Abstract
Although blocking vascular endothelial growth factor (VEGF) signaling is clinically beneficial in certain cancers, tumor regrowth in treated patients suggests that compensatory angiogenic programs may limit the efficacy of anti-VEGF treatment. We found that association of galectin-1 with complex N-glycans on VEGFR2 links tumor hypoxia to VEGFR2 signaling and preserves angiogenesis in response to VEGF blockade.
Keywords: angiogenesis, glycans, cancer immunotherapy, anti-VEGF, glycosylation, galectin, inflammation
In 1970 Judah Folkman proposed that angiogenesis -the process through which new blood vessels arise from pre-existing ones- could influence tumor progression.1 These pioneer experiments have inspired the design of novel therapeutic modalities in cancer aimed at blocking abnormal angiogenesis. Later, the identification of vascular endothelial growth factor (VEGF) as a major pro-angiogenic factor and the elucidation of its specific receptors (VEGFRs) and downstream signaling pathways have facilitated the development of selective inhibitors that blocked the vascularization process and suppressed tumor growth.2
Blockade of VEGF-VEGFR2 signaling with bevacizumab (a neutralizing anti-VEGF mAb) or with receptor tyrosine kinases inhibitors have improved the clinical outcome of several types of cancers including metastatic colorectal cancer, non-small cell lung carcinoma, renal cell carcinoma and hepatocarcinoma.2 However, patients respond to these therapies with varying degrees of sensitivity, and a number of these develop progressive resistance, suggesting that compensatory pathways may contribute to hypoxia-driven tumor angiogenesis.3,4 These include the secretion of alternative pro-angiogenic factors such as fibroblast growth factor-2 (FGF-2), placental growth factor (PIGF) and interleukin-17 (IL-17), as well as the recruitment of Bv8-expressing myeloid-derived suppressor cells.3-5
Glycosylation, the process responsible for creating specific glycan structures in glycoproteins and glycolipids, is a posttranslational modification that involves the coordinated action of glycosyltranferases (enzymes that catalyze the transfer of a saccharide from a nucleotide sugar donor or a lipid sugar to a substrate) and glycosidases that catalyze the hydrolysis of glycosidic bonds in glycan structures. The glycosylation machinery represents >1% of the total genome and more than 100 glycosyltransferases and glycosidases have been identified to date.6 Remarkably, differential glycosylation can control a variety of cellular processes by displaying or masking ligands for endogenous lectins which can subsequently activate or fine-tune receptor signaling.6,7 Although the importance of protein glycosylation in immune-mediated processes has been largely appreciated in the past few years,6 our awareness of the impact of glycosylation in vascular biology is much more limited.
Galectin-1 (Gal1), a member of a family of endogenous glycan-binding proteins is upregulated in a variety of tumors and contributes to tumor progression by influencing homotypic and heterotypic cell adhesion, tumor cell migration invasiveness and immune escape.6,8 Interestingly, Gal1 expression is upregulated in tumor hypoxic microenvironments and influences the development of aberrant vascular networks.8,9
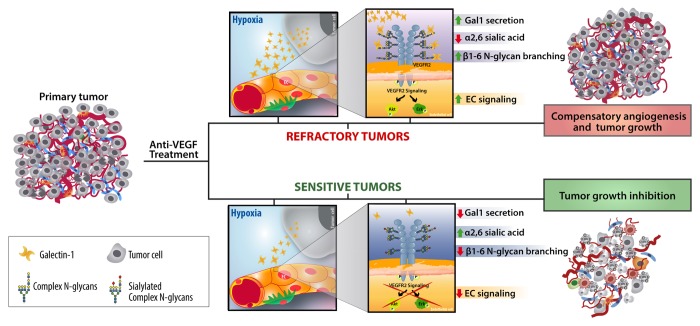
Recently we identified a mechanism based on the differential glycosylation of tumor-associated vessels that mimics VEGF signaling and preserves vascularization in anti-VEGF-refractory tumors.10 In response to immunosuppressive cytokines and hypoxic microenvironments, endothelial cells expressed all the repertoire of glycans that are critical for Gal1 binding and angiogenesis, including increased β1,6 N-glycan branching, higher poly-N-acetyllactosamine extension, and lower α2,6 sialylation. At the molecular level, we found that Gal1 co-opts the VEGFR2 signaling pathway through binding to non-sialylated complex N-glycans on Ig3, Ig4, and Ig7 domains of VEGFR2. These glycosylation-dependent interactions promoted segregation of VEGFR2 into membrane microdomains and prolonged residency of this receptor on the surface of endothelial cells.10
In vitro, serum-free conditioned medium from anti-VEGF refractory, but not anti-VEGF sensitive tumors, induced endothelial cell exposure of Gal1-specific ligands. More importantly, tumor-associated vessels from mice inoculated with tumors that were sensitive to anti-VEGF (B16-F0 melanoma and CT26 colon carcinoma), expressed high amounts of α2,6-linked sialic acid in response to VEGF blockade, which prevented Gal1 binding and angiogenesis (Fig. 1). In contrast, mice inoculated with anti-VEGF refractory tumors (Lewis lung adenocarcinoma; LLC1 and R1.1 T cell lymphoma) secreted increased Gal1 and their associated vasculature expressed higher amounts of β1–6GlcNAc-branched complex N-glycans and decreased α2,6 sialylation in response to VEGF blockade (Fig. 1). Programmed remodeling of the EC glycome facilitated Gal-1-N-glycan interactions and promoted compensatory angiogenesis in tumors with limited sensitivity to anti-VEGF.10 Lack of β1–6GlcNAc-branched N-glycans in endothelial cells or silencing of tumor-derived Gal1 converted refractory into anti-VEGF-sensitive tumors, whereas elimination of α2,6-linked sialic acid limited the efficacy of anti-VEGF treatment in sensitive tumors. This effect involved Gal1-VEGFR2 interactions, as it was prevented when Gal1 was silenced in tumor cells or when mice were treated with anti-VEGF monoclonal antibodies plus axitinib, a receptor tyrosine kinase inhibitor that preferentially perturbs VEGFR signaling.10
Figure 1. Association of Gal1 with complex N-gycans on VEGFR2 compensates for the absence of cognate ligand in anti-VEGF refractory tumors. In response to VEGF blockade, anti-VEGF refractory tumors (upper pannel) secrete higher amounts of Gal1 and their associated endothelial cells (ECs) express all the repertoire of glycans that are critical for Gal1 binding (increased β1,6 N-glycan branching, augmented poly-N-acetyllactosamine extension and lower α2,6 sialylation). This inducible EC glycophenotype facilitated Gal1 signaling, compensatory angiogenesis and tumor growth. In contrast, blood vessels associated with anti-VEGF sensitive tumors (lower panel) displayed higher amounts of α2,6-linked sialic acid which prevented Gal-1-VEGFR2 interactions.
Finally, we validated the therapeutic efficacy of an anti-Gal1 neutralizing monoclonal antibody that selectively inhibits the function of Gal1 but not other members of the galectin family.9,10 Administration of the anti-Gal1 antibody promoted tumor growth inhibition and circumvented compensatory angiogenesis induced by VEGF blockade. Interruption of Gal1-N-glycan interactions promoted transient normalization of the tumor-associated vasculature in vivo. This effect was reflected by decreased vessel diameter, fewer dilated and tortuous vessels and greater coverage by mature pericytes in vessels from anti-Gal1-treated tumors.10 Moreover, disruption of complex N-glycans or antibody-mediated Gal1 blockade contributed to alleviate tumor hypoxia and facilitated influx of immune cells into the tumor microenvironment early after treatment. This effect resulted in augmented T-cell proliferation and enhanced IFN-γ and IL-17 production by tumor-draining lymph node cells.10 These results emphasize the dual effects of blocking Gal1-N-glycan interactions, which influence tumor growth by attenuating aberrant angiogenesis and evoking T cell-mediated responses. Our findings offer novel opportunities for circumventing resistance to VEGF-targeted therapies and potentiating cancer immunotherapeutic modalities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Work in G.A.R.’s laboratory is supported by grants from the Argentinean Agency for Promotion of Science and Technology (PICT 2010; PICT 2012), CONICET, University of Buenos Aires, Sales Foundation and donations from Ferioli and Ostry families.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–22. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–23. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322–35. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139:1229–41. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thijssen VL, Rabinovich GA, Griffioen AW. Vascular galectins: regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013;24:547–58. doi: 10.1016/j.cytogfr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, García-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–58. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]