Abstract
The Wnt pathway is a conserved signal transduction pathway that contributes to normal development and adult homeostasis, but is also misregulated in human diseases such as cancer. The tumor suppressor adenomatous polyposis coli (APC) is an essential negative regulator of Wnt signaling inactivated in >80% of colorectal cancers. APC participates in a multiprotein “destruction complex” that targets the proto-oncogene β-catenin for ubiquitin-mediated proteolysis; however, the mechanistic role of APC in the destruction complex remains unknown. Several models of APC function have recently been proposed, many of which have emphasized the importance of phosphorylation of high-affinity β-catenin-binding sites [20-amino-acid repeats (20Rs)] on APC. Here we test these models by generating a Drosophila APC2 mutant lacking all β-catenin-binding 20Rs and performing functional studies in human colon cancer cell lines and Drosophila embryos. Our results are inconsistent with current models, as we find that β-catenin binding to the 20Rs of APC is not required for destruction complex activity. In addition, we generate an APC2 mutant lacking all β-catenin-binding sites (including the 15Rs) and find that a direct β-catenin/APC interaction is also not essential for β-catenin destruction, although it increases destruction complex efficiency in certain developmental contexts. Overall, our findings support a model whereby β-catenin-binding sites on APC do not provide a critical mechanistic function per se, but rather dock β-catenin in the destruction complex to increase the efficiency of β-catenin destruction. Furthermore, in Drosophila embryos expressing some APC2 mutant transgenes we observe a separation of β-catenin destruction and Wg/Wnt signaling outputs and suggest that cytoplasmic retention of β-catenin likely accounts for this difference.
Keywords: adenomatous polyposis coli (APC), β-catenin, Wnt signaling, axin destruction complex, colon cancer
THE Wnt signaling pathway represents one of six evolutionary conserved pathways that collectively regulate animal development, yet are frequently misregulated in human disease (Clevers and Nusse 2012). During development, Wnt signaling regulates cell fate decisions that influence a myriad of developmental events as diverse as establishment of the vertebrate axis, control of bone development, and the wiring of the neural circuitry (Cadigan and Peifer 2009; Regard et al. 2012; Salinas 2012; Hikasa and Sokol 2013). Wnt signaling continues to be critical in adult homeostasis, as it is involved in the maintenance of certain mammalian stem cells (Holland et al. 2013). While Wnt signaling is clearly essential for an array of developmental events, misregulation of the pathway is the initiating event in the vast majority of colorectal cases. Truncating mutations in the negative regulator of Wnt signaling, adenomatous polyposis coli (APC), are responsible for both the hereditary colorectal cancer syndrome familial adenomatous polyposis (FAP) and >80% of spontaneous colon cancer cases (Polakis 2012).
APC functions as a gatekeeper of the intestinal epithelium by mediating proteolytic degradation of the Wnt signaling effector β-catenin (βcat). At the molecular level, APC negatively regulates βcat by participating in a multiprotein “destruction complex” that consists of the core members Axin and the serine/threonine kinases glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1) (Stamos and Weis 2013). Once assembled into the destruction complex, βcat is sequentially phosphorylated by CK1 and GSK3, recognized by the SCFβTrCP E3 ubiquitin ligase, polyubiquitinated, and destroyed in the 26S proteasome. Thus in cells with an active destruction complex, βcat levels are low and Wnt target genes suppressed.
In cells receiving Wnt signal, the Wnt ligand binds to a coreceptor complex consisting of the seven-pass transmembrane protein frizzled (Fz) and the single-pass membrane protein LRP5/6 (MacDonald and He 2012). Wnt binding to the receptor complex results in phosphorylation of the cytoplasmic tail of LRP5/6, which recruits Axin to the membrane. Membrane recruitment of Axin inactivates the destruction complex through a mechanism involving Disheveled (Dvl) (Bilic et al. 2007). He and colleagues suggest that Wnt signaling promotes an intramolecular conformational change in Axin that prevents assembly of βcat into the complex (Kim et al. 2013), whereas Clevers and colleagues propose that Wnt signaling prevents recruitment of the E3 ligase to an intact destruction complex (Li et al. 2012). In either case, βcat remains hypophosphorylated and escapes ubiquitination, and its intracellular protein levels rise. βcat then translocates to the nucleus and associates with TCF/LEF proteins at Wnt-responsive elements (WREs) and displaces the Groucho repressor, thereby converting TCF/LEF from transcriptional repressors to activators. Ultimately these events result in expression of Wnt target genes such as c-myc and cyclin D1.
While substantial experimental evidence supports this general model of Wnt signal transduction, several fundamental questions remain. One question is, What mechanistic role does APC play in the destruction complex? APC is a large protein with several putative protein–protein interaction domains; therefore, APC was initially thought to be the scaffold for the complex. However, Axin was subsequently identified as a negative regulator of Wnt signaling that binds all core components of the destruction complex (βcat, GSK3, CK1, APC, and Dvl), and other less well-characterized players such as the catalytic subunit of protein phosphatase 2A (PP2A) (Zeng et al. 1997; Sakanaka et al. 1998; Fagotto et al. 1999; Hsu et al. 1999). In addition, Axin can enhance the rate of βcat phosphorylation by CK1/GSK3, consistent with a scaffolding effect, whereas APC does not (Ha et al. 2004). These findings make Axin a stronger candidate to be the scaffold, leaving the mechanistic role of APC in the destruction complex mysterious.
Recent biochemical and crystallographic data have suggested several models of APC function in the destruction complex, many of which have focused on the importance of βcat-binding sites on APC. APC contains two types of βcat-binding sites called 15-amino-acid repeats (15Rs) and 20-amino-acid repeats (20Rs), with vertebrate APC containing four 15Rs and seven 20Rs (Figure 1A). Importantly, the 20Rs of APC are substrates for CK1/GSK3 phosphorylation (Ikeda et al. 2000; Ferrarese et al. 2007), whereas the 15Rs remain unphosphorylated. The affinity of each site for βcat was determined using isothermal calorimetry, which established that the 20Rs are higher-affinity βcat-binding sites than the 15Rs (Liu et al. 2006). In addition, phosphorylation of the 20Rs increases affinity for βcat up to 1500-fold. These findings prompted Kimmelman and Xu to propose a cycle of catalytic activity in the destruction complex in which the complex first assembles with βcat bound to Axin due to Axin’s single βcat-binding site possessing higher affinity for βcat than unphosphorylated 20Rs (Kimelman and Xu 2006; Xu and Kimelman 2007). Upon assembly, the 20Rs of APC are phosphorylated by CK1/GSK3, resulting in higher-affinity βcat-binding sites. βcat thus transfers from Axin to APC, presumably resulting in phosphorylated βcat being recognized by the SCFβTrCP E3 ligase. PP2A-mediated dephosphorylation of the 20Rs would serve to reset the cycle. Thus, the Kimmelman and Xu model would argue that at least some subset of 20Rs should be essential for APC function in the destruction complex.
Figure 1.
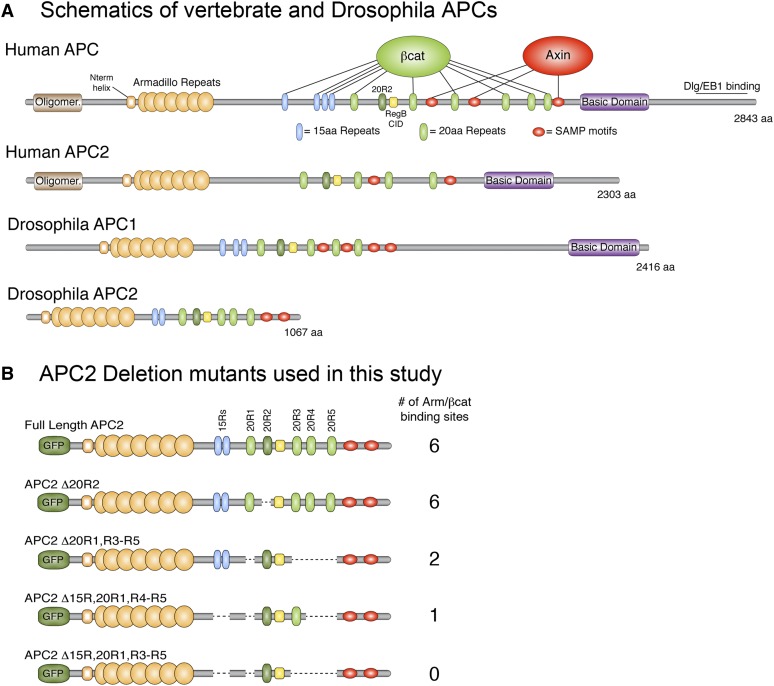
Schematics of human and Drosophila APCs. (A) Vertebrates and flies have two APC proteins that share a conserved core region consisting of Armadillo repeats, 15-amino-acid repeats (15Rs), 20-amino-acid repeats (20Rs), the catenin inhibitory domain (CID) (called region B in Drosophila), and SAMP motifs. The 15Rs and 20Rs both bind βcat, except 20R2. C-terminal sequences are more divergent, with some APCs containing a basic domain and an EB1-binding site. (B) Drosophila APC2 deletion constructs used in this study.
Liu and colleagues present an alternative view of APC’s function in the destruction complex, but maintain that phosphorylation of 20Rs should be essential (Su et al. 2008). They suggest that when βcat is phosphorylated by CK1/GSK3, it is subject to rapid dephosphorylation by PP2A. APC serves to protect βcat from PP2A-mediated dephosphorylation, ensuring that phosphorylated βcat is recognized by the SCFβTrCP ligase and destroyed. The authors further demonstrate that a fragment of human APC encompassing the 20Rs was sufficient for this protective function, whereas the 15Rs of APC were nonprotective. Moreover, phosphorylation of APC was critical to prevent βcat dephosporylation, suggesting that CK1/GSK3 phosphorylation of the 20Rs mediates this effect. Surprisingly, APC binding to βcat prevents dephosphorylaton even though the APC-binding site on βcat does not overlap the βcat phosphodegron.
Finally, Weis and colleagues suggest that the different βcat-binding sites on APC play an important role in fine-tuning βcat destruction in response to a gradient of Wnt signals (Ha et al. 2004). They propose that in the absence of Wnt signal, high-affinity βcat-binding sites (phosphorylated 20Rs) are important to maintain low levels of βcat, whereas low-affinity binding sites (15Rs) become critical in cells winding down from Wnt signal where βcat levels are relatively high. In such cells, the combination of low- and high-affinity βcat-binding sites could be important to rapidly destroy βcat and efficiently turn off Wnt signaling.
In this study, we perform functional studies to test these models of APC function by generating APC transgenes lacking various βcat-binding sites. Vertebrate APCs are large proteins (approaching 300 kDa), thereby precluding the ability to reasonably perform structure/function studies in the context of a full-length protein. Instead, we use Drosophila as a model system. Flies and vertebrates have two highly conserved APC proteins (APC1 and APC2) that participate redundantly in Wnt regulation (Ahmed et al. 1998, 2002; Akong et al. 2002). APC1 and APC2 proteins share a conserved core region consisting of an N-terminal set of Armadillo repeats, a combination of 15Rs and 20Rs, and a series of SAMP motifs that bind the scaffold protein Axin (Figure 1A). In addition to this core region, APC1 proteins also contain a basic domain and an EB1 binding motif that are necessary for its localization to the plus end of microtubules. Prior studies have established that sequences C-terminal of the SAMP motifs are dispensable for proper Wnt regulation (Smits et al. 1999; Roberts et al. 2012b); thus we have selected Drosophila APC2 as a model to study APC function in the βcat destruction complex. Drosophila APC2 contains all components of the core region (including a set of Armadillo repeats, two 15Rs, five 20Rs, and two SAMP motifs) (McCartney et al. 1999; Yu et al. 1999); however, it is considerably shorter than other APC proteins, facilitating its use for structure/function studies.
In designing Drosophila APC2 mutants devoid of particular βcat-binding sites, we were also informed by biochemical work demonstrating that the second 20R of human APC (20R2) lacks any detectable affinity for βcat, even when phosphorylated (Liu et al. 2006; Kohler et al. 2008). Initially, this finding suggested that 20R2 is a degenerate 20R that lost affinity for βcat through evolution; however, we recently showed that 20R2 is highly conserved in species as diverse as sea snails (Lottia gigantean), fruit flies, and humans (Roberts et al. 2011). Furthermore, we established that 20R2 is the only 20R individually required for APC-mediated βcat destruction in both full-length Drosophila APC2 and fragments of human APC. Thus 20R2 has an essential role in mediating βcat destruction independent of the ability to directly bind βcat. Prior studies that sought to address the importance of high-affinity βcat-binding sites on APC for destruction complex function all eliminated 20R2 in addition to other combinations of 20Rs (Rubinfeld et al. 1997; Kunttas-Tatli et al. 2012). It is now clear that such APC mutants are defective in βcat destruction at least in part due to deleting or mutating 20R2 and thus may not accurately reflect the importance of direct βcat binding to APC. Therefore, in this study we test models of APC function by generating Drosophila APC2 transgenes lacking combinations of βcat-binding sites, but that keep 20R2 intact. We perform functional studies with these transgenes in both human colon cancer cell lines and APC null Drosophila embryos, allowing us to assess which aspects of APC biology are conserved throughout evolution.
Materials and Methods
APC2 deletion mutants
Drosophila APC2 deletion mutants were generated using the approach outlined previously (Roberts et al. 2011). Briefly, precise amino acid deletions were generated using a PCR-splicing approach, and the resulting PCR products were TOPO-TA cloned into the pCR8/GW/TOPO Gateway entry vector (Life Technologies). For cell culture experiments, APC2 entry vector constructs were gateway cloned (Life Technologies) into a modified ECFP-N1 destination vector (Clontech) containing the CMV promoter, an N-terminal EGFP tag, and a Gateway-3X STOP cassette. For transgenic fly lines, APC2 constructs were instead cloned into a modified pUAStattB vector (Basler laboratory, University of Zurich, Switzerland, GenBank accession no. EF362409) containing the endogenous APC2 promoter, an N-terminal EGFP tag, the Gateway-3X STOP cassette, and an attB site to facilitate targeted genomic integration using the PhiC31 approach. All transgenic flies were generated by BestGene (Chino Hills, CA), using the BL#9723 line, which results in transgene integration at cytogenetic position 28E7 on the second chromosome. The use of PhiC31 eliminates differences in expression levels caused by positional effects. All constructs were sequence verified.
Yeast two-hybrid analysis
Yeast two-hybrid (Y2H) analysis was performed using the Matchmaker System (Clontech). Briefly, the pGBKT7 and pGADT7 yeast vectors were engineered to be Gateway compatible by inserting a Gateway-3X STOP cassette downstream of the Gal4 DNA-binding domain or the Gal4 transcriptional activation domain, respectively. APC2 constructs were gateway cloned into the resulting pGBKT7-W, whereas full-length Armadillo was cloned into pGADT7-W. APC2 pGBKT7-W constructs were transformed into the Y2HGold yeast strain and Arm pGADT7-W into Y187, using the SC Easy Transformation kit (Life Technologies), and colonies were selected on –Trp or –Leu plates, respectively (Sigma Aldrich). Transformed yeast colonies were then mated in 2× YPAD media for 24 hr and plated on double-selection –Leu –Trp plates. Yeast colonies were inoculated in liquid –Leu –Trp media, and β-galactosidase assays were performed using the Yeast β-galactosidase Assay Kit (Thermo Scientific; Pierce Chemical, Rockford, IL). Several different colonies were tested per experiment, and each experiment was independently conducted three times.
Cell culture, transfections, and immunofluorescence
SW480 cells were cultured at 37° and 5% CO2 in DMEM-H supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1× Pen/Strep/Glutamine (GIBCO, Grand Island, NY). For transient transfections, SW480 cells were plated at a density of 2.5 × 105 cells per well in six-well plates and grown overnight. DNA constructs were then transfected using TurboFect (Thermo Fisher) according to the manufacturer’s instructions. For immunofluorescence studies, cells on coverslips were fixed 24 hr post-transfection with 4% formaldehyde in 1× phosphate-buffered saline (1× PBS) for 10 min. Cells were washed three times with 1× PBS, blocked for 15 min in 1× PBS containing 1% normal goat serum and 0.1% Triton X-100 (1× PBTN), and then antibody stained as previously described (Roberts et al. 2011). Primary antibodies were mouse anti-β-catenin (BD Transduction Laboratories, cat. no. 610153; 1:1000) and anti-Flag [Sigma (St. Louis), M2; 1:1000]. Secondary antibody was goat anti-mouse Alexa 568 (Life Technologies, 1:1000). To inhibit the proteasome, cells were treated with a cocktail of 25 μM MG132 and 25 μM ALLN for 6 hr prior to processing.
TOP/FOP luciferase reporter assay
The TOP/FOP Flash Luciferase constructs and the pRL Renilla transfection control were provided by Hans Clevers (Hubrecht Institute, Utrecht, The Netherlands). Luciferase assays were performed using the Dual Glo Luciferase System (Promega, Madison, WI) according to the manufacturer’s protocol. Briefly, SW480 cells were transiently cotransfected with 2 μg of the relevant APC2 construct, 1 μg of pRL, and 1 μg of either TOP or FOP Flash Luciferase reporter. After 24 hr, cells were lysed in a hypotonic 0.1× PBS solution and subjected to a 5-min freeze–thaw at −80°. Cells were scraped and cellular debris was pelleted in a microcentrifuge. Luciferase activity of each lysate was measured using a Perkin-Elmer (Norwalk, CT) EnSpire plate reader and normalized to Renilla signal. All samples were measured in triplicate per experiment, and three independent experiments were performed. None of the constructs displayed significant FOP flash activity.
Quantifying βcat protein levels in transfected SW480 cells
βcat protein levels in transfected cells were accomplished using flow cytometry. Transfected cells were first trypsinized and then fixed in 10% formaldehyde/1× PBS for 20 min. Cells were permeabilized with 1× Perm/Wash reagent (BD Biosciences) and then antibody stained in 1× Perm/Wash with mouse anti-β-catenin (BD Transduction, 1:200) followed by goat anti-mouse Alexa 647 (Life Technologies, 1:1000). Cells were analyzed on an Accuri C6 Flow Cytometer, and the mean fluorescence intensity of GFP-positive cells was determined. At least 10,000 total cells were measured per sample, and three independent experiments were performed. Values were normalized to the GFP-only control.
Immunoprecipitations and immunoblotting
Transfected SW480 cells were lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Igepal, and 0.25% Na deoxycholate) supplemented with protease inhibitors (SigmaFAST Protease Inhibitor tablet, Sigma Aldrich), 1 mM sodium fluoride, and 0.5 mM sodium orthovanadate. Immunoprecipitations were performed using the Protein A Dynabeads System (Life Technologies). Briefly, lysates were precleared with Protein A Dynabeads and then incubated with beads saturated with rabbit anti-GFP antibody (Abcam, catalog no. Ab290). Beads were washed extensively with RIPA buffer and immunoprecipitated proteins eluted from the beads, using the provided elution buffer. A total of 1 M Tris, pH 7.4, was added to neutralize the solution, prior to adding 2× Laemmli solution (Bio-Rad, Hercules, CA). Protein samples were resolved by SDS–PAGE and transferred to nitrocellulose membrane. Membranes were probed with mouse anti-GFP (Clontech, clone JL-8; 1:1000), mouse anti-β-catenin (BD Transduction, 1:1000), mouse anti-APC (Abcam, clone Ab58; 1:1000), and mouse anti-tubulin (Sigma Aldrich, DM1A; 1:5000). Signal was detected using SuperSignal West Dura Chemiluminescent Substrate (Pierce) and imaged on a Fluor Chem Q imager (Protein Simple). Quantification of band intensity was accomplished using the Protein Simple software.
Drosophila genetics, assessing embryonic lethality, and analyzing cuticle patterning
Drosophila APC2 transgenes were crossed into the APC2g10 single-mutant and the APC2g10APC1Q8 double-mutant backgrounds. Transgene function was assessed in the single-mutant background by analyzing embryos expressing the transgene, but maternally/zygotically null for APC2. These embryos were generated by crossing APC2 Tn; APC2g10 males and females. Transgene function in the APC2g10APC1Q8 double-mutant background was assessed using the FLP/FRT ovoD dominant female-sterile technique. Heat-shocked APC2 Tn/+; FRT82B APC2g10APC1Q8/FRT82B ovoD females were crossed to FRT82B APC2g10 APCQ8/TM3 twiGFP males. Heat shocks were performed 3 days after egg laying for 1 hr in a 37° water bath. Embryonic lethality assays and cuticle preparations were performed as previously described (Wieschaus and Nüsslein-Volhard 1986). For experiments in the double-mutant background, 50% of the progeny inherit the TM3 twiGFP chromosome and are viable (paternally rescued). The level of embryonic cuticle rescue was assessed using a previously established scoring scheme on a scale of 0–6 with 0 indicating a wild-type cuticle pattern (McCartney et al. 2006).
Drosophila immunofluorescence
Fly embryos were dechorionated in 50% household bleach for 5 min and fixed in an equal mixture of heptane and 10% formaldehyde/1× PBS for 20 min. The formaldehyde solution was removed and the vitelline membrane popped by vortexing in a 1:1 mixture of methanol:heptane. Embryos were washed with methanol and then rehydrated in 1× PBTN. Embryos were stained with either anti-Arm [N27A1, Developmental Studies Hybridoma Bank (DSHB); 1:200] or anti-Engrailed (4D9, DSHB; 1:200). Alexa 568 secondary antibody (Life Technologies) was used at 1:500. Images were collected on a Leica SP8 scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL). Adobe Photoshop 7.0 was used to adjust input levels to span the entire output grayscale and to adjust brightness and contrast. When protein levels were compared, images were adjusted equally. For quantification of Engrailed (En) stripe width, six measurements were made across En stripes 4, 5, and 6 and a collective average was determined for each embryo. At least three embryos were analyzed per genotype.
Results
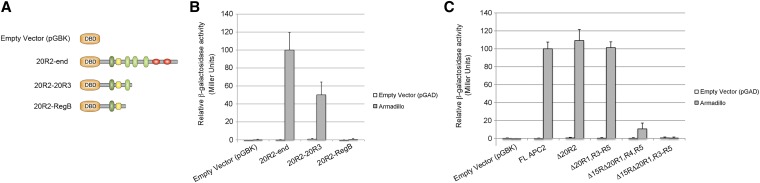
To test the importance of high-affinity βcat-binding sites on APC, we first generated a Drosophila APC2 mutant lacking all 20Rs that bind βcat. Drosophila APC2 has five 20Rs (Figure 1A); however, prior biochemical studies established that 20R2 of human APC does not bind βcat (Liu et al. 2006; Kohler et al. 2008). Human 20R2 shares considerable sequence identity with other βcat-binding 20Rs, but lacks an upstream acidic residue (Supporting Information, Figure S1) that contacts one of the “charged buttons” in βcat (Ha et al. 2004; Xing et al. 2004). Drosophila 20R2 also lacks this acidic residue, and yeast two-hybrid studies are consistent with the hypothesis that Drosophila 20R2 does not bind Armadillo (Arm equals Drosophila βcat) (Figure 2B). Given that 20R2 lacks affinity for βcat but is required for APC function (Roberts et al. 2011), we generated an APC2 mutant that lacks the βcat-binding 20Rs but keeps 20R2 intact (APC2Δ20R1, R3–R5) (Figure 1B).
Figure 2.
Yeast two-hybrid analysis comparing the ability of several Drosophila APC2 fragments to bind Armadillo (fly βcat). (A) Schematics of APC2 fragments containing various numbers of βcat-binding sites. (B) β-Galactosidase activity in Miller units normalized to R2-end × Arm. The fragment encompassing 20R2-B is not statistically different from the pGBK negative control in an unpaired Student’s t-test (P > 0.05). (C) Yeast two-hybrid analysis of Drosophila APC2 mutants containing various numbers of βcat-binding sites. β-Galactosidase activity is reported in Miller units normalized to FL APC2 × Arm. APC2 lacking all βcat-binding sites (APC2Δ15RΔ20R1, R3–R5) does not interact with Arm/βcat (P > 0.05).
An APC2 mutant lacking βcat-binding 20Rs is fully functional in SW480 cells
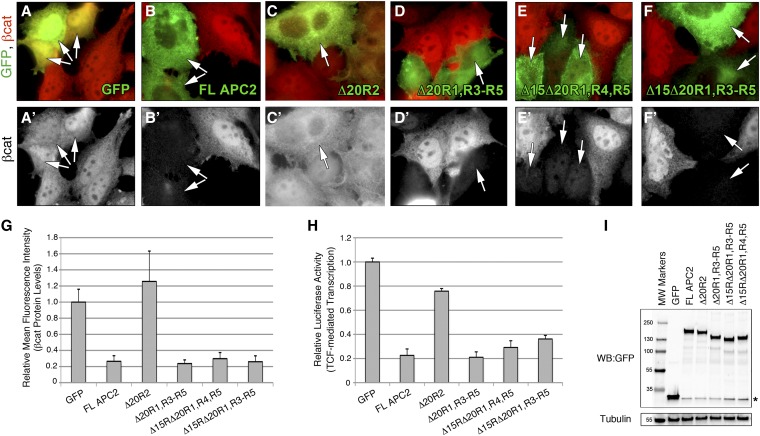
We first tested the APC2Δ20R1, R3–R5 mutant for function in the human colon cancer cell line SW480. SW480 cells contain a truncated APC protein that terminates after 20R1 (Nishisho et al. 1991), thereby inactivating the destruction complex and resulting in βcat hyperaccumulation. Transfection of exogenous human APC fragments encompassing 20R2 through the SAMP motifs can restore destruction complex activity in these cells (Munemitsu et al. 1995), and we recently demonstrated that full-length Drosophila APC2 or APC1 also fully rescues activity (Roberts et al. 2011, 2012b). We transiently transfected GFP-tagged APC2Δ20R1, R3–R5 into SW480 cells and assessed its ability to reduce elevated βcat protein levels, using both immunofluorescence and flow cytometry. Surprisingly, APC2Δ20R1, R3–R5 reduced βcat protein levels as efficiently as full-length APC2, whereas the GFP-only and APC2Δ20R2 negative controls were unable to facilitate βcat destruction (Figure 3, A–D and G). In addition to measuring βcat protein levels, we also evaluated the ability of APC mutants to reduce βcat-dependent transcription, using the well-established TOP/FOP Flash Luciferase system (Korinek et al. 1997). Again, APC2Δ20R1, R3–R5 reduced βcat-mediated transcription as well as full-length APC2, whereas APC2Δ20R2 was defective (Figure 3H). As noted previously, APC2Δ20R2 was able to moderately rescue βcat-mediated transcription, an observation we have attributed to cytoplasmic retention of βcat (Roberts et al. 2011). A Western blot confirmed that all proteins were stable and expressed at approximately the same level (Figure 3I). Overall, these findings suggest that direct binding of βcat to the 20Rs of APC2 is not required for APC2 activity in the destruction complex when expressed in SW480 cells.
Figure 3.
A Drosophila APC2 protein lacking all βcat-binding sites restores βcat destruction in SW480 cells. (A–F) GFP-tagged APC2 mutants lacking various βcat-binding sites were transiently transfected into SW480 cells and analyzed for βcat levels via immunofluorescence. Arrows indicate transfected cells. (G) Relative mean fluorescence intensity of βcat protein in transfected SW480 cells as determined by FACS analysis. APC2Δ20R1, R3–R5; APC2Δ15RΔ20R1, R4, R5; and APC2Δ15RΔ20R1, R3–R5 were not statistically different from wild-type (WT) APC2 (P > 0.05). (H) TOPFlash Luciferase assay to determine the level of TCF-mediated transcription. APC2Δ20R1, R3–R5 and APC2Δ15RΔ20R1, R4, R5 were not statistically different from WT APC2, using an unpaired Student’s t-test, whereas APC2Δ15RΔ20R1, R3–R5 was statistically different from WT APC2 (P = 0.02). (I) Western blot of transfected cells showing that all APC2 proteins are stable and expressed at approximately the same level.
An APC2 mutant lacking all βcat-binding sites is also functional in SW480 cells
We reasoned that APC2Δ20R1, R3–R5 might be able to reduce βcat protein levels in SW480 cells because it could still directly interact with βcat through the 15Rs. Biochemical studies of the mammalian 15Rs suggest that they are relatively low-affinity βcat-binding sites and are not substrates for phosphorylation (Liu et al. 2006). We previously showed that the 15Rs are not required for APC activity in the context of a full-length protein, as APC2Δ15Rs is fully functional in SW480 cells and Drosophila embryos (Roberts et al. 2011). However, others have established that the 15Rs can still provide function in truncated APC proteins lacking 20Rs (Kohler et al. 2010). To test the hypothesis that direct binding of βcat to the 15Rs of APC is sufficient for destruction complex activity, we generated a second APC2 mutant lacking all βcat-binding sites (APC2Δ15Rs, Δ20R1, R3–R5) (Figure 1B). In addition, to determine whether a single 20R could be sufficient for activity, we generated a third APC2 mutant that removed all βcat-binding sites except the highest-affinity site, 20R3 (APC2Δ15Rs, Δ20R1, R4, R5). In a yeast two-hybrid assay, these mutants interacted with Armadillo in the expected manner: we were unable to detect an interaction between Arm and APC2Δ15Rs, Δ20R1, R3–R5, whereas APC2Δ15Rs, Δ20R1, R4, R5 displayed an intermediate interaction (Figure 2C). Surprisingly, immunofluorescence and flow cytometry in SW480 cells indicated that both APC2 mutants fully reduced βcat protein levels (Figure 3, E–G). Moreover, they rescued βcat-mediated transcription in the TOP/FOP Flash assay (Figure 3H). Collectively, these findings suggest that direct binding of βcat to exogenous APC proteins is not essential for destruction complex activity in SW480 cells.
Exogenous APC2 proteins form a complex with endogenous truncated APC and βcat
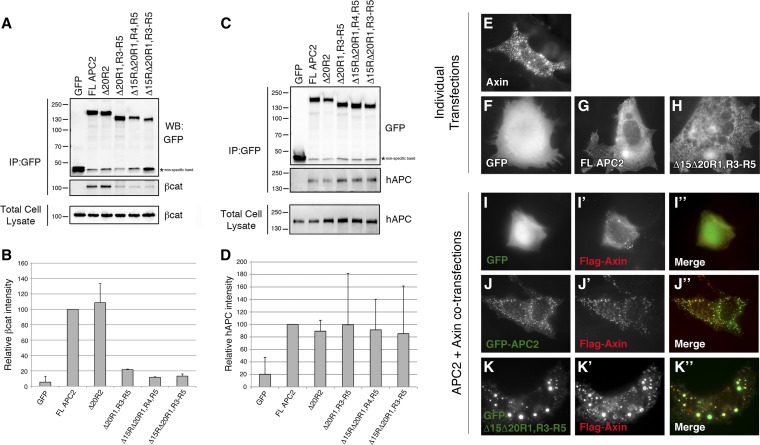
We were surprised to observe that an APC protein lacking all βcat-binding sites was still functional in the destruction complex, so we considered the possibility that the endogenous, truncated human APC protein in SW480 cells could provide function in trans. APC proteins are thought to dimerize/oligomerize as vertebrate APC proteins contain an N-terminal coiled-coil (Joslyn et al. 1993) and Drosophila APC2 has been shown to self-associate in immunoprecipitation studies (Zhou et al. 2011; Roberts et al. 2012a). In addition, a yeast two-hybrid study suggests that Drosophila APC1 and APC2 proteins can heterodimerize (Mattie et al. 2010). We therefore reasoned that our exogenous Drosophila APC2 proteins may interact with endogenous human APC proteins in SW480 cells in trans, therefore creating a functional molecular complex with the ability to bind and destroy βcat. [SW480s also express low levels of human APC2, which, when overexpressed, can reduce βcat protein levels and transcriptional output (van Es et al. 1999; Schneikert et al. 2013)]. Consistent with this idea, truncated APC proteins containing only the N-terminal Armadillo repeats were recently shown to co-immunoprecipitate βcat (Voloshanenko et al. 2013) and also appear to complement the activity of exogenously expressed hAPC2 (APCL) in inhibiting the transcriptional activity of β-catenin in Caco-2 cells (Schneikert et al. 2013).
To test the hypothesis that exogenous Drosophila APC2 proteins could associate with endogenous human APCs, we transiently transfected our panel of APC2 mutants into SW480 cells and analyzed the molecular complex through immunoprecipitation (IP). Both truncated human APC and βcat could be immunoprecipitated with the entire panel of Drosophila APC2 mutants (Figure 4, A and C), suggesting that Drosophila APC2 proteins may restore destruction complex function via complementation in trans with endogenous human APCs. We also quantified the amount of βcat and hAPC immunoprecipitated in each sample (Figure 4, B and D). hAPC levels were comparable across all samples, suggesting that the number of βcat-binding sites does not influence APC homo/hetero-dimerization (Figure 4D). In addition, the amount of immunoprecipitated βcat generally corresponded with the number of βcat-binding sites present in the APC2 mutants, with one notable exception: APC2 Δ15RΔ20R1, R4, R5 (1 βcat-binding site) and APC2 Δ15RΔ20R1, R3–R5 (0 βcat-binding sites) immunoprecipitated similar, low amounts of βcat. Given that these APC2 mutants associate with truncated hAPC in SW480 cells (Figure 4, B and D) and assemble into the Axin destruction complex (Figure 4, E–K), this low amount of βcat likely represents basal βcat assembled into the endogenous destruction complex and does not reflect direct binding to our exogenous APC2 mutants (truncated hAPC has four 15Rs and one 20R1, and Axin has a single βcat site). In support of this notion, truncated hAPC proteins containing only the Armadillo repeats were recently shown to immunoprecipitate βcat, Axin1, and GSK3 in colon cancer cell lines (Voloshanenko et al. 2013), indicating that immunoprecipitation of APC pulls down the entire molecular complex.
Figure 4.
Exogenous Drosophila APC2 proteins associate with endogenous human APC and are recruited into the Axin destruction complex. (A and C) SW480 cells were transiently transfected with the panel of GFP-tagged APC2 mutants, immunoprecipitation was performed for GFP, and an immunoblot was probed for (A) β-catenin and (C) human APC. Both human APC and β-catenin immunoprecipitated with all Drosophila APC2 proteins, suggesting they are in a complex. (B and D) Average band intensity quantified from two independent experiments. SW480 cells were also cotransfected with Flag-Axin in addition to GFP-APC2 mutants. (E) Singly transfected Flag-Axin has a punctate distribution that is thought to represent the localization of endogenous Axin (Schwarz-Romond et al. 2007). ((F–H) GFP is cytosolic with some nuclear enrichment (F), whereas GFP-APC2 proteins are cytoplasmic and largely nuclear excluded (G and H).) GFP is cytosolic with some nuclear enrichment (E), whereas GFP-APC2 proteins are cytoplasmic and largely nuclear excluded (F and G). (I-K) When cotransfected with Flag-Axin, GFP-APC2 proteins are relocalized into the Axin puncta, whereas the negative control GFP remains diffuse.
Drosophila as a model system to investigate APC function in the destruction complex
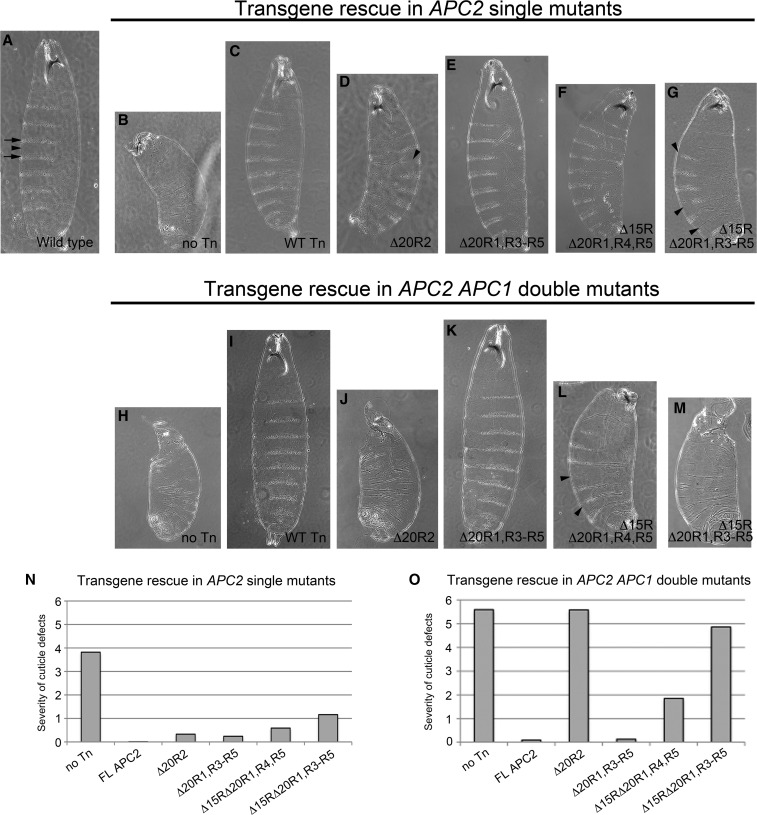
Given this apparent complementation in trans, it was necessary to assess APC2 mutant function in a background completely devoid of endogenous APC proteins. Small interfering RNA knockdown of endogenous APC proteins to a limiting level has proved difficult (Chandra et al. 2012; Schneikert et al. 2013), so we turned to the genetic system of Drosophila where null alleles of both APC proteins exist (APC2g10 and APC1Q8) (Ahmed et al. 1998; Akong et al. 2002; McCartney et al. 2006). Drosophila is a powerful system for structure/function studies of Wg/Wnt signaling components as it provides the ability to assess a broad spectrum of phenotypic outputs, including βcat (Armadillo) protein levels, transcriptional outputs, and ultimate cell fate decisions. Moreover, we can assess transgene function in both the APC2g10 single-mutant and APC2g10, APC1Q8 double-mutant backgrounds. APC2g10 and APC2g10, APC1Q8 maternally and zygotically mutant embryos have a fully penetrant embryonic lethal phenotype; however, they display differences in cell fate decisions consistent with the amount of elevated Wg/Wnt signaling (APC2 single mutants have less severe phenotypes due to residual APC1 activity) (McCartney et al. 1999; Ahmed et al. 2002; Akong et al. 2002). The ventral embryonic epidermis provides a particularly sensitive readout of Wg/Wnt activity as wild-type anterior cells in each segment of the animal are fated to secrete cuticle containing hair-like projections called denticles, while posterior cells secrete naked cuticle lacking denticles (Figure 5A). Activation of Wg/Wnt signaling through the loss of APC activity results in only posterior cell fate decisions with all epidermal cells secreting naked cuticle. Complete loss of APC activity in the APC2g10APC1Q8 double mutant results in a severe cuticle phenotype characterized by a complete lack of denticles, anterior head holes caused by failure of head involution, and an overall reduction in size caused by increased apoptosis (Figure 5H and McCartney et al. 2006). In contrast, APC2g10 single-mutant embryos have a more moderate cuticle phenotype characterized by a few remaining denticles, less severe anterior defects, and an intermediate overall size (Figure 5B). Assessing APC2 transgene function in both the single- and double-mutant backgrounds allows us to observe fine-scale differences in transgene function, as rescue of the double mutant requires a nearly completely functional APC2 protein, whereas rescue of the single mutant can occur with a partially functional protein (Roberts et al. 2011). Transgene function can be assessed using a previously established scheme that scores the cuticle phenotype on a scale of 0–6, with 0 indicating a wild-type cuticle pattern (McCartney et al. 2006).
Figure 5.
Direct binding of β-catenin to the 20Rs of APC is dispensable for proper Wg/Wnt-mediated cell fate decisions in Drosophila embryos. Shown is cuticle analysis of Drosophila APC2 transgenes expressed in the APC2g10 maternal/zygotic or APC2g10APC1Q8 maternal/zygotic background. Cuticle scores were determined using the previously established scale from 0 to 6 with 0 indicating a wild-type pattern and 6 a severe APC mutant pattern (McCartney et al. 2006). (A–M) Representative cuticles of APC2 transgenes expressed in the APC2g10 single-mutant (A–G) or APC2g10APC1Q8 double-mutant (H–M) background. (N and O) Graphical representation of cuticle scores. Arrows, denticle bands; arrowheads, naked cuticle.
To ensure consistent, physiological expression of our APC2 transgenes, we cloned the panel of APC2 deletion mutants into a Drosophila expression vector driven by the endogenous APC2 promoter (McCartney et al. 2006) and containing an attB site for targeted genomic integration (Venken et al. 2006; Bischof et al. 2007). Targeted integration avoids differences in transgene expression due to positional effects. We previously demonstrated that this system results in transgene expression approximating endogenous APC2 levels and that full-length GFP-APC2 can fully rescue all APC mutant phenotypes (Roberts et al. 2011). We confirmed uniform expression of our panel of APC2 mutants via immunoblotting (Figure S2), and each mutant was expressed at approximately the same level as the full-length APC2 transgene.
An APC2 mutant lacking all βcat-binding 20Rs fully rescues cell fate decisions in Drosophila embryos; however, deletion of all βcat-binding sites greatly impairs function
To test the importance of βcat binding to APC in the absence of complementation in trans, we generated transgenic flies expressing our panel of APC2 mutants and assessed embryonic viability and cuticle patterning in APC2 single and APC2, APC1 null embryos. Consistent with our findings in SW480 cells, the APC2 transgene lacking high-affinity βcat-binding sites (APC2Δ20R1, R3–R5) largely rescued embryonic lethality and cuticle patterning in both the single- and double-mutant backgrounds (Figure 5, E and K, Table 1). In fact, the APC2Δ20R1, R3–R5 transgene rescued APC2g10 mutants to adult viability (Table 1). These findings indicate that APC2Δ20R1, R3–R5 is fully functional even in the absence of endogenous APC proteins and that direct binding of βcat to the 20Rs of APC is not essential.
Table 1. Function of APC2 mutants in Drosophila.
| In APC2g10 single-mutant background | In APC2g10APC1Q8 double-mutant background | |||||
|---|---|---|---|---|---|---|
| Transgene | Deleted residues | Embryonic viability (%) | Cuticle score | Adult viable stock | Embryonic viability (%) | Cuticle score |
| No transgene control | NA | 8.9 (n = 245) | 3.82 (n = 435) | No | 46.7 (n = 541) | 5.59 (n = 196) |
| Full-length APC2 | NA | 94.7 (n = 415) | 0.01 (n = 37) | Yes | 96.5 (n = 481) | 0.09 (n = 32) |
| APC2Δ20R2 | 645–672 | ND | 0.38 (n = 100) | No | 49.0 (n = 296) | 5.58 (n = 128) |
| APC2Δ20R1, R3–R5 | 595–622, 737–889 | 70.8 (n = 490) | 0.24 (n = 213) | Yes | 81.7 (n = 279) | 0.13 (n = 101) |
| APC2Δ15RΔ20R1, R4, R5 | 497–535, 595–622, 784–889 | 58.7 (n = 252) | 0.59 (n = 157) | No | 53.2 (n = 233) | 1.85 (n = 228) |
| APC2Δ15RΔ20R1, R3–R5 | 497–535, 595–622, 737–889 | 41.9 (n = 346) | 1.16 (n = 355) | No | 52.2 (n = 599) | 4.86 (n = 186) |
NA, not applicable; ND, not determined.
However, our results with the other two APC2 deletion mutants differed from what was observed in SW480 cells, where they appeared largely functional. Instead, the APC2 transgene lacking all βcat-binding sites (APC2Δ15RΔ20R1, R3–R5) displayed substantial, but not complete activity in APC2 single-mutant fly embryos (restored embryonic viability to 42% and the cuticle phenotype to 1.2 in the APC2g10 single-mutant background as opposed to 9% viability and 3.8 cuticle score for the no transgene control) (Figure 5, G and N, Table 1). It is possible that this rescuing activity in the APC2g10 single mutant is at least partly due to complementation in trans with endogenous Drosophila APC1. In support of this hypothesis, APC2Δ15RΔ20R1, R3–R5 was less functional in the double-mutant background where it failed to rescue embryonic lethality (52.2% for APC2Δ15RΔ20R1, R3–R5 vs. 46.7% for the no transgene control, Table 1) and only modestly affected the cuticle phenotype (4.8 vs. 5.6, respectively) (Figure 5, M and O).
Finally, the APC2 protein with a single βcat-binding 20R (APC2Δ15RΔ20R1, R4, R5) substantially restored activity, resulting in intermediate phenotypes in both the single- and double-mutant backgrounds. In the APC2g10 single-mutant background, embryonic viability was restored to 59% and the cuticle score to 0.6, whereas in the double-mutant background, viability was 53% and the cuticle score 1.8 (Figure 5, F, L, N, and O, Table 1). Collectively, this analysis of APC2 mutants in Drosophila suggests that the βcat-binding 20Rs of APC are not essential for APC function in the destruction complex, but that the 15Rs are sufficient—a finding that is inconsistent with current models of APC activity. In addition, the observed differences in transgene function in APC mutant fly embryos vs. SW480 cells emphasize the importance of performing structure/function studies in a true null background and urge caution when interpreting results obtained in colorectal cell lines expressing endogenous truncated hAPC and wild-type hAPC2.
While βcat-binding sites are critical for cell fate decisions, they are largely dispensable for βcat destruction
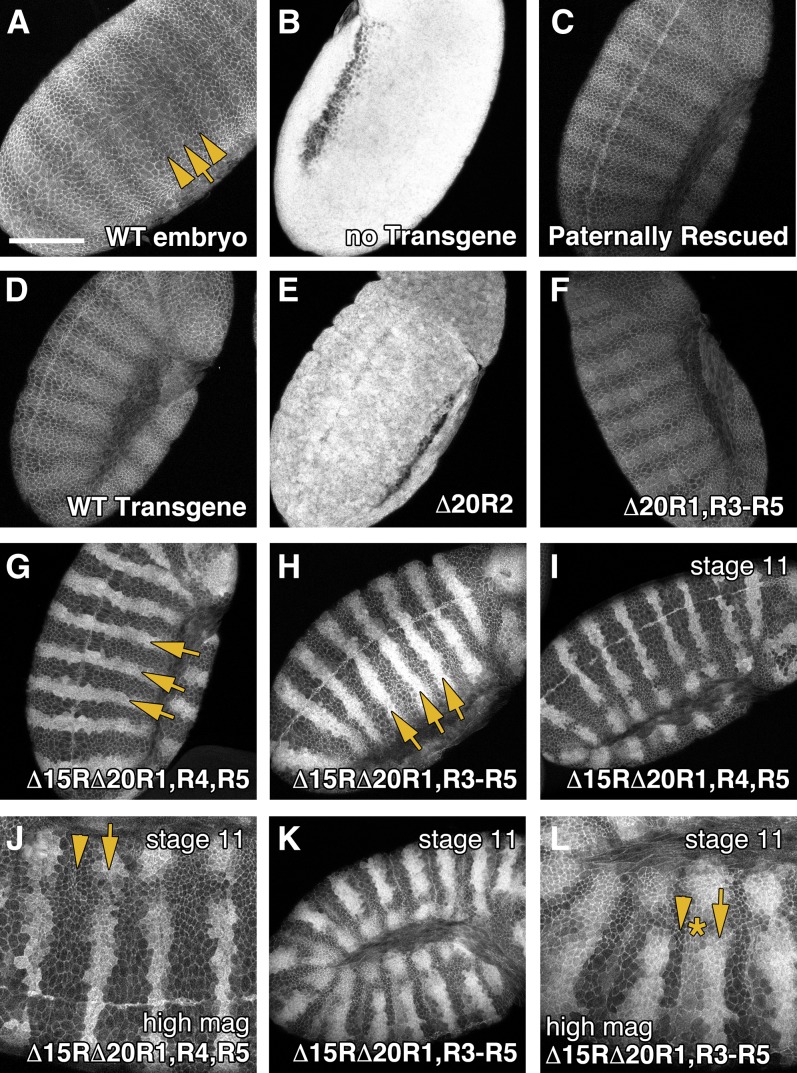
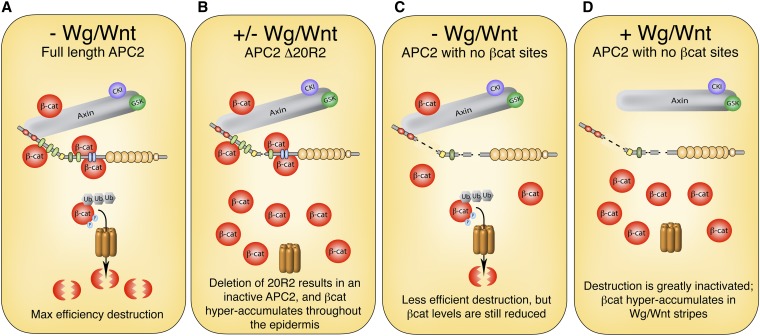
While cuticle patterning provides a sensitive readout of cell fate decisions in the Drosophila embryo, it does not necessarily correlate with the level of βcat destruction as we and others have suggested that cytoplasmic retention of βcat can modify target gene activation (Krieghoff et al. 2006; Roberts et al. 2011). To test the hypothesis that direct binding of βcat to APC is essential for βcat destruction, we assessed the ability of our Drosophila APC2 transgenes to regulate Arm protein levels in APC2g10APC1Q8 double-mutant embryos. In wild-type stage 9–10 embryos, Arm levels are kept low in cells without Wg signal (Figure 6A, arrowheads), but it accumulates modestly in stripes of epidermal cells that receive Wg signal (Figure 6A, arrow) (Peifer et al. 1994). APC2g10APC1Q8 double-mutant embryos hyperaccumulate Arm protein levels uniformly across the epidermis (Figure 6B) (Ahmed et al. 2002; Akong et al. 2002), while expression of full-length GFP-APC2 in this background fully restores the wild-type pattern (Figure 6D) (Roberts et al. 2011). The APC2 transgene lacking βcat-binding 20Rs (APC2Δ20R1, R3–R5) also restored proper Arm protein levels (Figure 6F), consistent with the observation that it fully rescued cuticle patterning.
Figure 6.
An APC2 mutant lacking all βcat-binding sites can partially regulate Armadillo destruction. Stage 9–10 Drosophila embryos expressing APC2 transgenes in the APC2g10APC1Q8 maternal/zygotic background were fixed and antibody stained for Armadillo. (A) In wild-type embryos, Arm localizes to the cell cortex, but also weakly accumulates in the cytoplasm of cells receiving Wg/Wnt signaling (arrows). Bar, 100 μm. (B) In APC2g10APC1Q8 maternal/zygotic mutants, the destruction complex is fully inactivated, and Arm hyperaccumulates uniformly throughout the embryo. (C) Paternal transmission of a wild-type chromosome restores destruction complex activity. (D and E) A full-length APC2 transgene restores Arm regulation (D), whereas deletion of 20R2 inactivates APC2 (E). (F) An APC2 transgene lacking all βcat-binding 20Rs (APC2Δ20R1, R3–R5) is fully functional. (G) APC2 with a single βcat-binding site (APC2Δ15RΔ20R1, R4, R5) restores Arm destruction, but results in Arm accumulation in pronounced stripes across the embryonic epidermis (arrows). (H) APC2 lacking all βcat-binding sites (APC2Δ15R, Δ20R1, R3–R5) also results in stripes of Arm accumulation (arrows). (I and J) Stage 11 APC2Δ15RΔ20R1, R4, R5 embryo. (K and L) Stage 11 APC2Δ15R, Δ20R1, R3–R5 embryo showing expansion of the Arm stripes and the presence of three levels of Arm accumulation (arrow, high level; asterisk, intermediate level; arrowhead, low level).
Given that the APC2 transgene lacking all βcat-binding sites (APC2Δ15RΔ20R1, R3–R5) was largely defective in rescuing cuticle patterning, we hypothesized that it would also be defective in mediating βcat destruction. However to our surprise, APC2Δ15RΔ20R1, R3–R5 displayed a substantial ability to regulate Arm protein levels, albeit in a patterned fashion. In stage 9–10 embryos expressing APC2Δ15RΔ20R1, R3–R5, Arm protein was reduced to near wild-type levels in most cells (Figure 6H); however, there was a dramatic accumulation of Arm in sharp stripes across the embryonic epidermis (Figure 6H, arrows). In stage 11 embryos, the Arm stripes are expanded (Figure 6K), and three populations of cells could be detected—those accumulating high levels, intermediate levels, and low levels of Arm (Figure 6L, arrow, asterisk, and arrowhead, respectively). These findings suggest that direct binding of βcat to APC is not essential for βcat destruction, although the interaction is required in certain developmental contexts.
Finally, the APC2 transgene containing a single 20R (APC2Δ15RΔ20R1, R4, R5), displayed an intermediate phenotype, with Arm protein still accumulating in sharp stripes across the epidermis, although at a slightly reduced level relative to the APC2 mutant lacking all βcat-binding sites (Figure 6G). The Armadillo stripes also did not appear to expand in stage 11 embryos, and only high- and low-accumulating cells were readily detectable (Figure 6, I and J). These observations may explain the intermediate denticle patterning phenotype observed in APC2Δ15RΔ20R1, R4, R5-expressing animals.
The level of Wg/Wnt target gene expression correlates with denticle patterning
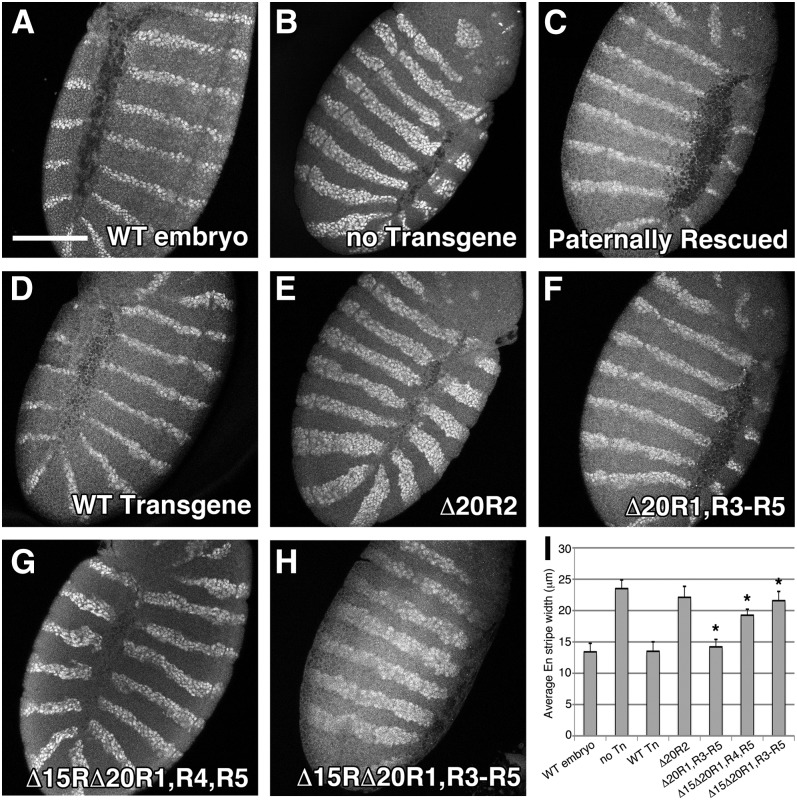
Given the apparent discontinuity between Arm destruction and cell fate decisions observed in APC mutants expressing APC2Δ15RΔ20R1, R3–R5, we sought to determine how the patterned accumulation of Arm affects Wg/Wnt target gene expression. Engrailed (En) is a well-characterized Wg/Wnt target expressed in narrow stripes of two to three cells across the wild-type embryonic epidermis (Figure 7A) (Hooper 1994). The Engrailed expression domain is expanded considerably in APC2g10APC1Q8 double mutants due to uniformly elevated Arm levels (Figure 7B), and full-length GFP-APC2 fully restores the wild-type En pattern (Figure 7D). In testing our panel of APC2 transgenes, APC2Δ20R1, R3–R5 fully rescued the En pattern (Figure 7F), APC2Δ15RΔ20R1, R3–R5 had an expanded En domain similar to the no transgene control (Figure 7H), and APC2Δ15RΔ20R1, R4, R5 had an intermediate En expansion (Figure 7G). We also quantified the En expression domain by measuring En stripe widths (Figure 7I). Overall, these En expression patterns are consistent with the observed cuticle patterns. In addition, even though the APC2 mutant lacking all βcat-binding sites (APC2Δ15RΔ20R1, R3–R5) was capable of destroying Arm in a developmental pattern, the En stripe width in these animals suggests that Arm is sufficiently elevated to activate Wg/Wnt target genes at a level comparable to that of the no transgene control.
Figure 7.
Analysis of Wg/Wnt target gene expression in Drosophila embryos expressing APC2 transgenes. Stage 9–10 Drosophila embryos expressing APC2 transgenes in the APC2g10APC1Q8 maternal/zygotic background were antibody stained for the Wg/Wnt target gene Engrailed (En). (A and B) En is normally expressed in stripes of two to three cells (A); however, the En expression domain is expanded considerably in APC2g10APC1Q8 maternal/zygotic mutants (B). (C–E) Inheritance of a wild-type paternal chromosome (C) or expression of full-length Drosophila APC2 (D) restores proper En expression, whereas deletion of 20R2 phenocopies the APC2g10APC1Q8 mutant (E). (F–H) The βcat-binding 20Rs are dispensable for proper En expression (F), whereas the presence of a single 20R is intermediate (G), and the lack of all βcat-binding sites phenocopies the APC2g10APC1Q8 mutant (H). (I) Quantification of average En stripe width (μm). Δ20R1, R3–R5 is not statistically different from WT APC2. Δ20R1, R3–R5; Δ15Δ20R1, R4, R5; and Δ15Δ20R1, R3–R5 are all statistically different from each other (P < 0.05). Δ15Δ20R1, R3–R5 is not statistically different from the no transgene control.
An APC2 mutant lacking all βcat-binding sites is defective in cytoplasmic retention
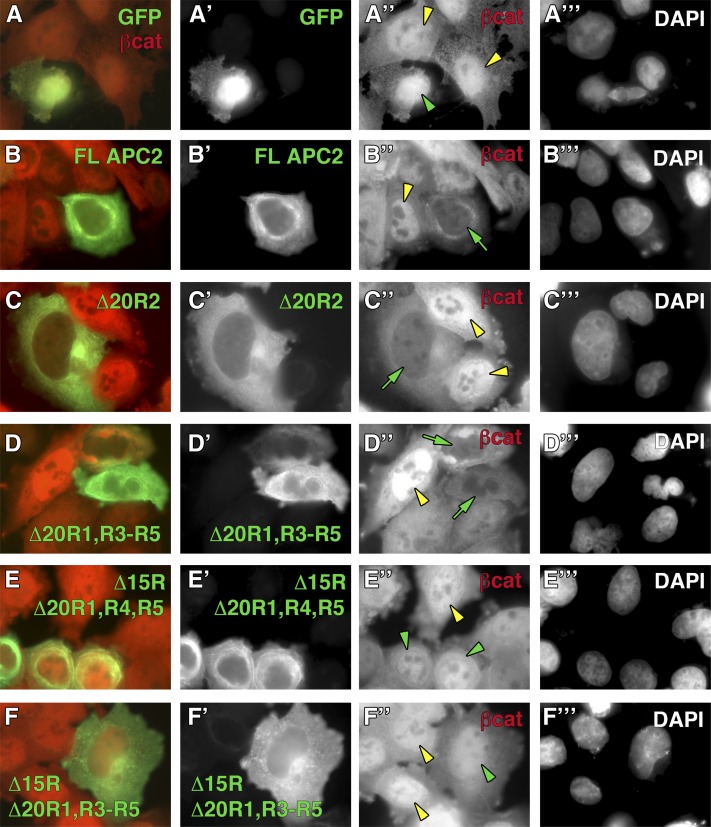
We and others have previously suggested that APC proteins can modulate Wg/Wnt signaling through cytoplasmic retention of βcat (Roberts et al. 2011; Wang et al. 2014). We reasoned that the phenotypes observed in APC mutant embryos expressing APC2Δ15RΔ20R1, R3–R5 may be explained if APC2Δ15RΔ20R1, R3–R5 is defective in cytoplasmic retention. To test this hypothesis, we transiently transfected SW480 cells with our panel of APC2 mutants, inhibited proteasomal activity through the addition of a cocktail of MG132 and ALLN (thereby preventing APC-mediated destruction of βcat), and assessed nuclear enrichment of βcat via immunofluorescence. We first confirmed that MG132/ALLN treatment was successful by measuring βcat protein levels, using flow cytometry (Figure S3). In immunofluorescence studies, untransfected SW480 cells typically display a pronounced nuclear enrichment of βcat (Figure 8, A′′–F′′, yellow arrowheads), whereas GFP-APC2 proteins are cytoplasmic and largely nuclear excluded (Figure 8, B′–F′). In the presence of MG132/ALLN, full-length APC2 sequestered βcat in the cytoplasm and dramatically reduced the amount of detectable nuclear βcat (Figure 8B′′, green arrow), whereas the GFP control did not influence βcat localization (Figure 8A′′, green arrowhead). Cytoplasmic retention of βcat was also observed in APC2Δ20R2- and APC2Δ20R1, R3–R5-expressing cells (Figure 8, C′′and D′′, green arrows); however, APC2Δ15RΔ20R1, R4, R5 and APC2Δ15RΔ20R1, R3–R5 were defective in retention (Figure 8, E′′ and F′′, green arrowheads). Thus, the inability to sequester βcat may explain the observation that Drosophila embryos expressing APC2Δ15RΔ20R1, R3–R5 have cells that appear to destroy Arm/βcat to wild-type levels, yet still activate Wg/Wnt target genes.
Figure 8.
(A–F) APC2 lacking all Arm/βcat-binding sites is defective in cytoplasmic retention. The panel of GFP-tagged APC2 mutants was transfected into SW480 cells and treated with a cocktail of MG132 and ALLN to prevent βcat destruction. βcat is typically nuclear enriched in untransfected control SW480 cells (A′′–F′′, yellow arrowheads), whereas APC2 proteins appear nuclear excluded (B′–F′). Expression of FL APC2 (B′′, green arrow), Δ20R2 (C′′, green arrow), and Δ20R1, R3–R5 (D′′, green arrow) prevents nuclear enrichment of βcat, whereas Δ15RΔ20R1, R4, R5 (E′′, green arrowhead) and Δ15RΔ20R1, R3–R5 (F′′, green arrowhead) are defective in this function. DAPI staining was used to label nuclei for all experiments (A′′′–F′′′).
Discussion
The tumor suppressor APC is a well-established negative regulator of the Wnt signaling pathway, which functions as a core member of the βcat destruction complex. Despite nearly 25 years of research on APC, its mechanistic role in the destruction complex remains unknown. Recent work investigating its role in the βcat destruction complex has focused on the importance of phosphorylation of the 20Rs (Ha et al. 2004; Xing et al. 2004; Su et al. 2008; Kunttas-Tatli et al. 2012). Prior studies designed to test the importance of the 20Rs in APC biology deleted or mutated all the 20Rs and concluded that direct binding of βcat to at least a subset of 20Rs is essential for function (Rubinfeld et al. 1997; Kunttas-Tatli et al. 2012). However, biochemical studies recently established that 20R2 does not bind βcat, likely due to the absence of an upstream acidic residue that contacts βcat in the crystal structure (Liu et al. 2006; Kohler et al. 2010). Initially, it was assumed that 20R2 is simply a degenerate motif; however, 20R2 is highly conserved and is required for APC function in both SW480 cells and Drosophila embryos (Roberts et al. 2011). Thus, it remained possible that past APC proteins that mutated all the 20Rs were defective due to lack of 20R2 activity and not because direct βcat binding to the 20Rs is essential. In this study, we therefore reevaluated the importance of direct βcat binding to the 20Rs.
Exogenous APC2 proteins cooperate with endogenous APCs in SW480 cells
To test the importance of βcat binding to the 20Rs of APC, we generated a series of Drosophila APC2 mutants that remove various βcat-binding sites but keep 20R2 intact. Surprisingly, all APC2 mutants (including an APC2 lacking all βcat-binding sites) rescued βcat destruction and reduced transcriptional activity in SW480 cells (Figure 3, G and H). SW480s express truncated hAPC and a full-length hAPC2; thus we considered the possibility that our exogenously expressed APC2 mutants could cooperate with endogenous APCs. In support of this hypothesis, truncated hAPC and βcat could both be co-immunoprecipitated with the entire panel of Drosophila APC2 mutants (Figure 4). These findings further support the hypothesis that APC proteins function as dimers/oligomers in the βcat destruction complex and also prompted us to test transgene function in a genetic null background.
The βcat-binding 20Rs are dispensable for APC activity
We assessed transgene function in APC null Drosophila embryos, using four different criteria: (1) embryonic viability, (2) cuticle patterning, (3) Armadillo (Arm) protein levels, and (4) expression of the Wg/Wnt target gene Engrailed. Similar to what was observed in SW480 cells, the APC2 transgene lacking all βcat-binding 20Rs (APC2Δ20R1, R3–R5) rescued all these criteria in fly embryos to the level of the full-length control, suggesting that direct binding of βcat to the 20Rs of APC is not essential for destruction complex activity. This finding is inconsistent with several current models of APC function, and we propose an alternative model below.
Direct binding of βcat to APC is essential for proper cell fate decisions, but largely dispensable for βcat destruction
Given that the βcat-binding 20Rs are dispensable, we asked whether a direct APC–βcat interaction is required for APC activity. The Drosophila APC2 transgene lacking all βcat-binding sites (APC2Δ15RΔ20R1, R3–R5) was partially active in the APC2 single-mutant background, but failed to rescue cuticle patterning in APC2, APC1 null embryos (Figure 5, G and M). We previously demonstrated that an APC2 protein lacking the 15Rs is fully functional (Roberts et al. 2011); thus our findings collectively argue that a direct APC–βcat interaction is essential for proper cell fate decisions, but that either the 15Rs or the 20Rs are sufficient for this activity.
Although APC2Δ15RΔ20R1, R3–R5 was largely nonfunctional in denticle patterning, it surprisingly showed substantial activity in Arm destruction (Figure 6H). APC mutant embryos expressing this transgene displayed hyperaccumulated stripes of Arm protein, whereas cells neighboring the stripes were able to reduce Arm to near wild-type levels. This finding suggests that a direct APC–βcat interaction is not required for βcat destruction, but also that there are in vivo mechanisms that modulate destruction complex activity at certain developmental positions in the embryo that do require βcat binding to the 15Rs or 20Rs. One interpretation is that the stripes of accumulated Arm correspond to cells receiving Wg/Wnt signal and that this pattern of Arm staining could be generated by a hypofunctional APC2 protein capable of targeting Arm for destruction in the absence of Wg/Wnt signaling. However, in cells that receive Wg/Wnt signal, the combination of inactivating the destruction complex and a marginally functional APC protein results in Arm hyperaccumulation (Figure 9). A prediction of this model would be that cells positioned at the peak of the Wg/Wnt morphogen gradient should accumulate the most Arm and that Arm levels would dissipate in a graded fashion as cells are positioned farther along the morphogen gradient. In stage 11 embryos, we did observe stripes of cells with intermediate Arm accumulation (Figure 6L); however, it was interesting that the pattern typically consisted of sharp stripes composed of cells with high Arm accumulation immediately adjacent to cells with low Arm levels. Consequently, this pattern suggests the presence of additional in vivo regulatory mechanisms that require further investigation to fully elucidate.
Figure 9.
Model showing the βcat-binding sites on APC serve to simply dock βcat in the destruction complex. (A) In a wild-type scenario, βcat is recruited into the destruction complex by both Axin and APC to provide maximal turnover of βcat. (B) Deletion of 20R2 inactivates APC; thus βcat hyperaccumulates throughout the epidermis. (C) In the absence of βcat-binding sites on APC, the efficiency of the destruction complex is greatly reduced; however, Axin can still recruit βcat into the destruction complex, allowing sufficient βcat degradation in the absence of Wg/Wnt signaling. (D) In the presence of Wg/Wnt signal, the destruction complex is further inactivated, resulting in hyperaccumulation of βcat.
Implications for APC’s role in the βcat destruction complex
Collectively, our findings in Drosophila embryos suggest an alternative view of APC’s mechanistic function in the destruction complex. We propose that direct binding of βcat to APC does not provide a mechanistic role, but rather serves to dock βcat in the destruction complex, thereby increasing the efficiency of βcat phosphorylation and subsequent degradation (Figure 9). Such a model is consistent with our observations that either the 15Rs or the 20Rs are sufficient for APC activity, that APC lacking all βcat-binding sites is capable of mediating Arm destruction (Axin has a single βcat-binding site and may recruit reduced levels of βcat into the complex), and that restoring a single βcat-binding site improves APC function. Our findings are also consistent with prior work from the Birchmeier laboratory demonstrating that mutant forms of βcat incapable of binding APC can still be destroyed (von Kries et al. 2000). Moreover, a recent structure/function analysis of Axin in Drosophila embryos demonstrated that the single βcat-binding site on Axin is largely dispensable for Wnt regulation (Peterson-Nedry et al. 2008). Combined with our findings here, these results suggest that βcat-binding sites on Axin and APC may be redundant in recruiting and docking βcat in the complex—a hypothesis that can be tested using Axin, APC1, APC2 triple mutants expressing Axin and APC2 transgenes lacking/adding back various βcat-binding sites.
While this study helps address the importance of a direct APC–βcat interaction in destruction complex function, the question remains what mechanistic role APC provides in targeting βcat for degradation. Combined with our prior structure-function analysis of Drosophila APC2, we now suggest that a minimal APC protein necessary to target βcat for destruction includes the N-terminal Arm repeats, a small subset of βcat-binding 15Rs or 20Rs (two 15Rs are sufficient for complete APC activity, whereas a single 20R is intermediate), 20R2 and the adjacent region B/catenin inhibitory domain (CID), and a SAMP motif. The Arm repeats of vertebrate APCs are known to bind a handful of partners, including the B56 regulatory subunit of PP2A phosphatase (Seeling et al. 1999), yet it is unclear how these interactions contribute to Wnt regulation. In addition, 20R2 and region B/CID are highly conserved motifs that are likely binding sites for essential protein partners. Identification of such partners is likely to elucidate the role of these particular binding sites and will collectively help establish APC’s mechanistic role. One exciting possibility is that 20R2 and region B/CID recruit members of the E3 ubiquitin ligase into the destruction complex to facilitate efficient ubiquitination of βcat (Li et al. 2012; Stamos and Weis 2013). Furthermore, it was recently reported that α-catenin binds the CID and is surprisingly required to mediate βcat destruction and regulate transcriptional activity at WREs (Choi et al. 2013). An interaction between Axin and 20R2 has also been suggested (Schneikert et al. 2014). It will be exciting to identify additional novel APC interactors and synthesize a holistic view of this molecular machine.
Implications for mechanisms of Wg/Wnt signal transduction
In addition to providing insight into the inner workings of the destruction complex, our data also have implications regarding the mechanism of Wg/Wnt signal transduction. Given the dramatic reduction in Arm levels in the Wg-interstripe zone of APC mutant embryos expressing APC2Δ15RΔ20R1, R3–R5 (Figure 6H), we were surprised to observe that these cells still express Wg/Wnt target genes (Figure 7H) and acquire a cell fate consistent with Wg/Wnt activation (Figure 5M). One possible explanation is that the observed accumulation of Arm in response to the Wg gradient is irrelevant for activation of Wg/Wnt outputs, but that Arm is somehow modified to be competent to signal. Work from the Gumbiner laboratory has suggested that cessation of βcat destruction (and the subsequent accumulation of βcat protein) is not what promotes Wnt signal transduction; rather, a small subset of βcat is made competent to promote Wnt target gene activation through conformational changes in the C-terminal tail of βcat (Gottardi and Gumbiner 2004). Thus, it is possible that APC2Δ15RΔ20R1, R3–R5-expressing cells in the posterior of each segment somehow generate a signaling-competent version of Arm that promotes naked cuticle fate despite the dramatic reduction in Arm levels.
An alternative possibility, which is not mutually exclusive, is that cytoplasmic retention of βcat could account for the observed phenotypes. Prior studies have suggested that βcat retention can modulate Wg/Wnt signaling (Roberts et al. 2011; Wang et al. 2014), and our current studies in transfected SW480 cells suggest that APC2Δ15RΔ20R1, R3–R5 is defective in this function (Figure 8F). In fact, the moderate differences in TOPFlash reporter activation observed in SW480 cells expressing APC2Δ20R1, R3–R5; APC2Δ15RΔ20R1, R4, R5; and APC2Δ15RΔ20R1, R3–R5 (Figure 2H) could be explained by differences in βcat retention [note that each of these APC2 mutants destroys βcat to comparable levels in SW480 cells (Figure 2G)]. To our knowledge, APC2Δ15RΔ20R1, R3–R5 represents the first example of a retention-defective, but destruction-competent APC mutant, allowing us to assess the importance of cytoplasmic retention of βcat in Wg/Wnt signaling regulation in vivo.
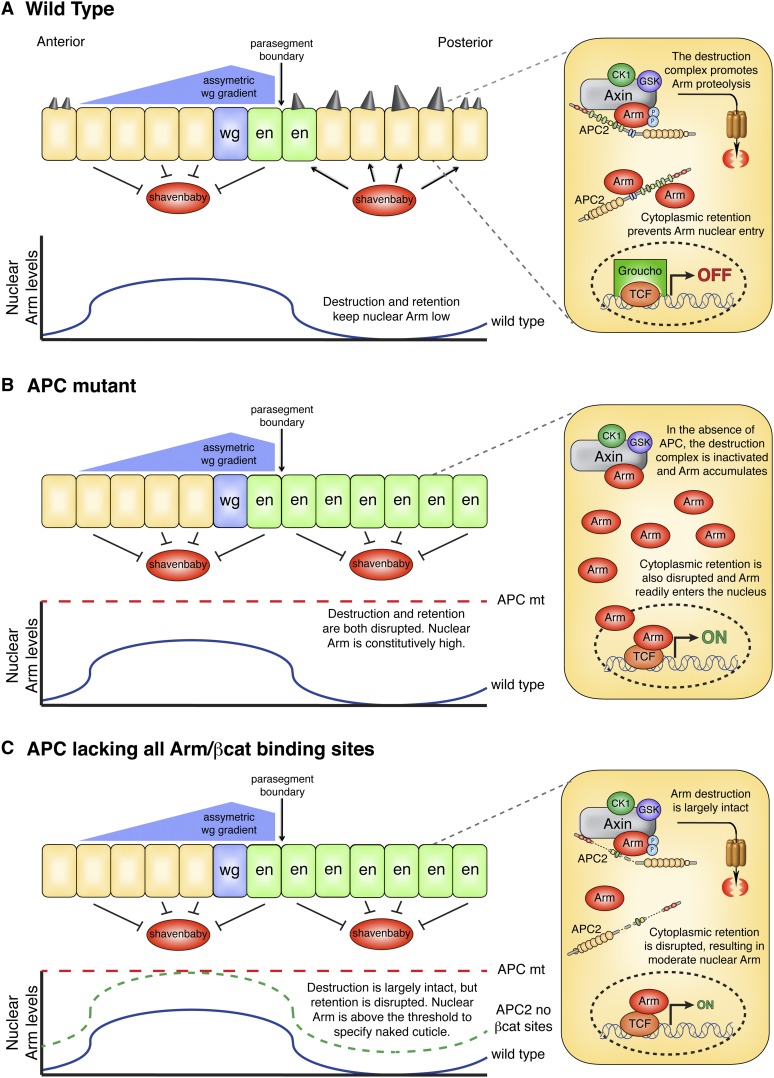
We propose that βcat retention is a physiologically important mechanism to regulate Wnt signal transduction and that activation of Wg/Wnt target genes can still occur in cells where βcat destruction is intact, but retention disrupted. In this model, APC thus regulates activation of Wg/Wnt target genes through dual mechanisms of βcat destruction and retention. In wild-type embryos (Figure 10A), both mechanisms are intact; thus in posterior cells (which do not receive Wg signal), βcat is destroyed to a low, basal level and APC sequesters additional βcat in the cytoplasm. Nuclear βcat levels consequently remain low, Wg/Wnt genes are repressed, and cells are fated to produce denticles. However, in anterior cells that receive Wg/Wnt signal, the destruction complex is inactivated and βcat protein levels accumulate modestly. The stoichiometry of cytoplasmic βcat may exceed that of APC, permitting some βcat to translocate into the nucleus, where it promotes target gene expression and a naked cuticle cell fate. In contrast, APC null embryos (Figure 10B) are defective for both destruction and retention, resulting in high nuclear βcat levels and a naked cuticle cell fate throughout the epidermis. Finally, in embryos expressing an APC2 mutant lacking all βcat-binding sites (Figure 10C), βcat destruction is largely intact but retention disrupted. Cells in the anterior of the segment hyperaccumulate βcat (as discussed above); thus they have high nuclear βcat levels and acquire a naked cuticle fate. However, posterior cells destroy βcat to levels indistinguishable from wild type. Although destruction is intact in these cells, the inability of APC2Δ15RΔ20R1, R3–R5 to sequester βcat allows enough residual βcat to transport into the nucleus and activate Wg/Wnt target genes above the threshold for naked cuticle fate. A small increase in nuclear βcat is likely sufficient to specify naked cuticle fate, as APC2 null Drosophila embryos accumulate modest levels of Arm throughout the epidermis yet produce largely naked cuticle (McCartney et al. 1999, 2006).
Figure 10.
Model showing APC regulates Wg/Wnt signaling outputs through dual mechanisms of Arm/βcat destruction and retention. (A) In Drosophila embryos, a single cell in each segment secretes Wg ligand, resulting in an asymmetric Wg gradient due to the effects of Hedgehog signaling at the parasegment boundary (Sanson et al. 1999). Cells in the anterior of the segment respond to Wg signal, resulting in Arm/βcat accumulation and repression of the transcription factor Shavenbaby (Payre et al. 1999), thus establishing a naked cuticle phenotype. In contrast, the dual actions of Arm/βcat destruction and retention in posterior cells result in low nuclear Arm/βcat levels, Shavenbaby activation, and denticle cell fate. Moreover, cells immediately adjacent to the Wg-secreting cell express the Wg target gene engrailed. (B) In APC mutants, Arm/βcat destruction and retention are both disrupted, resulting in high nuclear Arm/βcat levels throughout the epidermis. The Engrailed expression domain is expanded in the posterior compartment, Shavenbaby is repressed, and cells acquire a naked cuticle fate. (C) In embryos expressing an APC lacking all Arm/βcat-binding sites, Arm hyperaccumulates in anterior cells, but is destroyed to seemingly wild-type levels in posterior cells (Figure 6H). Anterior cells thus have high nuclear Arm/βcat levels and repress Shavenbaby, whereas posterior cells accumulate nuclear Arm/βcat above the threshold to specify naked cuticle fate due to the lack of Arm/βcat retention.
From an evolutionary perspective, duplication of the 15R and 20R βcat-binding sites would therefore provide two major selective advantages: (1) additional βcat-binding sites would increase the efficiency of βcat destruction by docking more βcat in the complex for rapid turnover, but (2) they would also blunt Wg/Wnt target gene activiation via retention of βcat in the cytoplasm, permitting fine-tuning of signaling outputs. Furthermore, cytoplasmic retention of βcat could have important implications for APC’s role in colon cancer, as truncated alleles of APC that are destruction defective may modulate Wnt signaling through retention (Roberts et al. 2011), creating a “just right” level of Wnt activation to promote tumorigenesis (Albuquerque et al. 2002). It will be exciting to explore these possibilities further.
Supplementary Material
Acknowledgments
We thank M. Peifer and B. Davis for helpful comments on the manuscript and R. Moon and H. Clevers for reagents. We are grateful to P. Robinson, B. Greaves, and R. von Kleeck for generous experimental assistance with Figure 2, as well as to K. Jacob, B. Davis, J. Roberts, G. Roberts, and C. Roberts for technical support and advice. This work was supported by National Institutes of Health grant R15 GM107796 (to D.M.R.), startup funds from Franklin & Marshall College (to D.M.R.), and an Undergraduate Science Education Award from the Howard Hughes Medical Institute to Franklin & Marshall College (52007538).
Footnotes
Communicating editor: R. Duronio
These authors contributed equally to this work.
Literature Cited
- Ahmed Y., Hayashi S., Levine A., Wieschaus E., 1998. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93(7): 1171–1182. [DOI] [PubMed] [Google Scholar]
- Ahmed Y., Nouri A., Wieschaus E., 2002. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development 129(7): 1751–1762. [DOI] [PubMed] [Google Scholar]
- Akong K., Grevengoed E. E., Price M. H., McCartney B. M., Hayden M. A., et al. , 2002. Drosophila APC2 and APC1 play overlapping roles in wingless signaling in the embryo and imaginal discs. Dev. Biol. 250(1): 91–100. [DOI] [PubMed] [Google Scholar]
- Albuquerque C., Breukel C., van der Luijt R., Fidalgo P., Lage P., et al. , 2002. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum. Mol. Genet. 11(13): 1549–1560. [DOI] [PubMed] [Google Scholar]
- Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., et al. , 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316(5831): 1619–1622. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104(9): 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K. M., Peifer M., 2009. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb. Perspect. Biol. 1(2): a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S. H., Wacker I., Appelt U. K., Behrens J., Schneikert J., 2012. A common role for various human truncated adenomatous polyposis coli isoforms in the control of beta-catenin activity and cell proliferation. PLoS ONE 7(4): e34479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Estaras C., Moresco J. J., Yates J. R., 3rd, Jones K. A., 2013. alpha-Catenin interacts with APC to regulate beta-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 27(22): 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Nusse R., 2012. Wnt/beta-catenin signaling and disease. Cell 149(6): 1192–1205. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Jho E., Zeng L., Kurth T., Joos T., et al. , 1999. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J. Cell Biol. 145(4): 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese A., Marin O., Bustos V. H., Venerando A., Antonelli M., et al. , 2007. Chemical dissection of the APC Repeat 3 multistep phosphorylation by the concerted action of protein kinases CK1 and GSK3. Biochemistry 46(42): 11902–11910. [DOI] [PubMed] [Google Scholar]
- Gottardi C. J., Gumbiner B. M., 2004. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J. Cell Biol. 167(2): 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha N. C., Tonozuka T., Stamos J. L., Choi H. J., Weis W. I., 2004. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15(4): 511–521. [DOI] [PubMed] [Google Scholar]
- Hikasa H., Sokol S. Y., 2013. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 5(1): a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. D., Klaus A., Garratt A. N., Birchmeier W., 2013. Wnt signaling in stem and cancer stem cells. Curr. Opin. Cell Biol. 25(2): 254–264. [DOI] [PubMed] [Google Scholar]
- Hooper J. E., 1994. Distinct pathways for autocrine and paracrine Wingless signalling in Drosophila embryos. Nature 372(6505): 461–464. [DOI] [PubMed] [Google Scholar]
- Hsu W., Zeng L., Costantini F., 1999. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J. Biol. Chem. 274(6): 3439–3445. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida M., Matsuura Y., Usui H., Kikuchi A., 2000. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene 19(4): 537–545. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Richardson D. S., White R., Alber T., 1993. Dimer formation by an N-terminal coiled coil in the APC protein. Proc. Natl. Acad. Sci. USA 90(23): 11109–11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. E., Huang H., Zhao M., Zhang X., Zhang A., et al. , 2013. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 340(6134): 867–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Xu W., 2006. beta-Catenin destruction complex: insights and questions from a structural perspective. Oncogene 25(57): 7482–7491. [DOI] [PubMed] [Google Scholar]
- Kohler E. M., Derungs A., Daum G., Behrens J., Schneikert J., 2008. Functional definition of the mutation cluster region of adenomatous polyposis coli in colorectal tumours. Hum. Mol. Genet. 17(13): 1978–1987. [DOI] [PubMed] [Google Scholar]
- Kohler E. M., Brauburger K., Behrens J., Schneikert J., 2010. Contribution of the 15 amino acid repeats of truncated APC to beta-catenin degradation and selection of APC mutations in colorectal tumours from FAP patients. Oncogene 29(11): 1663–1671. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., et al. , 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275(5307): 1784–1787. [DOI] [PubMed] [Google Scholar]
- Krieghoff E., Behrens J., Mayr B., 2006. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J. Cell Sci. 119(Pt 7): 1453–1463. [DOI] [PubMed] [Google Scholar]
- Kunttas-Tatli E., Zhou M. N., Zimmerman S., Molinar O., Zhouzheng F., et al. , 2012. Destruction complex function in the Wnt signaling pathway of Drosophila requires multiple interactions between Adenomatous polyposis coli 2 and Armadillo. Genetics 190: 1059–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li V. S., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., et al. , 2012. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 149(6): 1245–1256. [DOI] [PubMed] [Google Scholar]
- Liu J., Xing Y., Hinds T. R., Zheng J., Xu W., 2006. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J. Mol. Biol. 360(1): 133–144. [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., He X., 2012. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb. Perspect. Biol. 4(12): a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie F. J., Stackpole M. M., Stone M. C., Clippard J. R., Rudnick D. A., et al. , 2010. Directed microtubule growth, +TIPs, and kinesin-2 are required for uniform microtubule polarity in dendrites. Curr. Biol. 20(24): 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B. M., Dierick H. A., Kirkpatrick C., Moline M. M., Baas A., et al. , 1999. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 146(6): 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B. M., Price M. H., Webb R. L., Hayden M. A., Holot L. M., et al. , 2006. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development 133(12): 2407–2418. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P., 1995. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA 92(7): 3046–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., et al. , 1991. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253(5020): 665–669. [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent A., Carreno S., 1999. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature 400(6741): 271–275. [DOI] [PubMed] [Google Scholar]
- Peifer M., Sweeton D., Casey M., Wieschaus E., 1994. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120(2): 369–380. [DOI] [PubMed] [Google Scholar]
- Peterson-Nedry W., Erdeniz N., Kremer S., Yu J., Baig-Lewis S., et al. , 2008. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev. Biol. 320(1): 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P., 2012. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4(5): a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard J. B., Zhong Z., Williams B. O., Yang Y., 2012. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 4(12): a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. M., Pronobis M. I., Poulton J. S., Waldmann J. D., Stephenson E. M., et al. , 2011. Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol. Cell 22(11): 1845–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. M., Pronobis M. I., Alexandre K. M., Rogers G. C., Poulton J. S., et al. , 2012a Defining components of the ss-catenin destruction complex and exploring its regulation and mechanisms of action during development. PLoS ONE 7(2): e31284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. M., Pronobis M. I., Poulton J. S., Kane E. G., Peifer M., 2012b Regulation of Wnt signaling by the tumor suppressor adenomatous polyposis coli does not require the ability to enter the nucleus or a particular cytoplasmic localization. Mol. Biol. Cell 23(11): 2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Albert I., Porfiri E., Munemitsu S., Polakis P., 1997. Loss of beta-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res. 57(20): 4624–4630. [PubMed] [Google Scholar]
- Sakanaka C., Weiss J. B., Williams L. T., 1998. Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc. Natl. Acad. Sci. USA 95(6): 3020–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas P. C., 2012. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol. 4(2): a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B., Alexandre C., Fascetti N., Vincent J. P., 1999. Engrailed and hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell 98(2): 207–216. [DOI] [PubMed] [Google Scholar]
- Schneikert J., Vijaya Chandra S. H., Ruppert J. G., Ray S., Wenzel E. M., et al. , 2013. Functional comparison of human adenomatous polyposis coli (APC) and APC-like in targeting beta-catenin for degradation. PLoS ONE 8(7): e68072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneikert J., Ruppert J. G., Behrens J., Wenzel E. M., 2014. Different roles for the Axin interactions with the SAMP vs. the second twenty amino acid repeat of adenomatous polyposis coli. PLoS ONE 9(4): e94413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T., Metcalfe C., Bienz M., 2007. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120(Pt 14): 2402–2412. [DOI] [PubMed] [Google Scholar]
- Seeling J. M., Miller J. R., Gil R., Moon R. T., White R., et al. , 1999. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science 283(5410): 2089–2091. [DOI] [PubMed] [Google Scholar]
- Smits R., Kielman M. F., Breukel C., Zurcher C., Neufeld K., et al. , 1999. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 13(10): 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos J. L., Weis W. I., 2013. The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5(1): a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Fu C., Ishikawa S., Stella A., Kojima M., et al. , 2008. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol. Cell 32(5): 652–661. [DOI] [PubMed] [Google Scholar]
- van Es J. H., Kirkpatrick C., van de Wetering M., Molenaar M., Miles A., et al. , 1999. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr. Biol. 9(2): 105–108. [DOI] [PubMed] [Google Scholar]
- Venken K. J., He Y., Hoskins R. A., Bellen H. J., 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314(5806): 1747–1751. [DOI] [PubMed] [Google Scholar]
- Voloshanenko O., Erdmann G., Dubash T. D., Augustin I., Metzig M., et al. , 2013. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat. Commun. 4: 2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kries J. P., Winbeck G., Asbrand C., Schwarz-Romond T., Sochnikova N., et al. , 2000. Hot spots in beta-catenin for interactions with LEF-1, conductin and APC. Nat. Struct. Biol. 7(9): 800–807. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu X., Gusev E., Wang C., Fagotto F., 2014. Regulation of the phosphorylation and nuclear import and export of beta-catenin by APC and its cancer-related truncated form. J. Cell Sci. 127(Pt 8): 1647–1659. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nusslein-Volhard C., 1986. Looking at embryos, pp. 199-228 in Drosophila, A Practical Approach, edited by D. B. Roberts. IRL Press, Oxford, England. [Google Scholar]
- Xing Y., Clements W. K., Le Trong I., Hinds T. R., Stenkamp R., et al. , 2004. Crystal structure of a beta-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol. Cell 15(4): 523–533. [DOI] [PubMed] [Google Scholar]
- Xu W., Kimelman D., 2007. Mechanistic insights from structural studies of beta-catenin and its binding partners. J. Cell Sci. 120(Pt 19): 3337–3344. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer L., Bienz M., 1999. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1(3): 144–151. [DOI] [PubMed] [Google Scholar]
- Zeng L., Fagotto F., Zhang T., Hsu W., Vasicek T. J., et al. , 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90(1): 181–192. [DOI] [PubMed] [Google Scholar]
- Zhou M. N., Kunttas-Tatli E., Zimmerman S., Zhouzheng F., McCartney B. M., 2011. Cortical localization of APC2 plays a role in actin organization but not in Wnt signaling in Drosophila. J. Cell Sci. 124(Pt 9): 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.