Abstract
Panicle type has a direct bearing on rice yield. Here, we characterized a rice clustered-spikelet mutant, sped1-D, with shortened pedicels and/or secondary branches, which exhibits decreased pollen fertility. We cloned sped1-D and found that it encodes a pentatricopeptide repeat protein. We investigated the global expression profiles of wild-type, 9311, and sped1-D plants using Illumina RNA sequencing. The expression of several GID1L2 family members was downregulated in the sped1-D mutant, suggesting that the gibberellin (GA) pathway is involved in the elongation of pedicels and/or secondary branches. When we overexpressed one GID1L2, AK070299, in sped1-D plants, the panicle phenotype was restored to varying degrees. In addition, we analyzed the expression of genes that function in floral meristems and found that RFL and WOX3 were severely downregulated in sped1-D. These results suggest that sped1-D may prompt the shortening of pedicels and secondary branches by blocking the action of GID1L2, RFL, and Wox3. Moreover, overexpression of sped1-D in Arabidopsis resulted in the shortening of pedicels and clusters of siliques, which indicates that the function of sped1-D is highly conserved in monocotyledonous and dicotyledonous plants.
Keywords: clustered spikelet, map-based cloning, pedicel, pollen fertility, pentatricopeptide repeat, rice
PEDICEL length is one of the most important properties of rice yield. However, little is known about the mechanisms that control pedicel elongation. Here, we report a novel rice panicle mutant with shortened pedicels (sped1-D), which results in the formation of spikelet clusters on secondary branches, and we present evidence that the sped1-D gene encodes a pentatricopeptide repeat protein involved in the gibberellin (GA)-signaling pathway. In addition, RFL and WOX3, which play important roles in inflorescence branch elongation, are downregulated in sped1-D.
Inflorescence architecture is an important evolutionary characteristic in the reproductive processes of flowering plants. Many studies have focused on inflorescence development, and many mutants and corresponding genes related to flowering have been isolated in model dicotyledonous plants, such as LEAFY (LFY) (Schultz and Haughn 1991), APETALA1 (AP1) (Mandel et al. 1992), CLV1, CLV2, CLV3 (Clark et al. 1993, 1995), WUSCHEL (WUS) (Laux et al. 1996; Mayer et al. 1998), BREVIPEDICELLUS (BP) (Venglat et al. 2002), and BELLRINGER (BLR) (Byrne et al. 2003) in Arabidopsis. In addition, the well-known WUS–CLV feedback loop was proposed, which regulates inflorescence structures generated from inflorescence meristems (Brand et al. 2000; Schoof et al. 2000; Nardmann and Werr 2006). In monocotyledonous plants, inflorescences are commonly referred to as panicles, and studies about these inflorescences have mainly focused on maize and rice. To date, a dozen mutants have been identified from maize, and their corresponding genes have been cloned, such as KNOTTED1 (Kn1) (Greene et al. 1994; Jackson et al. 1994), INDETERMINATE SPIKELET1 (IDS1) (Chuck et al. 1998), FASCIATED EAR2 (FEA2) (Taguchi-Shiobara et al. 2001), BRANCHED SILKLESS1 (BD1) (Chuck et al. 2002), and ZFL1, ZFL2 (Bomblies et al. 2003). In addition, more than 20 panicle-type mutants have been identified in rice. However, only lax (Komatsu et al. 2002), fzp9 (Yi et al. 2005), sp1 (Li et al. 2009), aberrant panicle organization 1 (APO1), (Ikeda-Kawakatsu et al. 2012), and TAWAWA1 (Yoshida et al. 2012) have been isolated. Cloning of additional genes that control different stages of inflorescence growth will provide further insights into the molecular mechanisms underlying rice panicle development.
The pentatricopeptide repeat (PPR) protein family is one of the largest and most perplexing families in plants (Small and Peeters 2000). There are 450 PPR proteins in Arabidopsis thaliana (Lurin et al. 2004) and 477 in rice (Oryza sativa) (O’Toole et al. 2008). The large number of PPR proteins in these plants is consistent with the role of these proteins in organelle gene expression and transcriptional regulation in plants (Delannoy et al. 2007; Schmitz-Linneweber and Small 2008). PPR proteins govern various steps in RNA metabolism, such as cleavage, splicing, stability, editing, and translation, by forming sequence-specific associations with RNA (Schmitz-Linneweber and Small 2008; Fujii and Small 2011). Increasing evidence indicates that PPR proteins play important roles in plant development (Ding et al. 2006; Sung et al. 2010; Sosso et al. 2012; Yuan and Liu 2012).
In this study, we characterized the rice mutant sped1-D, a clustered-spikelet dominant mutant with shortened pedicels and secondary branches and demonstrated that the mutant phenotype of sped1-D is due to two nucleotide substitutions in SPED1. The sped1-D gene encodes a mitochondrion-localized PPR-like protein, which indicates that this gene may regulate the development of inflorescence branches through a novel pathway.
Materials and Methods
Plant material and culture
The rice spontaneous mutant sped1-D, which contains clustered spikelets, was isolated in the breeding line 9311. A F2 mapping population with 1929 wild-type and 5598 mutant-type plants (χ2 test value is 1.576, <χ20.95 = 3.84, indicating that the segregation ratio is 1:3) was generated from crosses between the sped1-D mutant and the japonica variety TP309. All of these plants, including the parents and their offspring (Supporting Information, Table S5), were grown in the fields or greenhouse.
Scanning electron microscopy analysis
Samples were fixed overnight in FAA at 4°. After dehydration in a graded ethanol series and substitution with 3-methyl-butyl-acetate, the samples were critical-point dried, sputter-coated with gold, and observed under a scanning electron microscope (S-3000N; Hitachi Ltd., Tokyo, Japan) at an accelerating voltage of 10 kV.
Positional cloning and complementation test of sped1-D
The sped1-D locus was mapped to a 19.2-kb DNA fragment between two closely linked sequence-tagged site (STS) markers, csp30 and csp25, on chromosome 6 using an F2 population of sped1-D and TP309 (spp. japonica). Three putative genes were identified in this region using a gene prediction program, Rice Automated Annotation System (http://RiceGAAS.dna.affrc.go.jp). The genomic sequences of the sped1-D candidate gene in the sped1-D mutant were determined by performing direct sequencing after PCR amplification. For complementation, a 2855-bp fragment, which included 775 bp upstream of the initiation codon and 567 bp downstream of the stop codon of sped1-D, was cloned into the binary vector pCAMBIA1301 and introduced into wild-type, TP309, and 9311 rice by Agrobacterium-mediated transformation (Hiei et al. 1994). The full-length cDNA of sped1-D was prepared and cloned into pCAMBIA1300 under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The overexpression construct was also transformed into TP309 and 9311 using Agrobacterium-mediated transformation.
DGE analysis
Immature wild-type and mutant panicles were harvested at heading time, and their total RNAs were extracted using Trizol (Invitrogen). DGE (digital gene expression profiles) library construction and sequencing were carried out at Beijing Genomics Institute (BGI-Shenzhen, Shenzhen, China). Raw sequences were transformed into clean tags by removing dirty raw reads. The total numbers of clean tags from the wild-type and mutant library were 5,678,386 and 5,737,757, respectively. All clean tags were mapped to the reference sequences and only 1-bp mismatch was considered. Clean tags mapped to reference sequences from multiple genes were filtered. The remaining clean tags were designed as unambiguous clean tags. The number of unambiguous clean tags for each gene was calculated and normalized to TPM (number of transcripts per million clean tags) (‘t Hoen et al. 2008; Morrissy et al. 2009). The rigorous algorithm p(χ) = e−λλχ/χ! (λ and χ represent the real transcripts and clean tags of the gene, respectively) was used to identify differentially expressed genes (DEGs) between two samples (Audic and Claverie 1997). P-value corresponds to the differential gene expression test, and the false discovery rate (FDR) method was used to determine the threshold of P-values. The DEGs were identified using the criteria FDR ≤ 0.001 and absolute value of the log2 ratio ≥ 1. Cluster analysis of gene expression patterns was performed with Cluster (Eisen et al. 1998) and Java Treeview (Saldanha 2004) software. Significantly enriched metabolic pathways and signal transduction pathways were identified using the KEGG database to perform pathway enrichment analysis of DGEs compared with the whole genome background.
Bioinformatics analysis
The full-length cDNA of SPED1 was obtained from the Rice Genome Resource Center (http://www.rgrc.dna.affrc.go.jp/). Domain prediction for SPED1 was performed using the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). A search for SPED1 homologs in plants was performed using the NCBI BLAST server (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence alignment was performed using Multalin (http://multalin.toulouse.inra.fr/multalin/). Unrooted neighbor-joining trees of SPED1 homologs were generated using MEGA5. Bootstrap values of >50% are shown. Prediction of the 3-D structure of SPED1 and sped1-D was carried out using phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/). The template for homology modeling was the crystal structure of the pentatricopeptide repeat protein ppr10 from maize.
Subcellular localization
The SPED1 and sped1-D coding regions were amplified without their stop codons and inserted into the multiple cloning site (MCS) of the CaMV 35S promoter–MCS–YFP coding sequence-NOS terminator cassette of the pSAT6-EYFP-C1 vector (Tzfira et al. 2005). This construct generated SPED1::YFP and sped1-D::YFP fusion proteins. MitoTracker Red (Invitrogen, http://www.invitrogen.com/), a mitochondrion-specific dye, was used to label the mitochondria. Protoplast preparation and transformation procedures were performed as previously described (Bart et al. 2006).
RT–PCR analysis
Total RNA was extracted from leaf tissue using Trizol (Invitrogen), and first-strand cDNA was synthesized using MMLV reverse transcriptase (Promega) and the oligo(dT) 15 primer. RT–PCR was performed using the following conditions: an initial 5-min denaturation at 95° followed by 95° for 30 sec, 56° for 30 sec, and 72° for 40 sec (35 cycles for SPED1 and the other genes, 26 cycles for Actin1).
Design and construction of artificial miRNA
WMD (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi) was used to predict artificial miRNAs targeting SPED1. Two 21-nt miRNA sequences were selected to construct the artificial miRNA (SPED-1, GTGAGCAAGATTCCCAAATTA; SPED-2, TGACGTTCCAATTAACAAGGA). Artificial miRNA precursors of SPED-1 and SPED-2 were then amplified and subcloned into the plant binary vector pCambia2300 under the control of the maize ubiquitin promoter, using the pNW55 plasmid (which contains a natural osa-MIR528 precursor) as the template, to simultaneously replace the 21 bases of the natural osa-MIR528 miRNA and miRNA*, as described previously (Warthmann et al. 2008). The primers used in this experiment include the following: KPN1-OSMIR528-F, 5′-TCGGTACCCAGCAGCAGCCACAGCAAA-3′; BAMH1-OSMIR528-R, 5′-TCGGATCCGCTGCTGATGCTGATGCCAT-3′; osMi528-SPED-1-F, 5′-TTGGCTGTAGCAGCAGCAGTAATTTGGGAATCTTGCTCACCAGGAGATTCAGTTTGAAG-3′; osMi528-SPED-1-R, 5′-AACAGCCTAGCAGCAGGAATAATTTAGGAAACTTCCTCACAGAGAGGCAAAAGTGAAGT-3′; osMi528-SPED-2-F,5′-TTGGCTGTAGCAGCAGCAGTCCTTGTTAATTGGAACGTCACAGGAGATTCAGTTTGAAG-3′; osMi528-SPED-2-R, 5′-AACAGCCTAGCAGCAGGAATCCTTGCTAATAGGATCGTCAAGAGAGGCAAAAGTGAAGT-3′.
Analysis of RNA editing
Total RNAs extracted from seedling leaves were treated with RQ1 DNase (Promega). Then, cDNAs were synthesized using MMLV reverse transcriptase. These cDNAs were used as templates for PCR amplification of mitochondrial genes. Information about sequences and editing sites was obtained from the RNA Editing Database (REDIdb; http://bio-logia.unical.it/py_script/search.html) (Picardi et al. 2007). Primers were designed to cover all 491 mitochondrial editing sites, and PCR was performed using Taq polymerase. The RT–PCR products were directly sequenced. Sequencing chromatograms were manually compared between the wild type and mutant.
To analyze RNA editing efficiency, PCR was performed with Pfu DNA polymerase (Promega) using the Nad9 primer set. The product was recovered from the agarose gel and ligated into the pGEMTeasy vector (Promega). A total of 100 independent positive clones per sample were sequenced.
Results
Shortened pedicels or secondary branches in rice sped1-D mutant
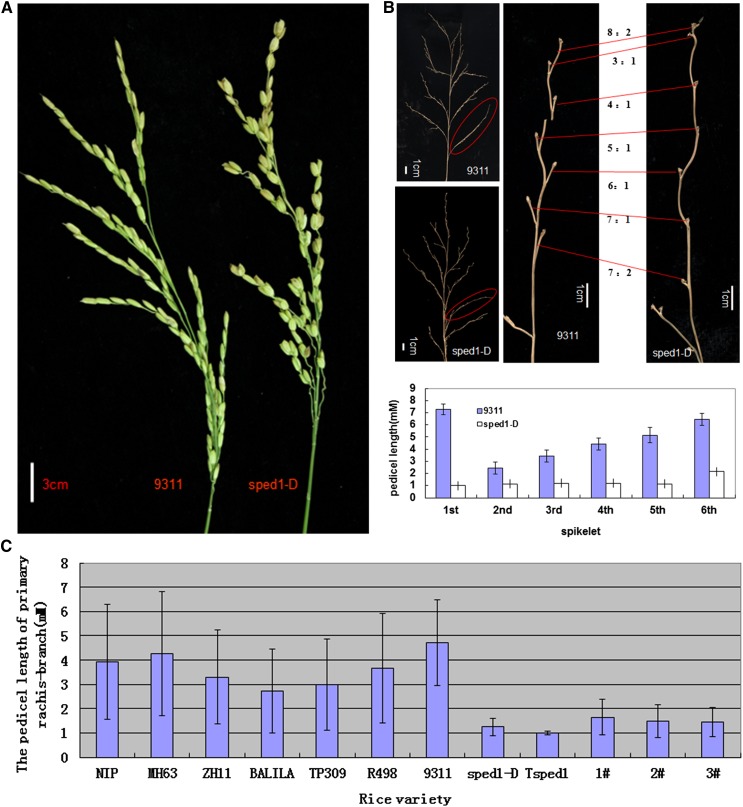
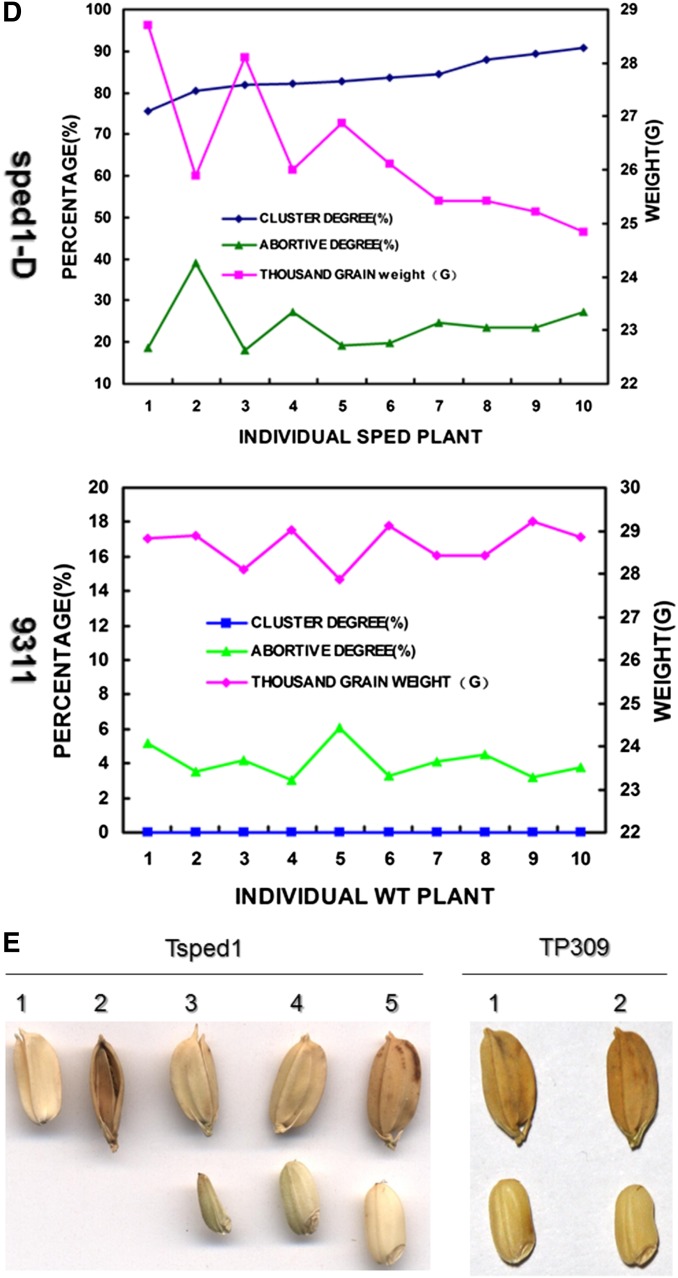
In this study, we identified a rice mutant of the O. sativa ssp. indica cultivar 9311. All of its pedicels and some secondary branches were significantly shortened, which caused varying degrees of spikelet clustering (Figure 1A). All of the pedicels in the mutant were ∼1 mm long, regardless of whether they were clustered (Figure 1B). The degree of spikelet clustering mainly depended on the extent to which the secondary branches were shortened. Namely, when the secondary branches were shortened in one to three segments from the top of the panicle, a two- to four-spikelet cluster was produced. The pedicel lengths of the first to sixth spikelet of a primary branch of the mutant were much shorter than those of the wild-type rice varieties (Figure 1C). A decrease in pollen fertility was also found in the mutant compared with the wild type (Figure S1). Moreover, as the degree of clustering increased in a panicle, the degree of abortion increased, while the thousand-grain weight of the rice decreased (Figure 1, D and E). In light of this discovery, we name the mutant sped1 (SHORTENED PEDICELS AND/OR SECONDARY BRANCHES 1).
Figure 1.
The phenotype of the panicles of the sped1-D mutant. (A) Cluster spikelets of sped1-D (right) vs. the wild type, 9311 (left). (B) Comparison of pedicel lengths in secondary branches between sped1-D and 9311. The ratio of the lengths of pedicels 1–6 on one secondary branch from the same position in TP309 and sped1-D (left); statistical analysis of the length of six pedicels on secondary branches (right). Error bars indicate standard error (SE) for sevenreplicate experiments. (C) Pedicel lengths of secondary branches in seven rice varieties, Tsped1, sped1-D, and three hygromycin-positive T0 complementation transgenic plants (1–3). Error bars indicate SE for seven replicate experiments. (D) The degree of abortion and thousand-grain weight of sped1-D seeds are negatively correlated with the degree of clustering in spikelets. (E) Five types of seeds are present in the mutant Tsped1, normal glumes, and paleas but without kernels; 2, abnormal paleas and without kernels; 3–5, seeds with normal kernels but altered grain filling.
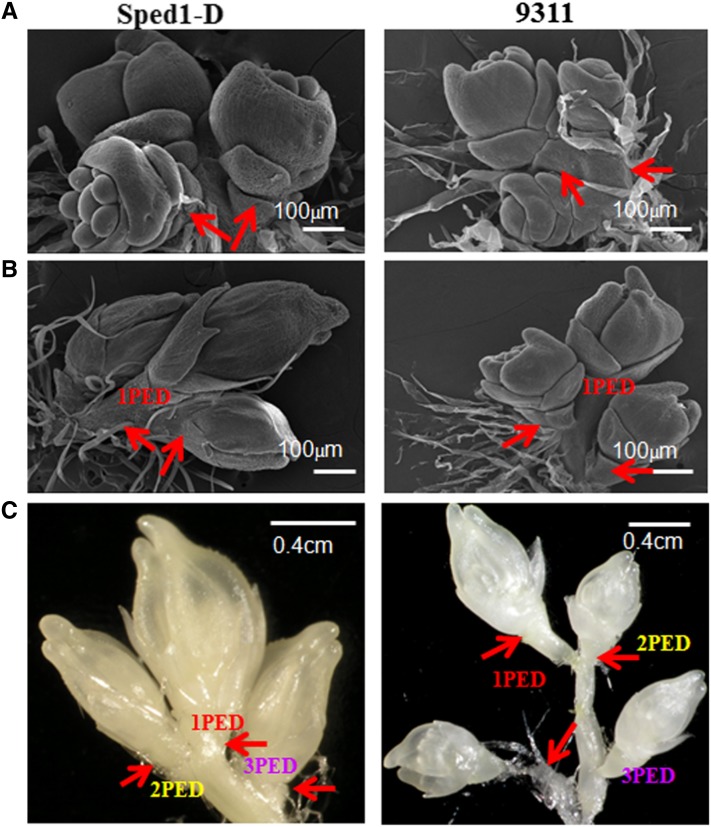
Rice inflorescences normally go through nine stages of development, from the establishment of the rachis meristem to heading and flowering (Ikeda et al. 2004). SEM and light microscopy revealed that there was no difference in panicle development between wild-type and sped1 plants from stage 1 to stage 6 (data not shown); the spikelet pedicels and secondary branches were not elongated in either plant (Figure 2, A and B). However, after stage 7, the pedicels of the spikelets and secondary branches of the wild type elongated quickly, while those of sped1 did not (Figure 2C). We applied nine hormones including GA3 to sped1 during panicle initation once a week for a month. However, none of the shortened pedicels or secondary branches in the mutant plants was affected (Table S1).
Figure 2.
Morphology of sped1-D and 9311 panicles, as observed by light and scanning electron microscopy (SEM). (A) SEM images showing that there was no difference in the development of spikelets between sped1-D and 9311 during the early stages of floral organ differentiation.Bars, 100 µm. (B) SEM images showing that the pedicels and top peduncles did not elongateduring the advanced stage of floral organ differentiation in sped1-D and 9311. Bars, 100 µm. (C) The pedicels and top peduncles of 9311 rapidly elongated during the time of rapid elongation of rachis and branches, while those of sped1-Ddid not elongate. Bars, 120 µm; red arrows indicate the pedicels.
To study the heredity of the mutant sped1, we crossed the mutant with dozens of rice varieties, including TP309, R549, R498, R527, and Balila. The F1 plants all exhibited clustered spikelets to varying degrees, and three-quarters of the F2 plants exhibited two- to four-spikelet clusters, depending on the genetic background. These results indicate that the clustered spikelet phenotype is controlled by an incompletely dominant gene in the indica background (Table S2). Therefore, the sped1 mutant was formally designated sped1-D (dominant) relative to wild type (SPED1, recessive), and a series of sped1-D identical mutants in different genetic backgrounds were also designated, such as TP309-sped1-D (Tsped1), Kitaake-sped1-D (Ksped1), R549-sped1-D (R549sped1), R498-sped1-D (R498sped1), R527-sped1-D (R527sped1), and Balila-sped1-D (Balilasped1; Figure S2 and Table S5). The grain plumpness and thousand-grain weight were affected in each of these mutants (Figure 1, D and E).
Isolating the sped1-D gene by map-based cloning and identical-gene mutant analysis
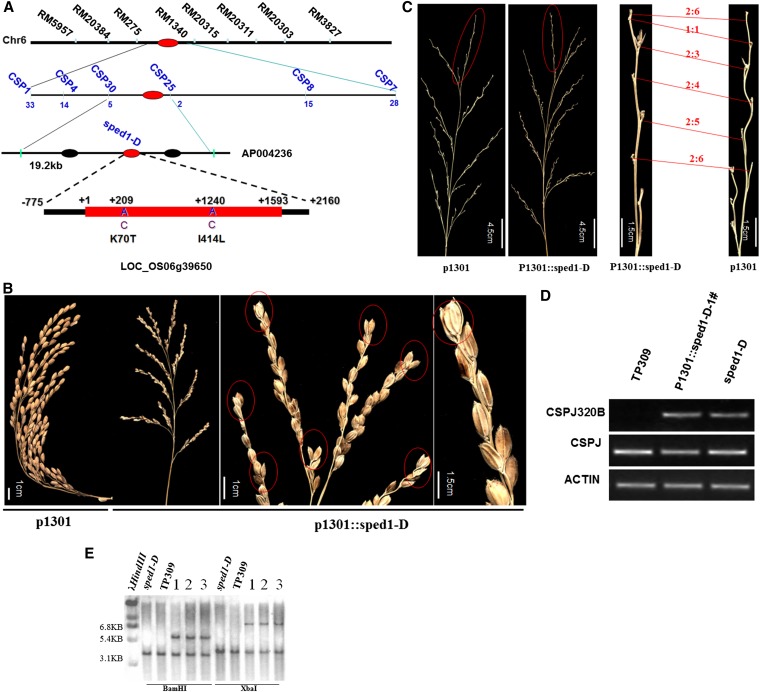
Genetic analysis showed that the sped1-D phenotype is controlled by a dominant gene. We isolated the sped1-D gene using map-based cloning. First, a primary mapping experiment, which employed 349 F2 wild-type plants obtained by crossing sped1-D with TP309, revealed that the sped1-D gene was anchored to a 0.3-cM interval between two SSLP markers, RM5957 and RM3827, on chromosome 6. These two flanking markers were used to screen 1580 F2 wild-type individuals to detect recombination in the regions flanking sped1-D. RM5957 identified 30 recombinants, and RM3827 identified 19 recombinants. Next, six SSLP markers and six STS markers (Table S3) were further analyzed in the 49 recombinants to narrow down the region containing sped1-D. Two recombinants between sped1-D and CSP25 and 4 recombinants between sped1-D and CSP30 were detected. Thus, sped1-D was fine-mapped to a 19.2-kb region on the P1-derived artificial chromosome clone (PAC), AP004236. There were three open reading frames located in this region, namely LOC_Os06g39640, LOC_Os06g39650, and LOC_Os06g39660. Next, we sequenced all three genes from six identical sped1-D mutants (Balilasped1, Tsped1, Ksped1, R549sped1, R498sped1, and R527sped1) and the corresponding wild-type plants (Balila, TP309, Kitaake, R549, R498, and R527). We found that there were two nucleotide substitutions, namely, A209C and A1240C, in the coding region of LOC_Os06g39650 in all six allelic mutants, which resulted in changes in two amino acids (K70T and I414L). These results strongly suggest that LOC_Os06g39650 is the best candidate for the sped1-D gene (Figure 3A).
Figure 3.
Map-based cloning ofsped1-D. (A) After the initial mapping of sped1-D on chromosome 6 using SSLP markers, the sped1-Dlocus was further narrowed down and limited to a 19.2-kb region of the PAC clone AP004236, between the STS marker CSP30 and CSP25, by analyzing 1580 F2 plants; three genes were predicted to be located in this region. The number of recombination events between the markers and the sped1-D locus is shown under the horizontal line; sequence analysis of the three genes between sped1-D and wild type showing that the second gene, LOC_OS06g39650, with two nucleotide substitutions, is the candidate gene, which was again confirmed by analyzing the sequence of the other seven isogenic sped1-D mutants. The three nucleotide substitution loci are marked in this figure, and below these, the corresponding amino acid substitutions are indicated. (B) Functional complementation of the wild type, TP309, with the candidate mutant transgene. The hygromycin-positive transgenic plants containing p1301-sped1-D exhibited the two- to three-spikelet cluster phenotype, while those with p1301(CK) showed the normal panicle phenotype I. Scale bar, 1.5 cM. (C) The pedicels of transgenic plants with p1301-sped1-D were shortened compared with those of control plants; the ratios of the lengths of pedicels 1–6 on one secondary branch from the same position in p1301 (right) and p1301::sped1-D (left)are indicated. (D) RT–PCR analysis with SPED1 or sped1-D-specific primers, CSPJF (5-GAAGCAATTCCATGCAATGAGG-3), CSPJR (5-GGTCCAGTCAAACTAATGG-3′), and the discriminating STS markerprimers, CSPJ320BF (5′-CAATGAGGGAGGTTTATCAGC-3′), CSPJ320BR (5′-CCAAACAGTGGCATAGCATCCTTT-3′). All plants produced a 400-bp band; the 1 T0 transgenic plant and sped1-D mutant both produced a 144-bp band, while no band was seen in the control plants (TP309). Actin was used as a control. (E) Three T2 plants of the 1 transgenic line were analyzed by Southern analysis. Genomic DNA was digested with restriction enzyme BamHI and XbaI, respectively, and hybridized with the sped1-D gene probe. Two bands were observed in transgenic plants, and only one band was present in the control plants.
The candidate gene was further confirmed by genetic complementation. A 2855-bp fragment containing the entire LOC_Os06g39650 gene from sped1-D plants was cloned into pCAMBIA1301 and introduced into rice cultivars 9311 (O. Sativa ssp. indica) and TP309 (O. Sativa ssp. Japonica) via Agrobacterium tumefaciens-mediated transformation. A total of 12 hygromycin-positive T0 transgenic plants from TP309 were obtained, while none was obtained from 9311. Of these TP309 transgenic plants, 5 plants showed the clustered spikelet phenotype, with two or three spikelets clustering on secondary branches due to the shortening of their pedicels and secondary branches (Figure 3, B and C and Figure S3). All of the T1 plants were further examined by performing PCR amplification of the hygromycin phosphotransferase gene and Southern blot analysis using the candidate gene as a probe (Figure 3, D and E). The results confirmed that we indeed cloned the rice sped1-D gene.
sped1-D encodes an uncharacterized protein containing five PPR groups and a truncated E-motif
The sped1-D gene, which is intronless, contains one 1593 nucleotide coding sequence and encodes a 530-amino-acid protein. The middle region of sped1-D contains five groups of PPR motifs, which demonstrates that sped1-D encodes a PPR-like protein. Phylogenetic analysis among sped1-D and other PPR proteins showed that sped1-D shares the highest sequence similarity with Arabidopsis proteins such as at1g31430 and SLO1 (Sung et al. 2010). Sequence alignment showed that sped1-D protein contains a truncated E-motif and two conserved altered amino acids, 70K and 414I (Figure S4). Secondary and 3-D structural analysis with Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2) showed that the two point mutations in sped1-D resulted in an increase in the proportion of alpha helices, which altered the conformation of this protein (Figure S4, File S3, and File S4). These results suggest that the alteration of two amino acids affects the organelle targeting or nucleotide binding of sped1-D.
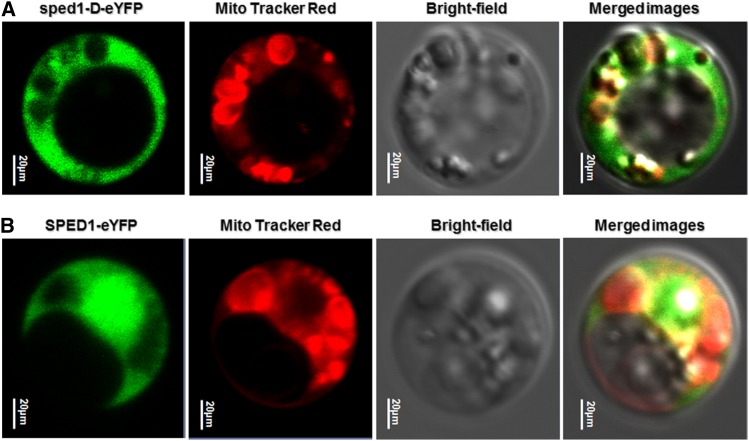
SPED1/sped1-D is predicated to be targeted to the mitochondria, with a cleavage site located at the 24th-amino-acid residue (probability is 0.874 and 0.9136, respectively) by TargetP1.1 (http://www.cbs.dtu.dk/services/TargetP/) and MitoProtP (http://ihg.gsf.de/ihg/mitoprot.html). SPED1::YFP and sped1-D::YFP plasmid were then transferred into protoplasts of the rice variety TP309, and the transformed protoplasts were treated with Mito Tracker Red and examined by confocal laser scanning microscopy. The yellow fluorescent signal was mainly localized to the cytoplasm, indicating that the SPED 1-YFP and sped1-D-YFP fusion proteins are mainly targeted to the cytoplasm (Figure 4).
Figure 4.
Subcellular localization of SPED 1 and sped1-D. Transient expression of sped1-D::eYFP (A) and SPED1::eYFP (B) in rice protoplasts.
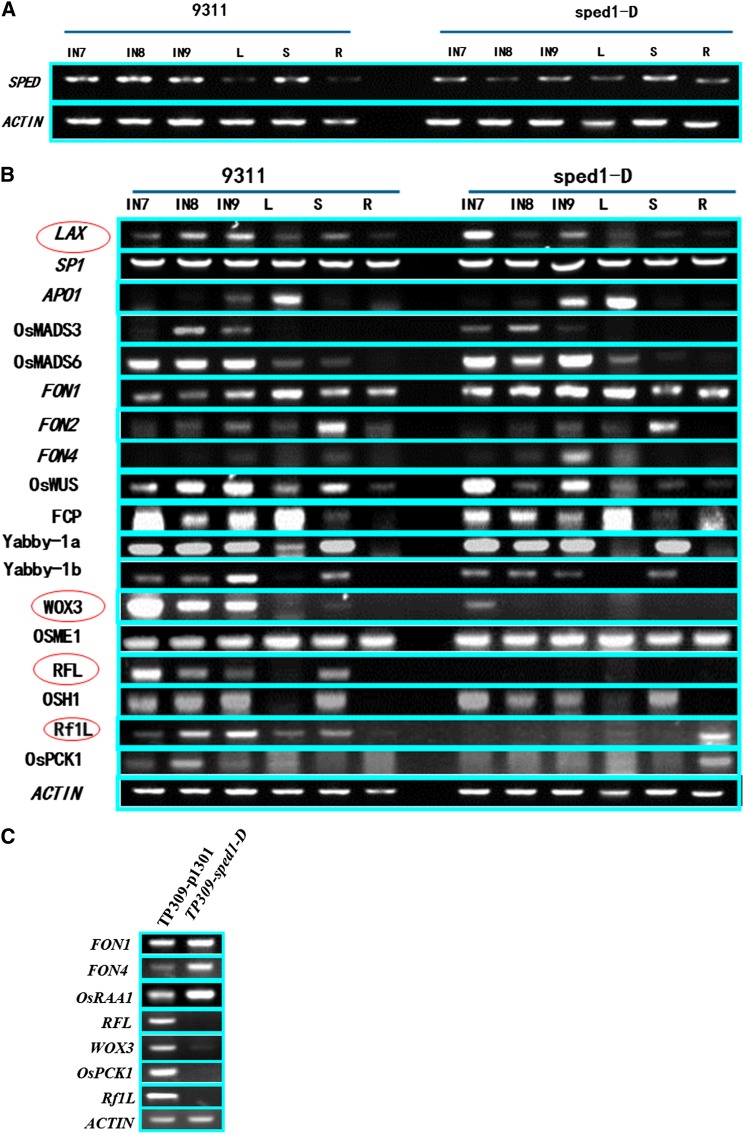
The expression patterns of sped1-D and inflorescence development-related genes
SPED 1 or sped1-D was constitutively expressed in young inflorescences and other tissues or organs (Figure 5A). However, sped1-D had some effects on the expression of other genes associated with flower development (Figure 5B and Table S4). For example, OsWUS (Nagasaki et al. 2005), FON1(Suzaki et al. 2004), and FON4 (Chu et al. 2006) were slightly upregulated in the mutant, while WOX3 (Dai et al. 2007) and Rf-1-like (Rf-1L, LOC_Os01g42620) (Komori et al. 2004) were strongly downregulated; RFL (Kyozuka et al. 1998) was entirely silenced. OsWUS, FON1, and FON4 are involved in the determination of meristem size, while WOX3 is involved in meristem activation, Rf1L is involved in fertility and RFL plays an important role in inflorescence branch elongation. These results indicate that sped1-D promotes the shortening of pedicels and secondary branches, perhaps through the CLV-WUS pathway, and influences pollen fertility through the action of Rf1L. Other genes, such as OsME1 (Nomura et al. 2005) in stress–response pathways and OsPCK1 (Nomura et al. 2005) in photosynthetic pathways, were also regulated by sped1-D. The similar expression patterns were observed in transgenic TP309 plants containing the mutant gene LOC_Os06g39650 (Figure 5C), which confirm that the mutant LOC_Os06g39650 is sped1-D.
Figure 5.
Expression analysis of flowering-related genes in wild-type, sped1-D, and transgenic plants containing p1301-sped1-D. (A) RT–PCR analysis shows that SPED1 or sped1-D constitutivelyexpressed in young inflorescences from stage 7 to stage 9 (IN7 to IN9) and in the shoot (S), with slight downregulation in leaves (L) and roots (R). (B) RT–PCR analysis of 17 flowering-related genes. RFL, WOX3, and Rf-1L were strongly downregulated or entirely silenced in young inflorescences. (C) The expression pattern of these flowering-related genes in young, stage 9 TP309 panicles containing p1301-sped1-D was the same as that of the sped1-D mutant.
Transgenic rice and Arabidopsis overexpressing sped1-D have clustered spikelets and siliques, respectively
To investigate how the transcription of sped1-D affects panicle development, a binary plasmid with sped1-D driven by the CaMV 35S promoter (sped1-D-OX) was introduced into wild-type TP309 rice via Agrobacterium-mediated transformation. Twenty independent transgenic lines showed varying degrees of spikelet clustering; RT–PCR analysis revealed that the levels of sped1-D transcript increased accordingly (Figure S5). These results indicate that sped1-D is a dominant gene, and the function of SPED1 is inhibited in these transgenic plants.
SPED 1 is highly homologous to some Arabidopsis proteins, such as SLO1, CRR4, CRR2, and especially At1g31430 (Figure S4). We produced transgenic Arabidopsis Col-0 plants that overexpressed sped1-D, and observed a series of novel phenotypes, including impaired meristem activation and defective elongation of terminal pod pedicels in T0 transgenic plants (Figure 6, A and B). These results indicate that the function of sped1-D is highly conserved in monocotyledonous and dicotyledonous plants.
Figure 6.
The function of sped1-D is conserved between Arabidopsis and rice. (A) Overexpression of sped1-D in Arabidopsis Col-0 results in the defective elongation of terminal pedicels and pods (II and III, wild type; I, IV–VII, transgenic plant and its pod). (B) PCR check of sped1-D (CSPJ320B)and hygromycin-resistance gene (HPT) in T0 transgenic Arabidopsis plants.
SPED 1 and sped1-D also affect pollen fertility in rice spikelets
Because the fertility of the sped1-D mutant was negatively correlated with the presence of spikelet clusters (Figure 1) and sped1-D encodes a PPR-like protein, we speculated that sped1-D is somehow related to rice spikelet fertility. To verify this notion, we first searched international rice mutant resources for suitable TOS17 and T-DNA insertion lines for SPED1, but no such line was available. Therefore, we generated SPED1 knockdown transgenic lines by cleaving SPED1 transcripts using an artificial miRNA method (Warthmann et al. 2008). The precursor of osa-MIR528 (accession no. MI0003201) was selected as the endogenous stemloop backbone for the artificial miRNA transgenes. Then, two artificial miRNA-directed sped1-D RNA silencing binary vectors, miR528-sped-1 and miR528-sped-2, were constructed and introduced into TP309 via Agrobacterium-mediation transformation. A total of five and nine G418-positive transgenic T0 lines were obtained, respectively. All five transgenic T0 lines of miR528-sped-1 exhibited a normal phenotype during both the vegetative and reproductive stages, while six miR528-sped-2 lines showed poor spikelet fertility or even sterility (Figure S1). In addition, the six miR528-sped-2 lines exhibited a high level of miR528-sped-2 expression but no SPED 1 expression (Figure S5).
SPED 1 and sped1-D have no significant effect on RNA editing
Growing research suggests that PPR proteins with E motifs may participate in RNA editing in chloroplasts and mitochondria (Okuda et al. 2007, 2009; Sung et al. 2010; Takenaka 2010; Yuan and Liu 2012). SLO1, a homologous gene of SPED1, participates in two sites' editing of mitochondrial RNA (Sung et al. 2010), suggesting that SPED1 protein is involved in mitochondrial RNA editing. In rice mitochondria, there are ∼491 editing sites for 33 ORFs and one pseudogene (Notsu et al. 2002). We first analyzed the editing sites of the largest membrane-bound protein assemblies in rice mitochondria, complex I, because it covers the most editing sites. Complex I has more than 30 subunits, but only 9 (nad1–4, 4L, 5–7, and 9) are usually encoded by the mitochondrial genome. Therefore, we carried out bulk sequencing of the nine genes using the RT–PCR products from sped1-D and wild-type 9311. This analysis revealed that the editing efficiency of only three C residues (C92, C298, and C328) out of the 11 editing sites in Nad9 changed (from 20 to 40%, 25–45%, and 25–45%, respectively),while there was no difference in editing efficiency among the Nad1–4, 4L, or 5–7 genes. We then examined the editing efficiency of all 11 editing sites in SPED-RNAi transgenic plants, which exhibited low pollen fertility but no spikelet clusters, and found that their editing efficiency was increased, with editing events reaching 70–80% (Figure S6). No apparent difference in other editing sites of the mitochondrial genome was detected between sped1-D and wild-type 9311 (data not shown). These findings suggest that sped1-D is involved in regulating the editing efficiency of 11 editing sites of the Nad9 gene, but the latter is not involved in the shortening of pedicels and secondary branches.
GA signaling pathway involves in the elongation of pedicels and secondary branches
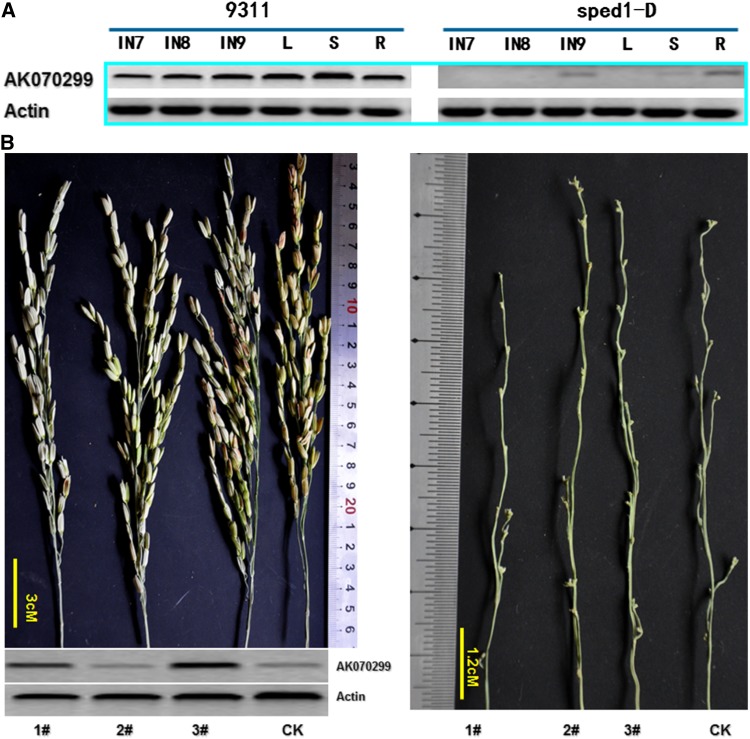
To elucidate the molecular mechanisms by which SPED1/sped1-D regulate the elongation of pedicels and secondary branches, we performed DEG analysis of panicles of sped1-D and wild-type 9311 using RNA-Seq technology. A total of 5,737,757 and 5,678,386 clean tags 21 bp in length were generated, respectively, which represent 52,845 genes, comprising ∼78.41% of rice genes. Sequencing saturation analysis indicated that the data were sufficient for quantitative analysis of gene expression. We used a FDR ≤ 0.001 and an absolute value of log2 ratio ≥ 1 as the threshold to judge the significance of differential gene expression. Of the 24,221 genes expressed in either sped1-D or 9311 plants, 1247 DEGs were detected, namely 672 genes that were upregulated and 574 that were downregulated in sped1-D relative to wild-type plants. Detailed gene lists (with fold changes, GO, gene descriptions, pathways, and other relevant information) are provided in File S1 and File S2. The expression of genes involved in hormone signaling and metabolism-related genes associated with cell growth and division such as carbohydrate metabolic process, aminoglycan metabolic process and cellulose synthase was primarily altered in the sped1-D mutant. Among these, gibberellin receptor GID1L2 family genes were downregulated in sped1-D, which may account for the mutant phenotype. The transcript level of LOC_Os06g11130 (AK070299) decreased 86.7% in sped1-D, which was further corroborated by RT–PCR analysis. A 450-bp specific band was amplified strongly from wild-type 9311 cDNA at the early panicle differentiation stage, while a very weak band was obtained from that of sped1-D (Figure 7A). In fact, LOC_Os06g11130 (AK070299) is expressed constitutively in organs and tissues of 9311. These results indicate that gibberellin receptor GID1L2 family genes, especially LOC_Os06g11130 (AK070299), may be involved in the elongation of pedicels and secondary branches. If the expression of LOC_Os06g11130 (AK070299) is required for the elongation of pedicels and secondary branches, its overexpression in sped1-D plants can restore the clustered spikelet phenotype to the normal phenotype. We used Agrobacterium-mediated transformation to produce rice harboring the full-length cDNA of LOC_Os06g11130 (AK070299) in the pZh01 vector (Xiao et al. 2003). We generated 55 plants from seven independent transgenic rice lines and confirmed the presence of the hygromycin-resistance gene in these plants by PCR. Of these, 9 plants showed the normal panicle phenotype, while 12 showed varying degrees of alleviation of spikelet clustering, which indicates that LOC_Os06g11130 (AK070299) is involved in the elongation of pedicels and secondary branches (Figure 7B).
Figure 7.
GA signal pathway involoves in the elongation of pedicels and secondary branches. (A) The expression pattern of gibberellin receptor GID1L2-like gene, AK070299 in sped1-D and wild type. (B) Overexpression of AK070299 gene in sped1-D mutant can restore its panicle phenotype. The recover level is associated with the expression level of AK070299 (1–3 refer the T0 sped1-D transgenic plants with overexpression of AK070299).
Discussion
Identical-gene mutant analysis is an effective strategy for cloning target genes
Map-based cloning has been widely used to help elucidate complex biological processes in plants, such as developmental regulation and gene expression cascades. However, critical bottlenecks of gene functional complementation analysis still exist, including the determination of candidate genes and the relatively low efficiency of genetic transformation. In this study, we succeeded in cloning sped1-D using map-based cloning in combination with identical-gene mutant analysis. First of all, we obtained identical-gene mutants of sped1-D in different backgrounds (Figure S2). Once the sped1-D gene was narrowed down to a limited region, we reliably obtained the target gene by comparing the sequences of this region among the identical-gene mutants. In addition, the functional complementation transformation can be accomplished easily in its identical-gene mutants with japonica background.
SPED1 might serve as a regulatory factor to affect genes expression
Most characterized PPR proteins serve as sequence-specific RNA-binding proteins inside organelles, regulating RNA processing, maturation, and translation. However, some PPR proteins are present outside of organelles. The Arabidopsis GLUTAMINE-RICH PROTEIN23 (GRP23) might interact physically with subunit III of RNA polymerase II through its C-terminal Gln-rich WQQ domain and bind directly to cis-regulatory elements of DNA through its N-terminal basic domain (Ding et al. 2006). PNM1 might be involved in the regulation of its own gene expression in the nucleus and play a role in gene expression adjustments between the mitochondria and the nucleus (Hammani et al. 2011). The two point mutations in sped1-D resulted in an increase in the proportion of alpha helices, leading to an altered protein conformation. These changes might affect the nucleotide binding abilities of sped1-D and the regulation of gene expression, such as LAX1, WOX3, RFL, Rf1L, and GID1L.
Pedicel and secondary branch elongation is mediated by gibberellin signaling
GAs regulate many phases of plant development, including seed germination, stem growth, floral induction, pollen development, and fruit growth (Kende and Zeevaart 1997; Olszewski et al. 2002). GA biosynthesis is mainly controlled by feedback regulation, which provides an important link between GA metabolism and GA-signaling pathways (Phillips et al. 1995; Hedden and Phillips 2000; Olszewski et al. 2002; Weston et al. 2008). GAs are synthesized from ent-kaurene via geranylgeranyl diphosphate, and all metabolic steps after ent-kaurene are oxidative. Among these oxidases, GA20-oxidase (GA20ox) is responsible for the removal of the C-20 of GA12 in the formation of C19-GAs (Mauriat and Moritz 2009). Arabidopsis plants harboring a mutant AtGA20ox1 (ga5) gene exhibit a semi-dwarf phenotype. In this study, gibberellin receptor GID1L2 family genes were downregulated in the sped1-D mutant. Therefore, treatment of sped1-D plants with GA during panicle initiation did not lead to elongation of secondary branches or pedicels, as the mutant meristems failed to respond to GA signaling. These results indicate that GA signaling plays an important role in the elongation of pedicels and secondary branches.
Supplementary Material
Acknowledgments
This research was supported by grants from the Ministry of Agriculture of China (2011ZX08009-001, 003) and the National Natural Science Foundation of China (30971545, 31071379, 31371590).
Footnotes
Communicating editor: J. A Birchler
Literature Cited
- Audic S., Claverie J. M., 1997. The significance of digital gene expression profiles. Genome Res. 7: 986–995. [DOI] [PubMed] [Google Scholar]
- Bart R., Chern M., Park C. J., Bartley L., Ronald P. C., 2006. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K., Wang R. L., Ambrose B. A., Schmidt R. J., Meeley R. B., et al. , 2003. Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130: 2385–2395. [DOI] [PubMed] [Google Scholar]
- Brand U., Fletcher J. C., Hobe M., Meyerowitz E. M., Simon R., 2000. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Groover A. T., Fontana J. R., Martienssen R. A., 2003. Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130: 3941–3950. [DOI] [PubMed] [Google Scholar]
- Chu H., Qian Q., Liang W., Yin C., Tan H., et al. , 2006. The floral organ number4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol. 142: 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Meeley R. B., Hake S., 1998. The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 12: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Muszynski M., Kellogg E., Hake S., Schmidt R. J., 2002. The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298: 1238–1241. [DOI] [PubMed] [Google Scholar]
- Clark S. E., Running M. P., Meyerowitz E. M., 1993. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119: 397–418. [DOI] [PubMed] [Google Scholar]
- Clark S. E., Running M. P., Meyerowitz E. M., 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121: 2057–2067. [Google Scholar]
- Dai M., Hu Y., Zhao Y., Liu H., Zhou D. X., 2007. A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development. Plant Physiol. 144: 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy E., Stanley W. A., Bond C. S., Small I. D., 2007. Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35: 1643–1647. [DOI] [PubMed] [Google Scholar]
- Ding Y. H., Liu N. Y., Tang Z. S., Liu J., Yang W. C., 2006. Arabidopsis GLUTAMINE-RICH PROTEIN23 is essential for early embryogenesis and encodes a novel nuclear PPR motif protein that interacts with RNA polymerase II subunit III. Plant Cell 18: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O., Botstein D., 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Small I., 2011. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 191: 37–47. [DOI] [PubMed] [Google Scholar]
- Greene B., Walko R., Hake S., 1994. Mutator Insertions in an intron of the maize Knotted1 gene result in dominant suppressible mutations. Genetics 138: 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K., Gobert A., Hleibieh K., Choulier L., Small I., et al. , 2011. An Arabidopsis dual-localized pentatricopeptide repeat protein interacts with nuclear proteins involved in gene expression regulation. Plant Cell 23: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P., Phillips A. L., 2000. Manipulation of hormone biosynthetic genes in transgenic plants. Curr. Opin. Biotechnol. 11: 130–137. [DOI] [PubMed] [Google Scholar]
- Hiei Y., Ohta S., Komari T., Kumashiro T., 1994. Efficient transformation of rice (Oryza sativa L.) mediated by agrobacterium and sequence-analysis of the boundaries of the T-DNA. Plant J. 6: 271–282. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Sunohara H., Nagato Y., 2004. Developmental course of inflorescence and spikelet in rice. Breed. Sci. 54: 147–156. [Google Scholar]
- Ikeda-Kawakatsu K., Maekawa M., Izawa T., Itoh J. I., Nagato Y., 2012. ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 69: 168–180. [DOI] [PubMed] [Google Scholar]
- Jackson D., Veit B., Hake S., 1994. Expression of maize Knotted1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413. [Google Scholar]
- Kende H., Zeevaart J., 1997. The five “classical” plant hormones. Plant Cell 9: 1197–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K., Okamoto H., Maekawa M., Shimamoto K., Kyouzuka J., 2002. Molecular cloning and analysis of LAX, a gene required for rice panicle branching. Plant Cell Physiol. 43: S219. [Google Scholar]
- Komori T., Ohta S., Murai N., Takakura Y., Kuraya Y., et al. , 2004. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J. 37: 315–325. [DOI] [PubMed] [Google Scholar]
- Kyozuka J., Konishi S., Nemoto K., Izawa T., Shimamoto K., 1998. Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proc. Natl. Acad. Sci. USA 95: 1979–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T., Mayer K. F., Berger J., Jurgens G., 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Li S. B., Qian Q., Fu Z. M., Zeng D. L., Meng X. B., et al. , 2009. Short panicle1 encodes a putative PTR family transporter and determines rice panicle size. Plant J. 58: 592–605. [DOI] [PubMed] [Google Scholar]
- Lurin C., Andres C., Aubourg S., Bellaoui M., Bitton F., et al. , 2004. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M. A., Gustafsonbrown C., Savidge B., Yanofsky M. F., 1992. Molecular characterization of the Arabidopsis floral homeotic gene Apetala1. Nature 360: 273–277. [DOI] [PubMed] [Google Scholar]
- Mauriat M., Moritz T., 2009. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 58: 989–1003. [DOI] [PubMed] [Google Scholar]
- Mayer K. F., Schoof H., Haecker A., Lenhard M., Jurgens G., et al. , 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Morrissy A. S., Morin R. D., Delaney A., Zeng T., McDonald H., et al. , 2009. Next-generation tag sequencing for cancer gene expression profiling. Genome Res. 19: 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki H., Matsuoka M., Sato Y., 2005. Members of TALE and WUS subfamilies of homeodomain proteins with potentially important functions in development form dimers within each subfamily in rice. Genes Genet. Syst. 80: 261–267. [DOI] [PubMed] [Google Scholar]
- Nardmann J., Werr W., 2006. The shoot stem cell niche in angiosperms: expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Mol. Biol. Evol. 23: 2492–2504. [DOI] [PubMed] [Google Scholar]
- Nomura M., Higuchi T., Ishida Y., Ohta S., Komari T., et al. , 2005. Differential expression pattern of C4 bundle sheath expression genes in rice, a C3 plant. Plant Cell Physiol. 46: 754–761. [DOI] [PubMed] [Google Scholar]
- Notsu Y., Masood S., Nishikawa T., Kubo N., Akiduki G., et al. , 2002. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol. Genet. Genomics 268: 434–445. [DOI] [PubMed] [Google Scholar]
- Okuda K., Myouga F., Motohashi R., Shinozaki K., Shikanai T., 2007. Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc. Natl. Acad. Sci. USA 104: 8178–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Chateigner-Boutin A. L., Nakamura T., Delannoy E., Sugita M., et al. , 2009. Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N., Sun T. P., Gubler F., 2002. Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14(Suppl.): S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole N., Hattori M., Andres C., Iida K., Lurin C., et al. , 2008. On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 25: 1120–1128. [DOI] [PubMed] [Google Scholar]
- Phillips A. L., Ward D. A., Uknes S., Appleford N. E., Lange T., et al. , 1995. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 108: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi E., Regina T. M., Brennicke A., Quagliariello C., 2007. REDIdb: the RNA editing database. Nucleic Acids Res. 35: D173–D177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha A. J., 2004. Java Treeview: extensible visualization of microarray data. Bioinformatics 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I., 2008. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 13: 663–670. [DOI] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K. F., Jurgens G., et al. , 2000. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Schultz E. A., Haughn G. W., 1991. Leafy, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell 3: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I. D., Peeters N., 2000. The PPR motif: a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 25: 46–47. [DOI] [PubMed] [Google Scholar]
- Sosso D., Mbelo S., Vernoud V., Gendrot G., Dedieu A., et al. , 2012. PPR2263, a DYW-subgroup pentatricopeptide repeat protein, is required for mitochondrial nad5 and cob transcript editing, mitochondrion biogenesis, and maize growth. Plant Cell 24: 676–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T. Y., Tseng C. C., Hsieh M. H., 2010. The SLO1 PPR protein is required for RNA editing at multiple sites with similar upstream sequences in Arabidopsis mitochondria. Plant J. 63: 499–511. [DOI] [PubMed] [Google Scholar]
- Suzaki T., Sato M., Ashikari M., Miyoshi M., Nagato Y., et al. , 2004. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131: 5649–5657. [DOI] [PubMed] [Google Scholar]
- ‘t Hoen P. A., Ariyurek Y., Thygesen H. H., Vreugdenhil E., Vossen R. H., et al. , 2008. Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res. 36: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi-Shiobara F., Yuan Z., Hake S., Jackson D., 2001. The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev. 15: 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka M., 2010. MEF9, an E-subclass pentatricopeptide repeat protein, is required for an RNA editing event in the nad7 transcript in mitochondria of Arabidopsis. Plant Physiol. 152: 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T., Tian G. W., Lacroix B., Vyas S., Li J., et al. , 2005. pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol. Biol. 57: 503–516. [DOI] [PubMed] [Google Scholar]
- Venglat S. P., Dumonceaux T., Rozwadowski K., Parnell L., Babic V., et al. , 2002. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 99: 4730–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthmann, N., H. Chen, S. Ossowski, D. Weigel and P. Herve, 2008 Highly specific gene silencing by artificial miRNAs in rice. PLoS One 3: e1829. [DOI] [PMC free article] [PubMed]
- Weston D. E., Elliott R. C., Lester D. R., Rameau C., Reid J. B., et al. , 2008. The Pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol. 147: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Wang Y., Liu D., Wang W., Li X., et al. , 2003. Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol. Biol. 52: 957–966. [DOI] [PubMed] [Google Scholar]
- Yi G. W., Choi J. H., Jeong E. G., Chon N. S., Jena K. K., et al. , 2005. Morphological and molecular characterization of a new frizzy panicle mutant, “fzp-9(t)”, in rice (Oryza sativa L.). Hereditas 142: 92–97. [DOI] [PubMed] [Google Scholar]
- Yoshida, A., M. Sasao, N. Yasuno, K. Takagi, Y. Daimon et al., 2012 TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc. Natl. Acad. Sci. USA 110: 767–72. [DOI] [PMC free article] [PubMed]
- Yuan H., Liu D., 2012. Functional disruption of the pentatricopeptide protein SLG1 affects mitochondrial RNA editing, plant development, and responses to abiotic stresses in Arabidopsis. Plant J. 70: 432–444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.