Abstract
Paramecium has long been a model eukaryote. The sequence of the Paramecium tetraurelia genome reveals a history of three successive whole-genome duplications (WGDs), and the sequences of P. biaurelia and P. sexaurelia suggest that these WGDs are shared by all members of the aurelia species complex. Here, we present the genome sequence of P. caudatum, a species closely related to the P. aurelia species group. P. caudatum shares only the most ancient of the three WGDs with the aurelia complex. We found that P. caudatum maintains twice as many paralogs from this early event as the P. aurelia species, suggesting that post-WGD gene retention is influenced by subsequent WGDs and supporting the importance of selection for dosage in gene retention. The availability of P. caudatum as an outgroup allows an expanded analysis of the aurelia intermediate and recent WGD events. Both the Guanine+Cytosine (GC) content and the expression level of preduplication genes are significant predictors of duplicate retention. We find widespread asymmetrical evolution among aurelia paralogs, which is likely caused by gradual pseudogenization rather than by neofunctionalization. Finally, cases of divergent resolution of intermediate WGD duplicates between aurelia species implicate this process acts as an ongoing reinforcement mechanism of reproductive isolation long after a WGD event.
Keywords: Paramecium, genome evolution, polyploidization, speciation, whole-genome duplication
THE genus Paramecium has been used as a model unicellular eukaryotic system for over a century, beginning with research by Jennings (1908) and leading to some of the earliest derivations of mathematical population-genetics properties (Jennings 1916, 1917). The subsequent discovery of mating types in members of the Paramecium aurelia complex (Sonneborn 1937) permitted the first systematic crossbreeding of different genotypes in any unicellular eukaryote. Later work provided fundamental insights into major issues in biology, including mutagenesis (Igarashi 1966), molecular and developmental genetics (Sonneborn 1947), symbiosis (Beale et al. 1969), mitochondrial genetics (Adoutte and Beisson 1972), aging (Siegel 1967), nuclear differentiation (Berger 1937), and gene regulation (Allen and Gibson 1972). More recently, Paramecium has been used to study maternal inheritance (Nowacki et al. 2005), programmed genome rearrangements and transposon domestication (Arnaiz et al. 2012), epigenetic inheritance (Singh et al. 2014), and whole-genome duplication (Aury et al. 2006; Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results). Although not as well-studied as the P. aurelia complex of species, P. caudatum also has a long history of research (Calkins 1902; Sonneborn 1933). Recent studies on P. caudatum involve investigations into quorum sensing (Fellous et al. 2012), thermal adaptation (Krenek et al. 2012), learning (Armus et al. 2006), endosymbiosis and parasite-mediated selection (Duncan et al. 2010, 2011), and ecotoxicology (Rao et al. 2007; Kawamoto et al. 2010; Hailong et al. 2011). P. caudatum cells are larger than those of the aurelia complex and have a single, larger micronucleus, whereas aurelia species have two smaller micronuclei (Fokin 2010). Like the P. aurelia species, P. caudatum appears to be widespread and cosmopolitan, with isolates identified on every continent except Antarctica (Fokin 2010).
With the discovery of at least three successive whole-genome duplications (WGDs) in the history of the P. tetraurelia lineage (Aury et al. 2006), there is renewed interest in the evolution and genetics of Paramecium. WGDs can be found in the ancestry of many model organisms, including zebrafish (Postlethwait et al. 2000), yeast (Wolfe and Shields 1997), Xenopus (Morin et al. 2006), and Arabidopsis (Simillion et al. 2002). Ancient WGDs are also likely to have occurred in the ancestor of all seed plants and the ancestor of all angiosperms (Doyle et al. 2008; Jiao et al. 2011), as well as the ancestor of all vertebrates (Panopoulou and Poustka 2005; Hughes and Liberles 2008; Putnam et al. 2008; Decatur et al. 2013). The evolutionary consequences of WGDs are far-reaching and include the possibilities of neofunctionalization and subfunctionalization of duplicated genes, as well as speciation through the process of divergent resolution of duplicates (Oka 1988; Werth and Windham 1991; Lynch and Conery 2000; Lynch and Force 2000). Retention of duplicate genes tends to be high after WGD compared to single-gene duplications, with 25–75% retention being typical (reviewed in Lynch 2007; Otto 2007). One explanation for this pattern is selection against breaking dosage balance among duplicate genes acting in the same pathway following WGD and opposing imbalances resulting from single-gene duplications (Yang et al. 2003; Davis and Petrov 2005; Veitia et al. 2008).
The sequencing of two additional aurelia species—P. biaurelia and P. sexaurelia—provided a detailed study of the consequences of the most recent WGD (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results), including the divergent resolution of duplicate genes between aurelia species. To further investigate all three WGDs (referred to here as recent, intermediate, and ancient) in the Paramecium lineage, we have sequenced the macronuclear genome of P. caudatum for use as a potential outgroup. Our results show that P. caudatum diverged from the aurelia complex before the two most recent Paramecium WGDs. We find that, although fewer duplicates from the intermediate WGD survive compared to the recent WGD, the forces governing retention have been largely similar for the two WGD events. In addition, we reveal that, despite the presence of the recent WGD, continuing divergent resolutions of intermediate WGD duplicates have occurred between species of the aurelia complex, further enforcing the reproductive isolation between these species.
Materials and Methods
DNA preparation and sequencing
To generate a fully homozygous stock of P. caudatum, a fresh isolate was derived from the wild and given the strain name 43. Cytogamy, a process similar to autogamy in aurelia species (Wichterman 1939), was then induced with 1.25% methyl cellulose (100 cP, Wako Pure Chemical Industries, Japan) for 5–8 hr (Yanagi and Haga 1995, 1998). Multiple lines were generated and screened for homozygosity, and line 43c3d was selected for sequencing. Roughly 2 liters of cells were then grown in Wheat Grass Powder (Pines International, Lawrence, KS) medium (Aury et al. 2006). Cells were subsequently starved, and Paramecium cells purified away from bacteria by filtration over a 10-μm Nitex membrane. Macronuclei were isolated away from other cellular debris by gentle lysis of the cell membrane and sucrose density separation (Aury et al. 2006). DNA was extracted and purified using a CTAB protocol (Doyle and Doyle 1987). We obtained 8-kb insert 454 FLX (454 Life Sciences, Branford, CT) mate-pair reads (24× coverage) and Illumina (Illumina, Inc., San Diego, CA) paired-end reads (162× coverage).
RNA preparation and sequencing
Roughly 1 liter of P. caudatum cells grown in Wheat Grass Powder medium to mid-log phase were purified away from bacteria by filtration over a 10-μm Nitex membrane. Whole-cell RNA was isolated using TRIzol (Ambion, Life Technologies, Carlsbad, CA) and the manufacturer’s suggested protocol for tissue culture cells. We then obtained Illumina paired-end reads to a coverage of ∼2000 reads/gene. RNAseq reads were mapped to the genome using Bowtie/TopHat (Langmead et al. 2009; Kim et al. 2013), and transcripts were predicted using Cufflinks (Trapnell et al. 2012). The abundance of each messenger RNA was predicted from a logarithm transformation of the FPKM (fragments per kilobase of exon per million fragments mapped) values reported by Cufflinks. Prior to the log transformation, we added 0.1 to the FPKM value of all genes to avoid log transformation of a null value.
Genome assembly and annotation
Illumina and 454 reads were co-assembled using the Celera assembler (Miller et al. 2008). Predicted transcripts from RNAseq reads as well as ab initio gene predictions from Augustus (Stanke et al. 2008), which had been previously trained on P. tetraurelia (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results), were used as hints for the final ab initio gene prediction by EuGene (Schiex et al. 2001; Foissac et al. 2008), which had also been previously trained on P. tetraurelia (Olivier Arnaiz, unpublished results). PANTHER (Mi et al. 2012) was used to predict gene functions for 9250 genes. Identification of genes with no homology to P. aurelia or Tetrahymena thermophila genes was conducted via BLASTP search (E-value cutoff = 0.05) against P. biaurelia, P. tetraurelia, and P. sexaurelia proteins (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results) or Tetrahymena proteins (Eisen et al. 2006).
Alignment of P. caudatum and P. aurelia orthologs
The P. caudatum genome was aligned with each of the three previously sequenced aurelia genomes using a modified version of the method used to align P. aurelia genomes with each other (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results). Specifically, an all-vs.-all BLASTP comparison (E-value cutoff = 10−5) between P. caudatum protein-coding genes and those from each P. aurelia genome identified best reciprocal hits (RBHs) between P. caudatum and each P. aurelia species. At this point, it became apparent that there were two rounds of WGD in the P. aurelia species after they diverged from P. caudatum, as there were up to four co-orthologs in each P. aurelia species for each P. caudatum protein. We counted a BLAST match as a RBH if a P. caudatum protein was the best hit for an aurelia protein and if that P. aurelia protein was also among the top four best hits for the P. caudatum protein. We used the alignments of the most recent P. aurelia WGD (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results) to predict an ancestral, prerecent WGD gene order for each aurelia species. We then used the RBH information to delineate orthologous regions between this ancestral (i.e., prerecent WGD) genome and the P. caudatum genome using a sliding-window analysis. Windows of 20 genes were examined at a time; if >60% of the P. aurelia genes in a window had a RBH to a single P. caudatum scaffold, we considered this an orthologous block. Contiguous windows that matched the same P. caudatum scaffold were merged. We then added additional non-RBH BLAST matches between P. caudatum and P. aurelia genes within the orthologous blocks that did not have RBH BLAST hits.
The above procedure produced alignments between P. caudatum scaffolds and prerecent WGD aurelia scaffolds. Because there was an additional round of WGD in the aurelia lineage, this produced two ancestral co-orthologous aurelia scaffolds (each one representing two present-day post-WGD scaffolds) aligned with each P. caudatum scaffold. We therefore identified P. caudatum regions that had two co-orthologous ancestral aurelia regions aligned and merged the information to allow alignment across the intermediate aurelia WGD. We further identified additional BLAST matches between aurelia genes within these regions that did not have a P. caudatum ortholog to align intermediate WGD paralogs even in the absence of a P. caudatum ortholog.
Alignment of ancient WGD paralogs in P. caudatum
The above procedure was adapted to align P. caudatum paralogs from the ancient WGD, with the only difference being that the percentage of genes required to have a RBH on the same scaffold was decreased to 30% due to the reduced retention rate following the ancient WGD.
Factors affecting intermediate and ancient WGD duplicate retention
Gene Ontology (GO) terms for P. caudatum genes [assigned by PANTHER (Mi et al. 2012)] and the gene-retention data from above were used to analyze functional categories of genes with an over- or underretention of intermediate WGD duplicates. The percentage of ancestral genes still duplicated for each GO term was compared to the percentage of ancestral genes still duplicated for all other GO terms within the same GO category (“Molecular Function,” “Biological Process,” or “Cellular Component”) with a χ2 test. A correction for multiple testing (Benjamini and Hochberg 1995) was applied to the resulting P-values to reduce the false discovery rate (FDR) to 5%.
To determine whether there was a correlation between retention of WGD duplicates and expression level or GC content, P. caudatum genes were sorted into bins based on their expression level or GC content, and then the fractional retention for each bin was calculated by the following equation: number of pairs still duplicated/(number of pairs still duplicated + number of singleton genes). For each of the aurelia species, we performed separate weighted least-squares regressions between this retention level and the expression level or GC content for duplicates arising at the intermediate and recent WGD events. In the latter case, we calculated retention across both WGDs and all species by adding up the number of extant orthologs for each P. caudatum gene (up to 4 in each of 3 species for a possible total of 12). We also used this last retention measure to determine whether both expression level and GC content were independent predictors of retention by performing a multiple regression of this retention level on both expression level and GC content of the P. caudatum gene.
Asymmetry of evolutionary rates between aurelia recent WGD paralogs
For all cases in which we could identify two aurelia intraspecific paralogs from the recent WGD and their P. caudatum ortholog, we produced protein alignments of the three genes using MUSCLE (Edgar 2004). We then performed Tajima’s relative-rate test (Tajima 1993) to determine the level of significance of rate asymmetry observed in each alignment and corrected for multiple tests to control the FDR at 5% (Benjamini and Hochberg 1995). We used codeml from the PAML package (Yang 2007) to compute synonymous and nonsynonymous substitution rates on the branches leading to the two paralogs. Expression data from RNA sequencing were used to determine whether the faster-evolving paralog was also the lower-expressed paralog and to determine candidates for neofunctionalization.
Results
The macronuclear genome sequence of P. caudatum
The DNA sequence of the macronuclear genome of P. caudatum was determined to a final mean depth of 186× coverage with a combination of Illumina and 454 mate-pair reads (see Materials and Methods). The genome assembly that comprises 30.5 Mb (including estimated gaps) is composed of 1202 scaffolds, 274 of which have lengths >2 kb and 96 of which are >50 kb (Supporting Information, Table S1). This is less than half the genome size of all members of the P. aurelia complex that have been sequenced (68–77 Mb) (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results) and is consistent with P. caudatum having diverged from the P. aurelia complex before the most recent genome duplication events.
The average lengths of exons and introns are similar between caudatum and the aurelias. However, the average intergenic length in caudatum is reduced and is one of the shortest for any eukaryote studied (Table 1). It is likely that the aurelia intergenic regions are longer because parts of these regions are composed of the remains of pseudogenized duplicates from the WGDs (Aury et al. 2006). The shorter average intergenic length of caudatum, therefore, likely represents the ancestral, preduplication intergenic length.
Table 1. Genome statistics for P. caudatum as compared to P. biaurelia, P. tetraurelia, and P. sexaurelia.
| P. caudatum | P. biaurelia | P. tetraurelia | P. sexaurelia | |
|---|---|---|---|---|
| Genome size (Mb) | 30.5 | 77.0 | 72.1 | 68.0 |
| Genes | 18,509 | 39,242 | 39,521 | 34,939 |
| Gene length (exons + introns) (bp) | 1,445.3 | 1,456.4 | 1,431.3 | 1,460.6 |
| Exons/gene | 3.5 | 3.6 | 3.3 | 3.6 |
| Average exon length (bp) | 399.0 | 377.9 | 418.8 | 379.3 |
| Average intron length (bp) | 24.7 | 31.4 | 24.2 | 30.3 |
| Intergenic length (bp) | 110.0 | 335.9 | 261.3 | 418.3 |
| Genomic GC content (%) | 28.2 | 25.8 | 28.0 | 24.1 |
Lengths given for genes, exons, introns, and intergenic regions are genome averages. Regions containing gaps were removed from the analysis before calculating averages.
The GC content of the P. caudatum genome is 28.2%, which is more similar to the GC content of P. tetraurelia (28.0%) than that of P. biaurelia (25.8%) or P. sexaurelia (24.1%). Similar differences in GC content are found in both coding and noncoding regions. Given the relationships between species (Figure 1), this complex pattern implies multiple changes of the GC content within the different lineages of Paramecium. Such changes could reflect a difference in the mutation spectrum or in processes such as biased gene conversion. In the future, sequencing the genomes of more P. aurelia species as well as additional Paramecium species outside of the aurelia complex should help reveal the complex evolution of genomic GC content in Paramecium.
Figure 1.
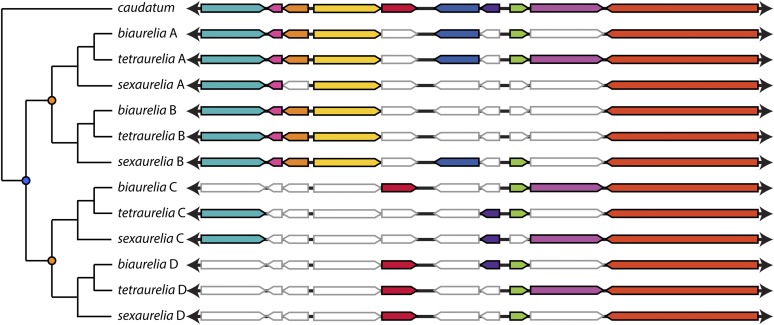
Example alignment of a region from a caudatum scaffold and 12 co-orthologous scaffolds from biaurelia, tetraurelia, and sexaurelia. Scaffolds designated with the same letter (A, B, C, or D) are orthologous to each other. Homologous genes are displayed in matching colors, and genes that have been lost on aurelia scaffolds are in white with gray outlines. Intergenic regions are not shown to scale to demonstrate the locations of gene losses. A phylogeny is given to demonstrate relationships between scaffolds with orange dots denoting the location of the recent WGD and a blue dot denoting the intermediate WGD. Regions illustrated include the following: caudatum—scaffold_0002:1786-13531; biaurelia—scaffold_0010:297344-307146, scaffold_0012:192506-199800, scaffold_0312:38737-46840, and scaffold_0297:20187-26050; tetraurelia—scaffold_15:289275-399069, scaffold_25:343442-351219, scaffold_30:236161-243107, and scaffold_31:210331-217756; sexaurelia—scaffold_015:487286-494871, scaffold_017:247245-256846, scaffold_026:298395-306376, and scaffold_027:313613-319815.
The P. caudatum genome contains 18,509 annotated protein-coding genes, about half the number of genes found in each P. aurelia species. PANTHER (Mi et al. 2012) functional annotations are available for 9250 (50.0%) of these genes. The genome sequence and annotations are available at ParameciumDB (http://paramecium.cgm.cnrs-gif.fr) (Arnaiz and Sperling 2011). Among the 18,509 P. caudatum predicted proteins, 1053 have no identifiable P. aurelia homolog, and 8456 have no identifiable homolog in another oligohymenophorean ciliate, T. thermophila (Eisen et al. 2006).
Retention and divergent resolution of aurelia intermediate WGD paralogs
Although previous data indicated that P. caudatum likely did not share the most recent WGD with the aurelia species (Aury et al. 2006), it was initially unclear whether P. caudatum shared the intermediate WGD. Upon aligning the P. caudatum genome with each of the P. aurelia genomes, it is apparent that there are up to four co-orthologs in each aurelia species for each P. caudatum protein (Figure 1), indicating that the intermediate WGD was also limited to the P. aurelia lineage. Although large-scale synteny has broken down to an extent between P. caudatum and the P. aurelia species, a substantial degree of local synteny remains (Figure 2). We were able to align 5781 P. caudatum genes with all 12 of their syntenic aurelia orthologs (when present), which includes 10,907 P. biaurelia, 10,970 P. tetraurelia, and 10,024 P. sexaurelia genes (File S1 and File S2). This represents ∼30% of the genes in each of the aligned genomes, as missing data in any of one of the 12 scaffolds being aligned caused a region to be excluded. We also identified 305 ancestral genes that existed in the P. aurelia ancestor before the intermediate WGD but that do not appear to have syntenic P. caudatum orthologs. These genes have either been lost in P. caudatum, were acquired by the aurelia ancestor before the intermediate WGD, or have evolved too rapidly to identify homology between P. caudatum and P. aurelia.
Figure 2.
Overview of synteny between a caudatum scaffold (scaffold_0001) and the four tetraurelia scaffolds that together make up one set of orthologs (e.g., “tetraurelia A” in Figure 1). Orthologs between caudatum and tetraurelia, as identified by our orthologous alignment process (see Materials and Methods), are connected by red lines. Scaffolds are represented by black lines around the outside of the circle.
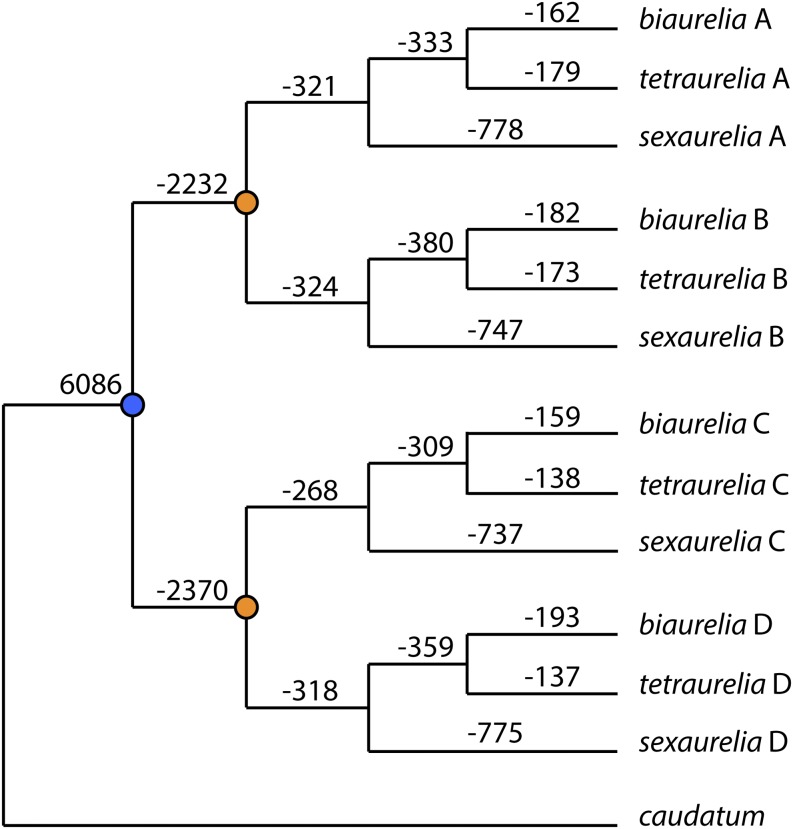
From these alignments, we estimate that 24% of duplicates that originated from the intermediate WGD were still present in two copies in the P. aurelia ancestor at the time of the recent WGD. Note that, when no descendant copies of an intermediate WGD duplicate remain in any P. aurelia species, we infer that this duplicate was lost before the recent WGD. This estimate of intermediate WGD retention is identical to that inferred from the P. tetraurelia genome alone (Aury et al. 2006) and is much lower than the 42–52% of recent WGD duplicates retained in each aurelia lineage (Lynn McGrath, Jean-Francois Gout, Parul Johri, Thomas Graeme Doak, and Michael Lynch, unpublished results). Using parsimony, we assigned gene losses to the P. aurelia phylogenetic tree for a complete picture of duplicate gene loss following the intermediate and recent WGDs (Figure 3).
Figure 3.
Gene losses following the intermediate and recent WGDs along the phylogenetic tree. The number of ancestral genes included in the analysis (6086) is given, followed by the number of losses occurring along each branch. Blue dot: intermediate WGD; orange dots: recent WGD. Note that branch lengths are not to scale.
The median synonymous site divergence (dS) between intermediate WGD duplicates (e.g., between genes along scaffold tetraurelia A and scaffold tetraurelia C in Figure 1) is much higher (median dS saturated at ∼8) than between recent WGD duplicates (P. tetraurelia A and P. tetraurelia B in Figure 1; median dS = 1.7; McGrath et al., unpublished results), indicating that the intermediate WGD occurred long before the recent WGD. It was previously observed that intraspecific paralogs, on average, have lower dS values than interspecific paralogs for the recent WGD, which was interpreted as evidence for frequent gene conversion between paralogs (McGrath et al., unpublished results). For duplicated genes derived from the intermediate WGD, intraspecific paralogs do not appear to be more similar to each other than interspecific intermediate WGD duplicates (Figure S1), suggesting that gene conversion between intermediate WGD duplicates does not occur frequently. However, because the dS between paralogs from the intermediate WGD is highly saturated, it is possible that gene conversion occurred immediately following the intermediate WGD and then gradually stopped, as observed for the most recent WGD (McGrath et al., unpublished results). The action of gene conversion on WGD duplicates therefore appears to be limited to a finite period of time following WGD.
Estimating the age of the intermediate WGD
Because synonymous substitutions are usually neutral, their accumulation should directly reflect the time of divergence between two homologous sequences. We previously relied on this principle to estimate the age of the recent WGD, based on the median dS between paralogs (1.7) and the mutation rate measured in P. tetraurelia [2.64 × 10−11 mutations per site per cell division (Sung et al. 2012)], and obtained an estimation of ∼230 million years (McGrath et al., unpublished results). Using the same strategy, and given that the median dS between paralogs from the intermediate WGD is ∼8 (indicating that each site in the P. caudatum genome has been hit by an average of approximately four mutations per site since the intermediate WGD), we derive an estimate of ∼150 billion cell divisions (4/2.64 × 10−11) since the intermediate WGD, which translates to ∼1.5 billion years, assuming 100 cell divisions per year (Pianka 2000). Because the estimation of such high dS values lacks precision and because both the mutation rate and the number of cell divisions per year could have varied substantially within this long period of time, these estimates need to be taken cautiously. However, it is clear that the intermediate WGD is much older than the recent one and that both events are at least hundreds of millions of years old.
Divergent resolution of intermediate WGD-derived paralogs is still ongoing
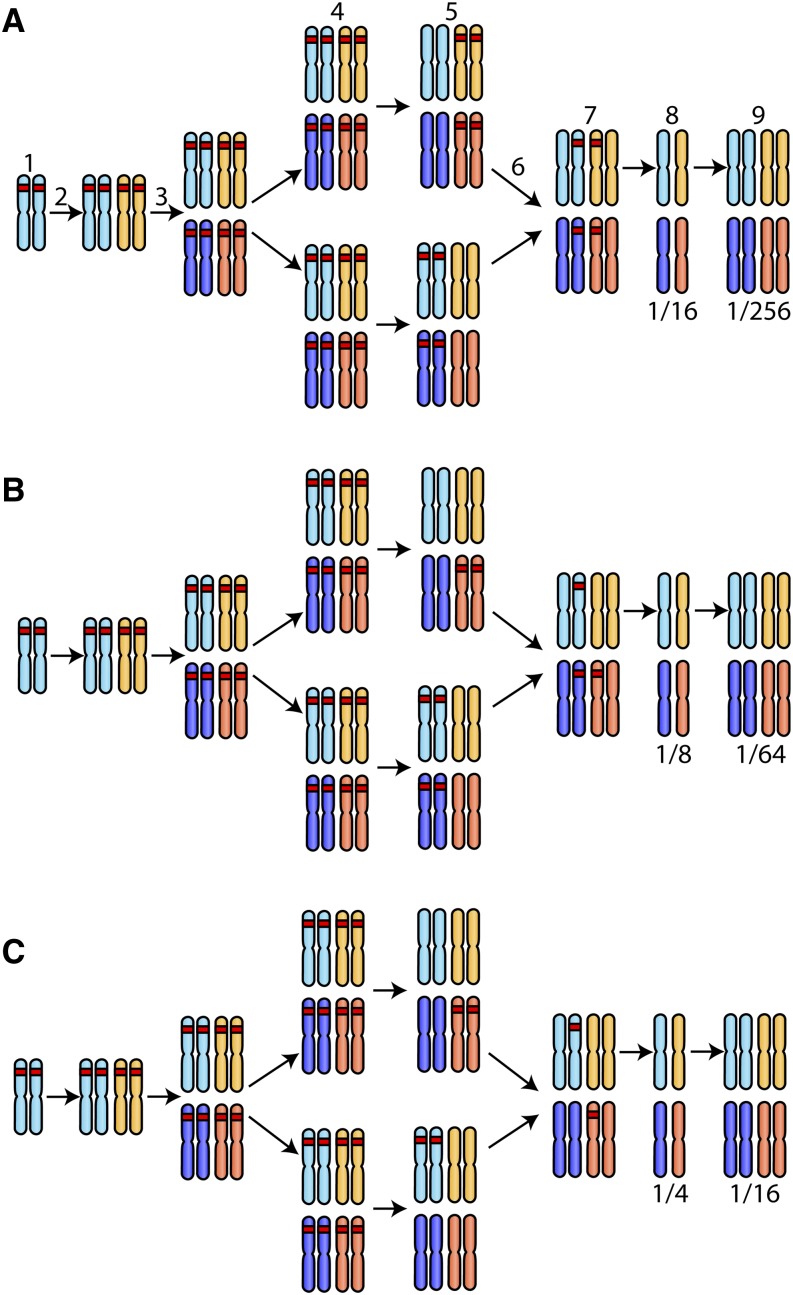
An important consequence of WGDs is their potential to cause reproductive isolation and speciation through divergent resolution of duplicate genes (Oka 1988; Werth and Windham 1991; Lynch and Conery 2000; Lynch and Force 2000). Although the intermediate WGD occurred long ago and there has been another round of WGD since, it is possible that divergent resolution of intermediate WGD duplicates is still ongoing between aurelia species (Figure 4). We found two instances of divergent resolution of intermediate WGD duplicates between P. tetraurelia and P. sexaurelia, four between P. biaurelia and P. sexaurelia, and two between P. biaurelia and P. tetraurelia (Table S2). Among these, there are two cases in which each species retained two recent copies of alternative intermediate duplicates (Figure 4A), three cases in which each species retained one copy (Figure 4C), and three cases in which one species retained two copies and the other species retained only one (Figure 4B). Although these represent only a handful of cases, it is surprising to observe that divergent resolution still occurs for such an old WGD event. Therefore, WGDs may still promote speciation even hundreds of millions of years after they arose.
Figure 4.
Divergent resolution of intermediate WGD duplicates. (A) In the preduplication ancestor (1), one gene (red) is shown on an ancestral, diploid chromosome. Following the intermediate WGD (2), the gene is present in two copies. Following the recent WGD (3), the gene is present in four copies. Two subpopulations become isolated from each other (4) and divergent resolution of the intermediate WGD duplicate occurs, with one population losing the gene from both the light and dark blue chromosomes and one population losing the gene from both the light- and dark-orange chromosomes (5). Hybridization then occurs between the two subpopulations (6). Hybridization leads to the creation of F1 hybrids (7). One-sixteenth of the gametes produced by the F1 hybrids have no functional copy of the gene (8). Of the F2 offspring, 1/256 have no functional copy of the gene (9). The amount of reproductive isolation increases when one (B) or both (C) subpopulations retain only one copy, instead of two copies, of the gene at stage 5.
GC content, expression level, and functional category influence duplicate-gene retention
Previously, we found that expression level, GC content, and functional category influenced the retention of duplicate genes from the recent aurelia WGD (Gout et al. 2010; McGrath et al., unpublished results). We investigated whether the same factors are correlated with retention of intermediate WGD duplicates. In the case of the recent WGD, we previously found that 10–17 functional categories were significantly overretained after the recent WGD in the three different P. aurelia species investigated, whereas five to seven functional categories were significantly underretained (McGrath et al., unpublished results). For the intermediate WGD, only three functional categories are overrepresented among duplicates (ribosomal proteins, proteins involved in amino acid phosphorylation, and proteins involved in response to stress), and no categories are significantly underrepresented (File S3). These three overretained categories were also found to be overretained after the recent WGD (McGrath et al., unpublished results). The relative scarcity of over- and underrepresented functional categories from the intermediate WGD could be due to the fact that biased retention of functional classes decreases over time, although the fact that fewer intermediate WGD duplicates survive reduces the power of the analysis.
We previously found that the GC content and expression level of P. aurelia genes are positively correlated with duplicate retention from the most recent WGD (McGrath et al., unpublished results). However, both these factors could be influenced by duplication itself. Frequent gene conversion between paralogs could increase their GC content via GC-biased gene conversion (Galtier et al. 2001; Duret et al. 2008; Pessia et al. 2012), making it unclear whether high-GC content drives gene retention or is a secondary consequence of gene retention. With the P. caudatum genome providing a proxy for the ancestral, preduplication genome, it is now possible to eliminate this ambiguity.
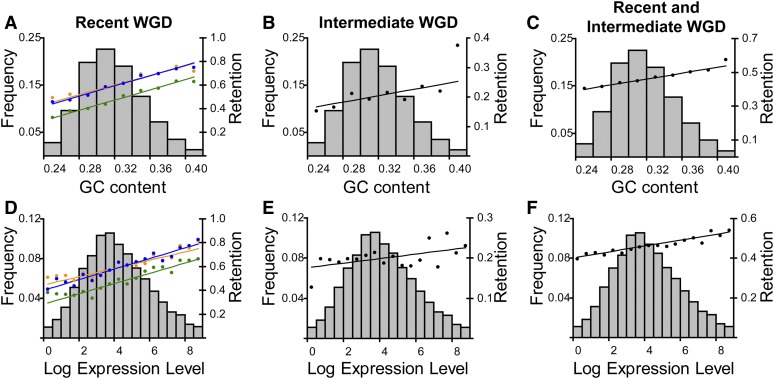
We separated P. caudatum genes into bins based on their GC content and then within each P. aurelia species determined the retention level for orthologous duplicates from the recent WGD using the following equation: retention rate = number of pairs of retained paralogs/(number of pairs of retained paralogs + number of singleton genes). A weighted least-squares regression of the retention levels on the P. caudatum GC content revealed a significant positive correlation for all three aurelia species (Figure 5A) (P. biaurelia r2 = 0.95, P. tetraurelia r2 = 0.92, P. sexaurelia r2 = 0.96; P < 0.0001 in each case).
Figure 5.
Relationships between duplicate retention and P. caudatum GC content or expression level. P. caudatum genes are sorted into bins based on GC content (A–C) or log-expression level (D–F). The frequency distribution of genes that fall into each GC content or expression level bin is shown (gray bars), along with the retention level of genes within each bin (dots) and the corresponding regression lines for retention on GC content or expression level (lines) (see Materials and Methods for more details). A and D show the retention of recent WGD duplicates in biaurelia (blue), tetraurelia (orange), and sexaurelia (green). B and E show the retention of intermediate WGD duplicates, and C and F show the retention of duplicates across both WGDs.
We repeated this procedure for the intermediate WGD, this time calculating a retention level for each bin based on the retention of intermediate WGD duplicates using the same formula as above (Figure 5B). Again, we found a significant positive correlation between GC content of P. caudatum genes and the retention of their orthologs following the intermediate WGD, although the correlation is weaker than for recent WGD duplicates (r2 = 0.32, P < 0.0001). Finally, we calculated a retention level for the P. caudatum GC bins across both the recent and intermediate WGDs (see Materials and Methods). We obtained a similar correlation between GC content and retention of duplicates across both WGDs (r2 = 0.88, P < 0.0001) (Figure 5C).
We applied the same analysis to expression levels (Figure 5, D–F). Like GC content, the retention rate of P. aurelia genes is positively correlated with the expression level of their preduplication orthologs in P. caudatum after both the intermediate (r2 = 0.21, P < 0.0001) and the recent WGD (P. biaurelia r2 = 0.88, P. tetraurelia r2 = 0.85, P. sexaurelia r2 = 0.88; P < 0.0001 for all three species) and after both WGDs combined (r2 = 0.86, P < 0.0001). Again, the correlation with the intermediate WGD is weaker than with the recent WGD, suggesting that the selective forces preventing gene loss weaken over time.
Because GC content and expression level are correlated with each other (Figure S2), we conducted a multiple regression analysis to determine whether both were independent predictors of duplicate retention. We calculated the number of extant aurelia orthologs across all species for each P. caudatum gene. Because there are up to four co-orthologs of each P. caudatum gene in each of three aurelia species, this retention value ranges from 1 to 12. We then determined whether this retention value was correlated with GC content and/or expression level of the P. caudatum genes. Although the overall correlation is low for this analysis (r2 = 0.05), the number of retained orthologs is significantly correlated with both GC content and expression level (GC-content partial regression coefficient = 5.43, P < 0.0001; expression-level partial regression coefficient = 0.15, P < 0.0001), indicating that the two variables, although correlated with each other, do have independent predictive power.
As suggested previously (Gout et al. 2010), it is likely that expression level promotes duplicate-gene retention through selection for increased dosage of the gene product. Because we observe a correlation between the preduplication GC content and the retention rate, we can rule out the hypothesis that the higher GC content of retained genes is simply a consequence of frequent GC-biased gene conversion between paralogs. Given the fact that the mutational spectrum of Paramecium is strongly biased toward GC→AT mutations (Sung et al. 2012), it is likely that a high-GC content in the coding region of ancestral genes reflects strong selective pressures at the sequence level, leading to the suggestion that genes under strong selective pressure at the sequence level are also under strong selective pressure against post-WGD changes in dosage (i.e., against gene loss). In agreement with this hypothesis, we observed a strong negative correlation between the speed of evolution of ancestral genes [measured as nonsynonymous site divergence (dN) between P. caudatum and P. multimicronucleatum orthologs] and post-WGD retention rate (Figure S3).
Asymmetry of evolutionary rate between recent aurelia paralogs
Although gene duplicates are 100% identical immediately after a WGD event, early mutational events may set them on differential courses of evolution, resulting in asymmetrical rates of evolution between the two copies. Using Tajima’s (1993) relative rate test, we found that 14% of P. biaurelia, 13% of P. tetraurelia, and 20% of P. sexaurelia paralogs exhibit significant rate asymmetry after correction for multiple testing (see Materials and Methods). This represents a significantly greater percentage of asymmetrical trees for P. sexaurelia than for P. biaurelia and P. tetraurelia (P < 10−15, χ2 test). Such asymmetries in the rate of evolution are often interpreted as evidence for neofunctionalization (Aury et al. 2006; Johnson and Thomas 2007; Han et al. 2009). However, both positive selection (expected in the case of neofunctionalization) and relaxed purifying selection (expected in the case of pseudogenization) can produce a signature of asymmetric evolution between the paralogs. Therefore, it is possible that a substantial fraction of duplicates showing asymmetrical evolutionary rates represent cases of ongoing pseudogenization, rather than neofunctionalization.
Because P. sexaurelia has a higher duplicate-gene loss rate than both P. biaurelia and P. tetraurelia, it is likely that the relative abundance of asymmetrical trees in this species is due to greater levels of pseudogenization occurring in P. sexaurelia, rather than to an increased level of neofunctionalization. To test this hypothesis, we compared the difference in expression level to the difference in the rate of evolution of the protein sequence between paralogs. While there is no reason to believe that genes undergoing neofunctionalization should preferentially become less expressed, genes undergoing pseudogenization are likely to experience decreased expression level due to random mutation and relaxed evolutionary constraints. We observed a significant negative correlation between the difference in expression level and the difference in dN between paralogs from the recent WGD in all three P. aurelia species (P. biaurelia: r = −0.32, P. tetraurelia: r = −0.26, P. sexaurelia: r = −0.30; P < 0.001 for all three species).
Further evidence that a pseudogenization process is responsible mainly for the sequence asymmetry comes from the fact that the faster-evolving paralog is also the lower-expressed paralog 77% of the time among significantly asymmetrically evolving pairs in P. biaurelia and P. tetraurelia and 81% of the time among P. sexaurelia asymmetrical pairs. Among symmetrically evolving pairs, on the other hand, this is true only slightly more than half the time (58% in P. biaurelia, 56% in P. tetraurelia, and 60% in P. sexaurelia), representing a significant difference between asymmetrical and symmetrical pairs for all three species (all P-values: <10−16, χ2 test). The proportion for symmetrical pairs, while lower than for asymmetrical pairs, is nonetheless significantly different from 50% (all P-values: <10−16, χ2 test), indicating that this process of increased sequence evolution and reduced expression level for one paralog of a pair has begun even for some of the pairs that do not yet demonstrate significant sequence evolution asymmetry. Finally, the percentage of both asymmetrically evolving and symmetrically evolving pairs where the faster-evolving paralog is also the lower-expressed paralog is greater in P. sexaurelia than in P. biaurelia or P. tetraurelia (P < 0.02 for all three species, χ2 test), consistent with the pseudogenization process happening more rapidly in P. sexaurelia, where the gene-loss rate is higher.
These observations do not preclude the possibility of some neofunctionalized paralogs among the set of asymmetrical pairs. To search for candidate genes possibly undergoing neofunctionalization, we identified paralog pairs in each species with significant asymmetry of coding sequence evolution, where the expression level difference between the two paralogs was above the 80th percentile, and where the more highly expressed paralog was the faster-evolving paralog. This analysis yielded 35 P. biaurelia genes, 18 P. tetraurelia genes, and 29 P. sexaurelia genes that are candidates for neofunctionalization (File S4). Interestingly, we found one gene (Pterin-4–α-carbinolamine) that is a candidate for neofunctionalization in both P. tetraurelia and P. sexaurelia. Because these species diverged soon after the recent WGD, it is likely that if neofunctionalization happened, it happened independently in the two species. Indeed, the faster-evolving copy in P. tetraurelia is the recent WGD paralog, not the ortholog, of the faster-evolving copy in P. sexaurelia. Similarly, a sodium-dependent phosphate transporter is a candidate in both P. sexaurelia and P. biaurelia, although in this case the faster-evolving copies are orthologs of each other, making it possible that only one event of neofunctionalization happened before the P. sexaurelia–P. biaurelia speciation. Because this analysis is based solely on sequence data, additional biochemical experiments will be necessary to test the function of these genes in the different aurelia species and confirm (or refute) the possibility of neofunctionalization.
Analysis of the ancient WGD in P. caudatum vs. P. aurelia
Although P. caudatum does not share the intermediate or recent WGDs with the aurelia complex, our genome alignments indicate that all species share the ancient WGD. We used a method similar to that of Aury et al. (2006) to identify ancient paralogous blocks within P. caudatum for comparison to the ancient paralogous blocks identified in P. tetraurelia. We hypothesized that, if gene dosage constraints play an important role in post-WGD retention, the subsequent rounds of WGD within the P. aurelia lineage may relax selective pressure for retention of paralogs derived from the more ancient WGD. Under this scenario, the retention rate for the ancient WGD is expected to be higher in P. caudatum compared to the P. aurelia species. Alternatively, if changes of function are responsible for most duplicate-gene retention, subsequent duplications should not affect the probability of retention, so that we would not expect to observe a difference in the post-ancient WGD retention rate between P. caudatum and the P. aurelia species. We find the post-ancient WGD retention rate in P. caudatum to be 16% (File S5), which is about twice the retention rate observed in P. tetraurelia (Aury et al. 2006), supporting the idea that gene dosage constraints play an important role in post-WGD retention for a majority of genes.
Like the intermediate and recent WGDs, P. caudatum genes retained in duplicate from the ancient WGD have significantly higher GC content (P < 10−6, t-test) and expression level (P < 10−8, t-test) than those whose paralogs have been lost (Table S3). Again, the most overretained functional category is ribosomal proteins, although due to the small number of genes included, the power of the GO analysis is diminished and no category remains significant after correcting for multiple tests (File S6).
Discussion
Previously, we examined the evolutionary consequences of the recent WGD predating the emergence of the P. aurelia species complex (McGrath et al., unpublished results). The sequencing of an outgroup species, P. caudatum, has allowed us to further investigate the recent and intermediate WGDs, both of which occurred after the divergence of P. caudatum and the aurelia lineage, as well as the ancient WGD that is shared between them. Although the percentage of retained duplicates differs across all three WGDs, we found similarities in the factors influencing retention, namely, GC content, expression level, and to a lesser extent, functional category. By correlating duplicate-gene retention in the aurelia species with gene features in P. caudatum, we demonstrate that the properties of the preduplication genes influence their post-WGD fate.
Interestingly, the correlation between all three of these gene features and retention is lower for the intermediate WGD than for the recent WGD, indicating that over time, as more duplicates are lost, biases in which genes are retained become weaker. This suggests that many paralogs are eventually able to escape the selective pressures that caused them to be initially maintained. For example, if a gene is preserved initially by selection for increased dosage or due to dosage balance constraints, subsequent mutations altering the gene’s expression could ultimately allow the gene to be lost. Moreover, these changes in preservational biases over time could complicate comparisons of WGDs across phyla, as such comparisons are generally not corrected for the amount of evolutionary time since the WGD.
It is still unclear what fraction of genes are retained for dosage constraints and what fraction are retained because of changes in function. However, our observation that P. caudatum has retained twice as many paralogs from the ancient WGD than the aurelia species suggests that subsequent WGDs can promote the loss of ancient paralogs. Because subsequent duplications are expected to relax the level of dosage constraints on genes, we interpret this observation as evidence for the intermediate and recent WGDs lowering the dosage constraints on ancient-WGD-derived paralogs in P. aurelia, therefore allowing a higher loss rate of these ancient paralogs. Considering the large difference in post-ancient WGD paralog retention rates between P. caudatum and the aurelia species, we suspect that selection on gene dosage is the major evolutionary force acting on post-WGD gene retention.
With P. caudatum as an outgroup, we were also able to conduct evolutionary analyses of the aurelia paralogs from the recent WGD. We found that, compared to P. biaurelia and P. tetraurelia, P. sexaurelia has a higher percentage of paralog pairs that are evolving asymmetrically and that this is likely reflective of the higher rate of gene loss through pseudogenization in this species. This does not preclude the possibility of some neofunctionalized paralogs among the asymmetrical gene pairs, and we have identified a list of candidate genes for neofunctionalization in each species. However, it should be emphasized that P. aurelia is a complex of cryptic species that are morphologically indistinguishable (Sonneborn 1975), so that if any new gene functions have evolved, they are not apparent from our current knowledge of the biology of P. aurelia. Therefore, it seems likely that neofunctionalization played only a minor role in the evolution of the P. aurelia lineage.
The underlying cause for the higher gene loss rate in P. sexaurelia remains unclear. One possibility is that the mutation rate in P. sexaurelia is higher than in P. tetraurelia and P. biaurelia, leading to an overall faster evolutionary rate. It is also possible that P. sexaurelia has a smaller effective population size than the other aurelia species studied, which would result in less efficient selection against gene loss. Data on the mutation rate and the effective population sizes of the different aurelia species will be required to test these hypotheses.
Perhaps the most surprising finding is the discovery of divergent resolutions of intermediate WGD duplicates between aurelia species. We previously showed that divergent resolution slowed rapidly over time after the recent aurelia WGD, as the majority of losses between P. biaurelia and P. tetraurelia represented parallel, rather than divergent, resolutions (McGrath et al., unpublished results). However, we now demonstrate that, although divergent resolutions may become increasingly rare, they can still occur after a large amount of time has elapsed following WGD. Divergent resolution of duplicates then has the potential to continue to contribute to reproductive isolation and speciation between lineages long after a whole-genome duplication event.
Finally, we point out that this study might help in understanding why the Paramecium lineage has been so permissive to WGDs. Paramecium cells contain two types of nuclei: the micronucleus (germline) is diploid and transcriptionally silent, while the macronucleus (soma) is generated de novo by amplification of a micronucleus after sexual reproduction. The macronucleus is highly polyploid (∼800 copies of each chromosome in P. aurelia) and is the site of all gene expression. Paramecium cells can sense the amount of DNA present in their macronucleus, so that they “synthesize similar amounts of macronuclear DNA, regardless of the number of macronuclei or their prereplication DNA content” (Berger and Schmidt 1978, pp. 116–126). Therefore, it is possible that a WGD would be immediately compensated by a homeostatic maintenance to the macronuclear mass (halving the ploidy of newly generated macronuclei relative to the now tetraploid micronucleus), and only the micronucleus would see its size initially doubling, before gradual gene loss slowly drives it toward its preduplication size. Because gene expression happens only in the macronucleus, most of the immediate deleterious effects of a genome duplication (Otto and Whitton 2000) would be absent under this scenario, making successive WGDs in the Paramecium lineage largely neutral events at the time of their establishment and their fixation via genetic drift more likely than in other species. After the successive WGDs, gradual gene loss might lead to a slow decrease in the total amount of macronuclear DNA present in the cell [if the mechanism described in Berger and Schmidt (1978) is not sensitive enough to detect small decreases of DNA content, as expected in the case of gene-by-gene losses], causing a reduction in the size of the macronucleus and therefore a reduction in the total cell size. This scenario would solve the paradoxical observation that, despite having undergone two extra rounds of WGDs and containing about twice as many genes, P. aurelia cells are smaller than P. caudatum cells. Therefore, the peculiarities of the Paramecium genome organization might have enabled this lineage to tolerate multiple rounds of WGDs, resulting in a greatly expanded gene repertoire and offering new evolutionary opportunities to the Paramecium lineage.
Supplementary Material
Acknowledgments
The authors thank O. Arnaiz and L. Sperling for sharing the EuGene annotation pipeline trained on P. tetraurelia. Funding for this work has been provided by a National Science Foundation (NSF) Graduate Research Fellowship (to C.L.M.), National Institutes of Health Genetics, Cellular, and Molecular Sciences Training Grant T32 GM007757 (to C.L.M.), and NSF grant EF-0328516-A006 (to M.L.). This research includes work supported by the NSF under grant no. ABI-1062432 to the National Center for Genome Analysis Support at Indiana University.
Footnotes
Communicating editor: G. Stormo
Literature Cited
- Adoutte A., Beisson J., 1972. Evolution of mixed populations of genetically different mitochondria in Paramecium aurelia. Nature 235: 393–395. [DOI] [PubMed] [Google Scholar]
- Allen S., Gibson I., 1972. Genome amplification and gene expression in the Ciliate macronucleus. Biochem. Genet. 5: 161–181. [DOI] [PubMed] [Google Scholar]
- Armus H. L., Montgomery A. R., Gurney R. L., 2006. Discrimination learning and extinction in Paramecia (P. caudatum). Psychol. Rep. 98: 705–711. [DOI] [PubMed] [Google Scholar]
- Arnaiz O., Sperling L., 2011. ParameciumDB in 2011: new tools and new data for functional and comparative genomics of the model ciliate Paramecium tetraurelia. Nucleic Acids Res. 39: D635–D636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz O., Mathy N., Baudry C., Malinsky S., Aury J.-M., et al. , 2012. The Paramecium germline genome provides a niche for intragenic parasitic DNA: evolutionary dynamics of internal eliminated sequences. PLoS Genet. 8: e1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aury J.-M., Jaillon O., Duret L., Noel B., Jubin C., et al. , 2006. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444: 171–178. [DOI] [PubMed] [Google Scholar]
- Beale G. H., Jurand A., Preer J. R., 1969. The classes of endosymbiont of Paramecium aurelia. J. Cell Sci. 5: 65–91. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57: 289–300. [Google Scholar]
- Berger J. D., 1937. Nuclear differentiation and nucleic acid synthesis in well-fed exconjugants of Paramecium aurelia. Chromosoma 42: 247–268. [DOI] [PubMed] [Google Scholar]
- Berger J. D., Schmidt H. J., 1978. Regulation of macronuclear DNA content in Paramecium tetraurelia. J. Cell Biol. 76: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins G. N., 1902. Studies on the life-history of Protozoa, I. The life-cycle of Paramaecium caudatum. Dev. Genes Evol. 15: 139–186. [Google Scholar]
- Davis J. C., Petrov D. A., 2005. Do disparate mechanisms of duplication add similar genes to the genome? Trends Genet. 21: 548–551. [DOI] [PubMed] [Google Scholar]
- Decatur W. A., Hall J. A., Smith J. J., Li W., Sower S. A., 2013. Insight from the lamprey genome: glimpsing early vertebrate development via neuroendocrine-associated genes and shared synteny of gonadotropin-releasing hormone (GnRH). Gen. Comp. Endocrinol.192: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Doyle J. L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19: 11–15. [Google Scholar]
- Doyle J. J., Flagel L. E., Paterson A. H., Rapp R. A., Soltis D. E., et al. , 2008. Evolutionary genetics of genome merger and doubling in plants. Annu. Rev. Genet. 42: 443–461. [DOI] [PubMed] [Google Scholar]
- Duncan A. B., Fellous S., Accot R., Alart M., Sobandi K. C., et al. , 2010. Parasite-mediated protection against osmotic stress for Paramecium caudatum infected by Holospora undulata is host genotype specific. FEMS Microbiol. Ecol. 74: 353–360. [DOI] [PubMed] [Google Scholar]
- Duncan A. B., Fellous S., Kaltz O., 2011. Reverse evolution: selection against costly resistance in disease-free microcosm populations of Paramecium caudatum. Evolution 65: 3462–3474. [DOI] [PubMed] [Google Scholar]
- Duret L., Cohen J., Jubin C., Dessen P., Gout J. F., et al. , 2008. Analysis of sequence variability in the macronuclear DNA of Paramecium tetraurelia: a somatic view of the germline. Genome Res. 18: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J. A., Coyne R. S., Wu M., Wu D., Thiagarajan M., et al. , 2006. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 4: e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous S., Duncan A., Coulon A., Kaltz O., 2012. Quorum sensing and density-dependent dispersal in an aquatic model system. PLoS ONE 7: e48436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissac S., Gouzy J., Rombauts S., Mathe C., Amselem J., et al. , 2008. Genome annotation in plants and fungi: EuGene as a model platform. Curr. Bioinform. 3: 87–97. [Google Scholar]
- Fokin S. I., 2010. Paramecium genus: biodiversity, some morphological features and the key to the main morphospecies discrimination. Protistology 6: 227–235. [Google Scholar]
- Galtier N., Piganeau G., Mouchiroud D., Duret L., 2001. GC-content evolution in mammalian genomes: the biased gene conversion hypothesis. Genetics 159: 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout J.-F., Kahn D., Duret L., 2010. The relationship among gene expression, the evolution of gene dosage, and the rate of protein evolution. PLoS Genet. 6: e1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailong Z., Guofeng L., Renliang H., Song C., Xiaoping D., 2011. Initial study on acute toxicity of two typical persistent organic pollutanta (POPs) against Paramecium caudatum. Asian J. Ecotoxicol. 6: 37–42. [Google Scholar]
- Han M. V., Demuth J. P., McGrath C. L., Casola C., Hahn M. W., 2009. Adaptive evolution of young gene duplicates in mammals. Genome Res. 19: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T., Liberles D. A., 2008. Whole-genome duplications in the ancestral vertebrate are detectable in the distribution of gene family sizes of tetrapod species. J. Mol. Evol. 67: 343–357. [DOI] [PubMed] [Google Scholar]
- Igarashi S., 1966. Temperature-sensitive mutation in Paramecium aurelia. I. Induction and inheritance. Mutat. Res. 3: 13–24. [DOI] [PubMed] [Google Scholar]
- Jennings H. S., 1908. Heredity, variation and evolution in Protozoa. 1. The fate of new structural characters in Paramecium, in connection with the problem of the inhertiance of acquired characters in unicellular organisms. J. Exp. Zool 5: 577–632. [Google Scholar]
- Jennings H. S., 1916. The numerical results of diverse systems of breeding. Genetics 1: 53–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. S., 1917. The numerical results of diverse systems of breeding, with respect to two paris of characters, linked or independent, with special relation to the effects of linkage. Genetics 2: 97–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y. N., Wickett N. J., Ayyampalayam S., Chanderbali A. S., Landherr L., et al. , 2011. Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97–100. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Thomas M. A., 2007. The monosaccharide transporter gene family in Arabidopsis and rice: a history of duplications, adaptive evolution, and functional divergence. Mol. Biol. Evol. 24: 2412–2423. [DOI] [PubMed] [Google Scholar]
- Kawamoto K., Oashi T., Oami K., Liu W., Jin Y. H., et al. , 2010. Perfluorooctanoic acid (PFOA) but not perffluorooctane sulfonate (PFOS) showed DNA damage in comet assay on Paramecium caudatum. J. Toxicol. Sci. 35: 835–841. [DOI] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., et al. , 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenek S., Petzoldt T., Berendonk T. U., 2012. Coping with temperature at the warm edge: patterns of thermal adaptation in the microbial eukaryote Paramecium caudatum. PLoS ONE 7: e30598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., 2007. The Origins of Genome Architecture. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Lynch M., Conery J. S., 2000. The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Lynch M., Force A. G., 2000. The origin of interspecific genomic incompatibility via gene duplication. Am. Nat. 156: 590–605. [DOI] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Thomas P. D., 2012. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 41: D377–D386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Delcher A. L., Koren S., Venter E., Walenz B. P., et al. , 2008. Aggressive assembly of pyrosequencing reads with mates. Bioinformatics 24: 2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin R. D., Chang E., Petrescu A., Liao N., Griffith M., et al. , 2006. Sequencing and analysis of 10,967 full-length cDNA clones from Xenopus laevis and Xenopus tropicalis reveals post-tetraploidization transcriptome remodeling. Genome Res. 16: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki M., Zagorski-Ostoja W., Meyer E., 2005. Nowa1p and Nowa2p: novel putative RNA binding proteins involved in trans-nuclear crosstalk in Paramecium tetraurelia. Curr. Biol. 15: 1616–1628. [DOI] [PubMed] [Google Scholar]
- Oka H. I., 1988. Functions and genetic bases of reproductive barriers, pp. 181–209 in Origin of Cultivated Rice, edited by Oka H. I. Japan Scientific Societies Press/Elsevier, Tokyo. [Google Scholar]
- Otto S. P., 2007. The evolutionary consequences of polyploidy. Cell 131: 452–462. [DOI] [PubMed] [Google Scholar]
- Otto S. P., Whitton J., 2000. Polyploid incidence and evolution. Annu. Rev. Genet. 34: 401–437. [DOI] [PubMed] [Google Scholar]
- Panopoulou G., Poustka A. J., 2005. Timing and mechanism of ancient vertebrate genome duplications: the adventure of a hypothesis. Trends Genet. 21: 559–567. [DOI] [PubMed] [Google Scholar]
- Pessia E., Popa A., Mousset S., Rezvoy C., Duret L., et al. , 2012. Evidence for widespread GC-biased gene conversion in eukaryotes. Genome Biol. Evol. 4: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka E. R., 2000. Evolutionary Ecology. Benjamin Cummings, San Francisco. [Google Scholar]
- Postlethwait J. H., Woods I. G., Ngo-Hazelett P., Yan Y. L., Kelly P. D., et al. , 2000. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 10: 1890–1902. [DOI] [PubMed] [Google Scholar]
- Putnam N. H., Butts T., Ferrier D. E. K., Furlong R. F., Hellsten U., et al. , 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453: 1064–1071. [DOI] [PubMed] [Google Scholar]
- Rao J. V., Gunda V. G., Srikanth K., Arepalli S. K., 2007. Acute toxicity bioassay using Paramecium caudatum, a key member to study the effects of monocrotophos on swimming behaviour, morphology and reproduction. Toxicol. Environ. Chem. 89: 307–317. [Google Scholar]
- Schiex, T., A. Moisan, and P. Rouze, 2001 EuGene: an eucaryotic gene finder that combines several sources of evidence, in Computational Biology, edited by O. Gascuel and M. -F. Sagot. Springer Berlin Heidelberg 2066: 111–125. [Google Scholar]
- Siegel R. W., 1967. Genetics of ageing and the life cycle in ciliates. Symp. Soc. Exp. Biol. 21: 127–148. [PubMed] [Google Scholar]
- Simillion C., Vandepoele K., Van Montagu C., Zabeau M., Van de Peer Y., 2002. The hidden duplication past of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 99: 13627–13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., Saudemont B., Guglielmi G., Arnaiz O., Gout J. F., et al. , 2014. Genome-defence small RNAs exapted for epigenetic mating-type inheritance. Nature. [DOI] [PubMed] [Google Scholar]
- Sonneborn T. M., 1933. Mendelian methods applied to the ciliate protozoan, Paramecium caudatum. Am. Nat. 57: 72. [Google Scholar]
- Sonneborn T. M., 1937. Sex, sex inheritance and sex determination in Paramecium aurelia. Proc. Natl. Acad. Sci. USA 23: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn T. M., 1947. Developmental mechanisms in Paramecium. Growth Symposia 11: 291–307. [Google Scholar]
- Sonneborn T. M., 1975. The Paramecium aurelia complex of fourteen sibling species. Trans. Am. Microsc. Soc. 94: 155–178. [Google Scholar]
- Stanke M., Diekhans M., Baertsch R., Haussler D., 2008. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24: 637–644. [DOI] [PubMed] [Google Scholar]
- Sung W., Tucker A. E., Doak T. G., Choi E., Thomas W. K., et al. , 2012. Extraordinary genome stability in the ciliate Paramecium tetraurelia. Proc. Natl. Acad. Sci. USA 109: 19339–19344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F., 1993. Simple methods for testing molecular clock hypothesis. Genetics 135: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L., et al. , 2012. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitia R. A., Bottani S., Birchler J. A., 2008. Cellular reactions to gene dosage imbalance: genomic, transcriptomic, and proteomic effects. Trends Genet. 24: 390–397. [DOI] [PubMed] [Google Scholar]
- Werth C. R., Windham M. D., 1991. A model for divergent, allopatric speciation of polyploid Pteridophytes resulting from silencing of duplicate-gene expression. Am. Nat. 137: 515–526. [Google Scholar]
- Wichterman R., 1939. Cytogamy: a new sexual process in joined pairs of Paramecium caudatum. Nature 144: 123–124. [Google Scholar]
- Wolfe K. H., Shields D. C., 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387: 708–713. [DOI] [PubMed] [Google Scholar]
- Yanagi A., Haga N., 1995. A simple method of induction of autogamy by methyl cellulose in Paramecium caudatum. Zoolog. Sci. 12: 26. [Google Scholar]
- Yanagi A., Haga N., 1998. Induction of conjugation by methyl cellulose in Paramecium. J. Eukaryot. Microbiol. 45: 87–90. [Google Scholar]
- Yang J., Lusk R., Li W.-H., 2003. Organismal complexity, protein complexity, and gene duplicability. Proc. Natl. Acad. Sci. USA 100: 15661–15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.