risk patients and selected patients with intermediate-risk disease, the combination of ADT and EBRT is a standard treatment option but is associated with a greater than 20% rate of biochemical failure at 5 y in patients with more aggressive disease.17 In our study, 30.4% of patients treated with ADT and EBRT developed an autoantibody response during or within 1 y of treatment. Among the autoantibody-positive patients, 71.4% experienced BF compared with only 25% of patients without autoantibody responses. Although it would have been preferable to perform the sera analysis on autologous tumor tissue, biopsy collection for this group of prostate cancer patients is not a standard of care at our institution. The presence of different autoantibody reactive bands among patients suggests that the target antigens are patient-specific rather than common autoantigens. Indeed, our previous serologic screening using SEREX identified a diverse repertoire of recognized antigens, among which only a small subset were known to be common autoantigens.15 A major limitation in our study is the sample size, and further studies are needed to validate these original findings in large well-defined cohorts with longitudinal blood samples. Nonetheless, our findings provide a rationale to investigate similar mechanisms in other settings where hormone therapy and radiation are used in combination, such as breast cancer.
The results presented here are consistent with our laboratory findings in the Shionogi tumor model, in which the detection of autoantibodies specific for poly(A) binding protein correlated with tumor recurrence after androgen deprivation by castration.18 Another study evaluating autoantibody responses in patients receiving a poxvirus-based vaccine and EBRT found that overall autoantibody responses had no impact on survival, although when stratified according to antigen specificity a trend toward worse outcomes for certain autoantibodies was observed.13 Thus, the specificity of the antigen response may determine whether autoantibody responses are beneficial or detrimental. Smith and colleagues noted that autoantibody responses to specific tumor antigens varied among individuals as well as with the type of treatment.12 We found that the majority of autoantibody responses were coincident with BF, suggesting that this type of immune response might promote disease recurrence. In this case, autoantibodies could serve as a surrogate marker of an underlying T-cell response, in which a strong Th2-mediated autoantibody response skews the pattern away from Th1-driven cytolytic T-cell responses. However, validation of these conclusions in a larger cohort of patients is required, in addition to further investigation to uncover the underlying mechanism of these autoantibody responses.
The increased rate of BF in patients with tumor-associated autoantibodies indicates that B-cell activation may promote tumor aggressiveness. It is possible that the combination of ADT with EBRT promotes a tumor microenvironment wherein stimulation of prostate-resident B cells promotes chronic production of inflammatory cytokines that, in turn, leads to tumor progression.19 Consistent with this alternative hypothesis, a previous report demonstrated that autoantibody production in the K14-HPV16 model of squamous carcinogenesis recruits and regulates tumor-associated macrophages and mononuclear phagocytes through interaction of IgG with Fcγ receptors, suggesting that the detection of autoantibodies in the serum of patients may represent an immunosuppressive tumor environment.20 Moreover, a recent finding by Karin and colleagues showed that tumor-infiltrating B cells can directly promote progression of androgen-independent prostate cancer via the production of pro-inflammatory cytokines, specifically lymphotoxin-α.21 In another study of colon cancer, depletion of B cells augmented antitumor responses and suppressed metastasis.22 Taken together, these data suggest that B cells may contribute to tumor pathogenesis in prostate cancer and that immunomodulation through B-cell depletion or blockade of inflammatory cytokines produced by B cells could be therapeutically beneficial in a subset of prostate cancer patients that receive ADT plus EBRT. Studies to determine the contribution of B cells to autoantibody responses and prostate cancer outcome are currently underway in mouse models.
Patients receiving ADT plus EBRT represent those with the highest risk for recurrence, and within this high-risk patient population we have identified a sub-group of patients with a deleterious immune response that has an even higher risk of recurrence. Randomized clinical trials have demonstrated that the addition of ADT to EBRT enhances, rather than compromises, prostate cancer control and survival, despite the apparent association between this bimodal therapy and high rates of biochemical failure.2 Understanding the immunologic profile of patients with unfavorable prognosis may help to stratify patients that would benefit most from additional treatments, such as B-cell depletion. Longitudinal monitoring of our prostate cancer patients is needed to shed light on the interactions between B and T cells and their respective contributions to treatment-associated immune responses.
Patients with prostate cancer frequently receive radiation therapy, with or without androgen-deprivation therapy. We show that treatment-associated autoantibodies correlate with poor outcome in patients receiving both androgen deprivation and external beam radiation therapies. Thus, current treatments for some prostate cancer patients could elicit undesirable immune responses. Our findings suggest that immunomodulation during hormone and radiation therapy to divert tumor immunity away from autoantibodies toward more beneficial cytolytic responses warrants further investigation.
Materials and Methods
Subjects
This study received approval by the Research Ethics Board of the British Columbia Cancer Agency and University of British Columbia. Fifty-six patients with non-metastatic prostate cancer who had elected to receive androgen deprivation and external beam radiation therapy with curative intent were recruited at the British Columbia Cancer Agency - Vancouver Island Centre in Victoria, British Columbia, between December 2003 and May 2006. The majority of patients (n = 16) had high-risk disease (defined as stage ≥ T3a, Gleason Score = 8–10, or PSA > 20), 6 patients had intermediate risk (stage T2b–T2c or Gleason Score = 7 or PSA 10–20), and one patient had low-risk disease but received ADT and EBRT because of bulky disease. Some patients were excluded from further analysis because they had received ADT prior to the first blood collection or were lost to follow up. Of the 56 patients recruited, 23 met the inclusion criteria for this study (Table 1).
Patients received neoadjuvant and concomitant ADT as single-agent or combination regimens at the discretion of the treating physicians. Regimens were typically a combination of a luteinizing hormone-releasing agonist and an anti-androgen. Neoadjuvant ADT was typically given for 1 y before and concurrently with EBRT. EBRT was planned and treated with 3D conformal EBRT techniques using CT simulation and multi-field beam arrangement to target the prostate gland and seminal vesicles. The prescribed dose of EBRT was 74 Gy, delivered in 2-Gy daily fractions over a period of 7.5 wk.
Blood Collection
Blood samples were collected with informed written consent from study participants. Serum was collected at the first visit, and subsequent serum samples were collected at approximately 3-mo intervals during treatment and then at 6-mo intervals for the first year after treatment, timed to coincide with clinical assessment of PSA. Blood was collected in serum separator tubes, centrifuged at 2,500 rpm for 10 min, aliquoted, and stored at -80 °C. PSA values were obtained from patient records. Biochemical failure was defined as nadir +2 ng/mL per the Phoenix definition.23
Detection of autoantibodies by immunoblotting
Western blotting was performed as previously described.15 The human prostate cancer cell line LNCaP was obtained from the American Type Culture Collection. Briefly, 400 μg of protein isolated from LNCaP cells was separated in a single lane by standard SDS-PAGE (Invitrogen, NP0336BOX) and transferred to nitrocellulose (VWR, CA27376–991) using a Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell (BioRad, 170–3940). Sera were diluted 1:500 in Blotto (5% dry milk powder, 0.1% Tween 20, 50 mmol/L Tris, 150 mmol/L NaCl) and incubated for 1 h at room temperature using the Mini Protean II MultiScreen multichannel immunoblotting device (Bio-Rad, 170–4017). Membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-human IgG secondary antibody (1:10,000; Heavy and Light chains; Jackson ImmunoResearch, 109–001–003) and visualized by enhanced chemiluminescence. All serum samples were assessed by immunoblot analysis at least twice. Autoantibody responses were considered positive if they developed within 1 y of completing EBRT.
Detection of actin by immunoblotting
Nitrocellulose membranes were rehydrated in water for 10 min. To remove the primary human sera and secondary IgG, blots were incubated for 10 min at room temperature with gentle shaking in RestoreTM Western Blot Stripping Buffer (Thermo Scientific, 21059) and washed thoroughly (five times for 5 min each at room temperature) in TBST. A 5% milk solution was used to block the blots before reprobing with mouse anti-actin (Sigma #A2228) antibody at a 1:50,000 dilution overnight at 4 °C with gentle shaking. Blots were washed five times for 5 min at room temperature in TBST followed by incubation with anti-mouse IgG IR800 secondary antibody (Rockland #610–132–121; 1:20,000) for 1 h. Blots were washed and imaged using a Li-Cor Odyssey Imager.
Statistical analysis
Log-rank tests were performed to determine significance for biochemical failure in autoantibody-positive and -negative patients. The relationship between risk (which incorporates stage, Gleason score, and PSA level) and the development of autoantibodies was analyzed by Cox regression analysis.
Disclosure of Potential Conflicts of Interest
The Author states he has no conflict of interest
Acknowledgments
The authors thank Kristy Dillon for performing phlebotomy and blood processing. We gratefully acknowledge the patients for their willingness and time to participate in this study as well as Dr. Jan Lim, the physicians, and clinic staff at the BC Cancer Agency Vancouver Island Centre. J.J. Lum was supported by CIHR New Investigator Award. L. Johnson was supported by the US. Department of Defense W81XWH-12–1-0035 and W81XWH-09–1-0169. Additional funding was provided by US. Department of Defense, Cancer Research Society, Ride for Dad (Comox Valley Chapter), West Coast Motorcycle Ride to Live (BC Chapter), Prostate Cancer Support Group (Vancouver Island), and the British Columbia Cancer Foundation.
Glossary
Abbreviations:
- ADT
androgen deprivation therapy
- BF
biochemical failure
- BFFS
biochemical failure-free survival
- EBRT
external beam radiation therapy
- IgG
immunoglobulin G
- MHC
major histocompatibility complex
- PSA
prostate-specific antigen
References
- 1.Alexander AS, Mydin A, Jones SO, Christie J, Lim JT, Truong PT, Ludgate CM. Extreme-risk prostate adenocarcinoma presenting with prostate-specific antigen (PSA)>40 ng/ml: prognostic significance of the preradiation PSA nadir. Int J Radiat Oncol Biol Phys. 2011;81:e713–9. doi: 10.1016/j.ijrobp.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 2.Ludgate CM, Lim JT, Wilson AG, Alexander AS, Wilson KS. Neoadjuvant hormone therapy and external beam radiation for localized prostate cancer: Vancouver Island Cancer Centre experience. Can J Urol. 2000;7:937–43. [PubMed] [Google Scholar]
- 3.Tendulkar RD, Reddy CA, Stephans KL, Ciezki JP, Klein EA, Mahadevan A, Kupelian PA. Redefining high-risk prostate cancer based on distant metastases and mortality after high-dose radiotherapy with androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2012;82:1397–404. doi: 10.1016/j.ijrobp.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res. 2011;17:1571–81. doi: 10.1158/1078-0432.CCR-10-2804. [DOI] [PubMed] [Google Scholar]
- 6.Hayden AJ, Catton C, Pickles T. Radiation therapy in prostate cancer: a risk-adapted strategy. Curr Oncol. 2010;17(Suppl 2):S18–24. doi: 10.3747/co.v17i0.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–93. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 10.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin Cancer Res. 2012;18:4522–5. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 12.Smith HA, Maricque BB, Eberhardt J, Petersen B, Gulley JL, Schlom J, McNeel DG. IgG responses to tissue-associated antigens as biomarkers of immunological treatment efficacy. J Biomed Biotechnol. 2011;2011:454861. doi: 10.1155/2011/454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesslinger NJ, Ng A, Tsang KY, Ferrara T, Schlom J, Gulley JL, Nelson BH. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res. 2010;16:4046–56. doi: 10.1158/1078-0432.CCR-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunphy EJ, McNeel DG. Antigen-specific IgG elicited in subjects with prostate cancer treated with flt3 ligand. J Immunother. 2005;28:268–75. doi: 10.1097/01.cji.0000158853.15664.0c. [DOI] [PubMed] [Google Scholar]
- 15.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 16.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickles T, Keyes M, Morris WJ. Brachytherapy or conformal external radiotherapy for prostate cancer: a single-institution matched-pair analysis. Int J Radiat Oncol Biol Phys. 2010;76:43–9. doi: 10.1016/j.ijrobp.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Hahn S, Nesslinger NJ, Drapala RJ, Bowden M, Rennie PS, Pai HH, Ludgate C, Nelson BH. Castration induces autoantibody and T cell responses that correlate with inferior outcomes in an androgen-dependent murine tumor model. Int J Cancer. 2009;125:2871–8. doi: 10.1002/ijc.24673. [DOI] [PubMed] [Google Scholar]
- 19.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr., Feng L, Sampsel JW. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–9. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach M, 3rd, Hanks G, Thames H, Jr., Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]


