Abstract
We have previously shown that HIV-1 superinfected Zambian seroconverters mount low binding and neutralizing antibody responses to their primary HIV-1 infecting virus, which could increase susceptibility to re-infection. Here, we investigated if antibody-dependent cellular cytotoxicity (ADCC), a process by which virus-infected cells are killed, was also reduced. Superinfected individuals exhibited low ADCC activity compared to non-superinfected individuals, but similar levels of CMV-reactive binding antibodies, suggesting superinfected individuals are capable of generating and maintaining virus-specific antibodies.
Keywords: HIV-1 superinfection, ADCC, HIV-1 ADCC, HIV dual infection
Introduction
Studies of HIV-1 superinfection (re-infection with a heterologous HIV-1 strain in an HIV+ individual) can provide a natural model by which to study the potential correlates of immune protection from HIV-1 acquisition. By identifying immune factors that differ between individuals that become superinfected versus those that may be at similar risk of superinfection but remain only singly-infected, it may be possible to define what a productive vaccine-mediated immune response requires. In a previous study of 22 individuals acutely infected with subtype C HIV-1, who received their infection from non-spousal partners, 3 were identified as superinfected during the first 12 months (3, 9, and 10 months) following their primary infection (1). We compared the anti-Env responses in the 3 superinfected individuals, prior to or at the time of superinfection, to those at equivalent time points following primary infection in 10 of the 19 individuals who remained free of superinfection, and demonstrated significantly lower pre-existing antibody responses to their primary HIV-1 infection (2). The ten nonsuperinfected controls were selected to have similar: 1) subtype of infection, 2) time from the last sero-negative to the first antigen or antibody positive sample, 3) seroconversion viral load, and 4) seroconversion within the same five-year interval (2002-2007) (2). Although, only 1/3 superinfected and 2/10 controls self-reported sex with outside partners, viral sequencing confirmed that they were initially infected by outside partners, consistent with under-reporting of this risk factor in this population (3). Thus, while with these small numbers of subjects it is not possible to perform case-control analyses, it is likely that all of the 19 nonsuperinfected individuals under study had similar sexual exposure to outside partnerships as those individuals who were superinfected (1, 2).
In our previous study of immune responses in the 3 superinfected and 10 control individuals (2) the following conclusions were made: 1) autologous neutralizing antibody responses that developed over the first 12 months to the primary infecting founder virus were significantly lower, 2) binding IgG antibodies to a Zambian subtype C gp120 protein derived from a founder virus infection in the same cohort were reduced, and 3) V1V2-reactive IgG antibodies were undetectable prior to superinfection in 3/3 individuals, whereas plasma from 6/10 non-superinfected controls, at a similar 5-8 months after primary infection, showed V1V2-reactive antibodies within the first year of infection (2). These data taken together suggested that potentially protective IgG neutralizing and binding antibodies were lower prior to re-infection in the superinfected group compared to similar time points for the non-superinfected group, representing potential correlates of HIV-1 protection. These data are also consistent with superinfection studies in intrasubtype B superinfected men having sex with men that have shown lower levels of neutralizing antibodies prior to superinfection (4, 5). However, a reduced antibody response was not observed in studies of multi-clade superinfected Kenyan female sex workers (6); although, in this same cohort, a significantly decreased risk of superinfection after the first year of primary infection was consistent with the development of resistance to re-infection (7). It remains to be seen what role antibody-mediated cellular cytotoxicity (ADCC) plays in protection or control of either primary HIV-1 infection or superinfection.
ADCC is a process by which virus-specific antibodies bind to viral antigen (e.g. Env) on the surface of infected cells, allowing FcR-bearing effector cells (e.g. natural killer cells, monocytes, etc.) to recognize them and trigger a degranulation cascade resulting in infected target cells death (8). ADCC-mediating antibodies have been shown to be present within days to weeks of acute HIV-1 infection symptom onset (9). Moreover, ADCC activity has been shown to correlate with slower disease progression, be enriched in HIV-1 infected elite controllers and may be associated with the initial decrease in viral load seen during acute infection (8-11). Recent studies have also implicated ADCC activity in the partial protection seen in the RV144 vaccine trial (12), in which a modest reduction in risk of HIV-1 acquisition was observed in vaccinees as compared to unvaccinated controls (13, 14). Although it is still unclear what potential functional role this effector activity may have in protection or amelioration of HIV-1 infection (15), ADCC is recognized to be a component of a productive antibody-mediated response to a viral infection. In this study, we therefore investigated whether the previously defined humoral antibody defect observed in superinfected individuals: 1) may functionally compromise the ability to elicit ADCC-mediated killing of virus-infected cells and 2) is HIV-1 specific or, rather, represents a global humoral defect in responding to viral pathogens.
Materials and Methods
Using a previously published rapid fluorometric ADCC assay (16, 17), we assessed the capacity of antibodies from pre-superinfection plasma (or similar time points for the controls) to mediate killing of an HIV-1 Env-coated target cell line. We first coated 106 CEM.NKR-CCR5 target cells with 15μg of a purified subtype C gp120 protein from ZM205F (that has previously been shown to be broadly recognized by antibodies in plasma from Zambian seroconverters (2)) to mimic a virus-infected cell expressing envelope on the outside. Target cells were then double-stained with the viability dye carboxyfluorescein succinimidyl ester (CFSE), to assess cell lysis, along with the lipid-membrane associated dye PKH-26 to distinguish target cells from effectors. Heat-inactivated plasma was diluted 1:1000 in complete R10 media and 100ul of diluted plasma was incubated with 104 double-stained target cells (in 50μl) in duplicate for 30 minutes, in order for antigen-antibody interactions to occur. Finally, 50μl of THP-1 monocyte cells, were added at a 20:1 effector to target ratio to the antibody-cell mixture and plates were incubated for 6 hours at 37°C. Primary monocytes or the THP-1 human monocytic cell line expressing FcγR1, capable of eliciting ADCC, have previously been used as effector cells in this same ADCC assay (17, 18).
Following incubation, cells were fixed and analyzed to determine the number of killed target cells by flow cytometry. Cells mixed with uninfected normal human plasma were used as a non-cytolytic control and were used to define the PKH-26+ target cell population (by gating on side-scatter by PKH-26). This strict PKH-26+ target cell gate was then applied to the test plasma samples in order to identify target cell populations that had lost CFSE upon cytolysis (PKH-26+CFSE-). Values from duplicate samples were averaged within each assay, and ADCC values were averaged between two independent experiments.
Mann-Whitney one-tailed test was used to compare the median ADCC responses in superinfected and non-superinfected groups, with the hypothesis that the superinfected group would have reduced levels of ADCC. Spearman rank correlation tests were performed in order to determine correlation between ADCC activity and antibody readouts (one-tailed) as well as viral load (two-tailed). Since a hypothesis regarding CMV-specific antibody levels and HIV-specific antibody levels was not predicted a priori, we applied a Mann-Whitney two-tailed test to compare CMV binding antibody levels in superinfected and nonsuperinfected groups.
Results and Discussion
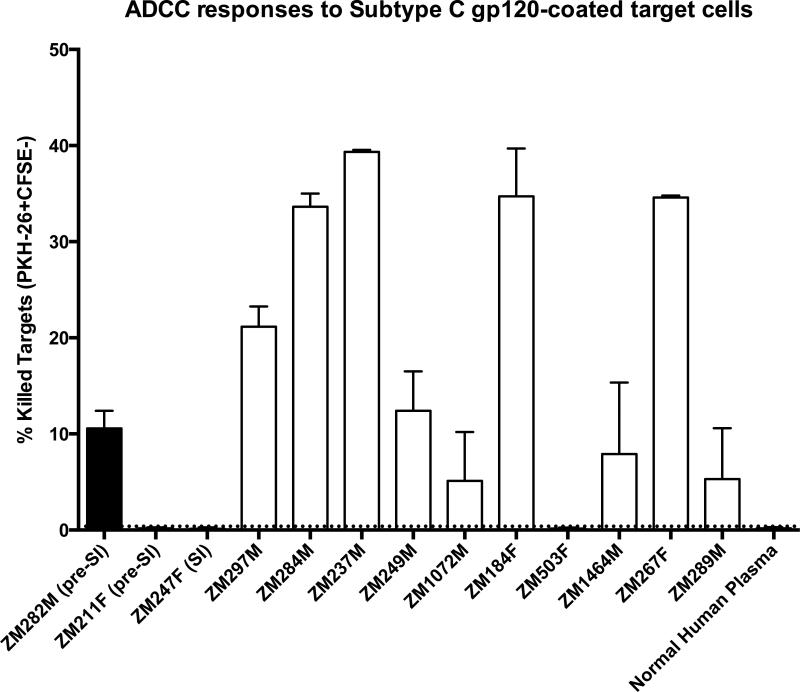
Whereas 9/10 non-superinfected controls showed detectable levels of ADCC-mediated killing, only 1/3 superinfected individuals showed any ADCC-mediated killing, and this single positive value was low compared to the median value of the controls – 10.6 vs 16.8 (Figure 1). Overall, the superinfected group showed a significantly lower level of ADCC-mediated killing activity compared to the non-superinfected group (Mann-Whitney test p = 0.049).
Figure 1. ADCC activity is low to absent in superinfected individuals prior to superinfection.
Pre-superinfection (pre-SI) plasma or similar time points for non-superinfected controls were used in a rapid fluorometric ADCC assay in order to assess ADCC-mediated killing, as measured by PKH-26+CFSE- killed target cells identified by flow cytometry. Percentage of killed target cells is shown for the three superinfected individuals (first three bars, filled: ZM282M, ZM247F and ZM211F) and ten non-superinfected controls (open bars). For ZM247F, in which superinfection was detected at 3-months post-seroconversion, we tested this 3-month plasma. Uninfected normal human plasma was used as a negative control for the assay. Error bars represent the SEM between two independent experiments.
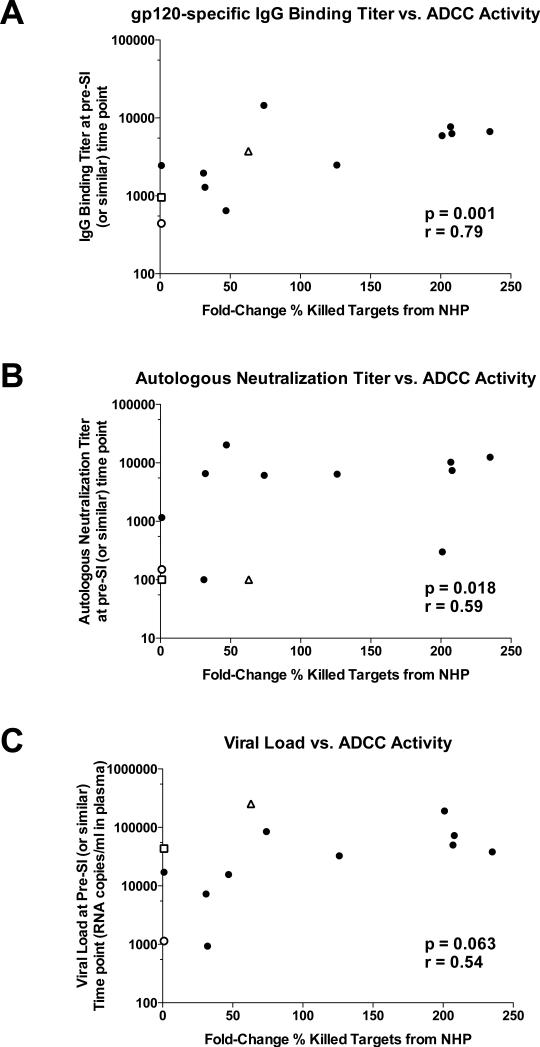
To ensure that this assay was accurately representing humoral antibody trends, average fold change of ADCC-mediated killing (compared to the normal human plasma control) amongst all study participants was compared to the other readouts of IgG-mediated antibody responses against the primary HIV virus that we have reported previously (2) (Figure 2A and B). ADCC activity was positively correlated with gp120 binding titer (Spearman correlation p = 0.001, r = 0.79) and titer of neutralization of founder Env pseudoviruses (Spearman correlation p = 0.018, r = 0.59). In addition, ADCC activity positively correlated with viral load, and this correlation trended toward statistical significance (Figure 2C; Spearman correlation p = 0.063, r = 0.54).
Figure 2. ADCC activity correlates with IgG-mediated humoral responses and viral load.
Average fold-change of ADCC-mediated killing (compared to the normal human plasma negative control) was compared with A) IgG binding titer to a subtype C gp120 protein (ZM205F), B) autologous neutralization titer to founder virus prior to superinfection and C) viral load prior to superinfection (pre-SI) or at similar time points for non-superinfected controls. Correlation values were calculated using Spearman rank correlation tests. Superinfected individuals are represented by open shapes where ZM282M is shown as a triangle, ZM247F is shown as a square, and ZM211F is shown as a circle.
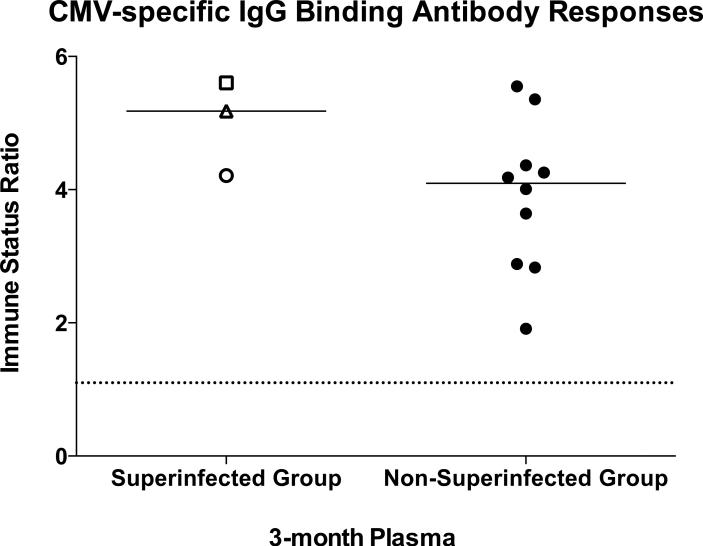
Finally, we wanted to evaluate whether superinfected individuals also have low binding antibodies to other viral pathogens potentially representing a global humoral response defect, or whether this trend was HIV-1 specific. Therefore, we compared levels of IgG binding antibodies (in 3-month post-seroconversion plasma) to Cytomegalovirus (CMV) using a commercially available CMV IgG antibody assay (Trinity Biotech Captia CMV IgG), as the CMV seroprevalence in both adults and infants is extremely high in Zambia and in most regions of the world (19). Immune status ratios, representing OD values adjusted for calibration controls, were calculated where a value ≥1.10 indicates the presence of CMV-specific IgG antibodies. All study subjects were CMV seropositive, and in contrast to their anti-HIV Env responses, the superinfected individuals had higher median values than the non-superinfected controls (Figure 3), although these differences were not significantly different (Mann-Whitney test p = 0.161).
Figure 3. Superinfected individuals are not globally-defective in the ability to generate virus-specific binding antibodies.
Cytomegalovirus-specific IgG binding antibodies were measured using 3-month post-seroconversion plasma in the Trinity Biotech Captia CMV IgG ELISA. Immune status ratios ≥1.10 (dashed line) indicate the presence of CMV-specific IgG antibodies. Superinfected individuals are represented as described for Figure 2. Immune status ratio values for superinfected and non-superinfected groups were compared using a Mann-Whitney test.
One recent study of Kenyan sex workers showed that there was no correlation between the risk of HIV-1 superinfection and antibody-dependent cell-mediated viral inhibition (ADCVI) activity - a cumulative effect of ADCC, release of β-chemokines and phagocytosis (20). This observation was consistent with previous studies from this cohort comparing antibody responses between superinfected and non-superinfected groups, in which no difference in breadth and potency of neutralizing antibodies was observed immediately prior to superinfection (6). We, by contrast, have shown that intrasubtype C superinfected Zambian seroconverters have low to undetectable levels of plasma antibodies that display ADCC-mediated killing of gp120-coated target cells. It is possible that a robust ADCC-mediated response might represent an early mechanism by which superinfection outgrowth may be quenched or limited post-superinfection, however this would require additional mechanistic studies.
The main limitation of this study is the small sample size, and conclusions made here will need to be confirmed in a larger ongoing study. However, in a recent review of the frequency of HIV-1 superinfections, it was shown that 12/16 observational studies of superinfection reported 3 or fewer superinfection cases (21). Identifying cases of superinfection in low-risk cohorts such as heterosexual couples is challenging due to inherently low behavioral risk factors (e.g. nonspousal partnerships, sex without condom, etc.). However, a majority of newly acquired HIV-1 infections occur in heterosexual couples (22), making this cohort type a crucial target group for an effective HIV-1 vaccine. A broader meta-analysis of data from multiple studies may allow for the characterization of potential correlates of protection from superinfection across multiple cohorts with similar risk factors.
Importantly, although the non-superinfected controls in this study had a variable range of ADCC responses, all three superinfected individuals clustered on the low end of the spectrum (two having no detectable ADCC activity). This difference in ADCC activity between superinfected and non-superinfected groups, despite the smallness of the sample size, was still statistically significant. Moreover, the low ADCC activity in these superinfected individuals was consistent with other HIV-1 specific antibody readouts measured previously, though it represents a novel defect in the functionality of the HIV-1 specific antibodies present prior to superinfection.
Conclusion
Our findings, in combination with previous studies showing limited neutralizing and non-neutralizing humoral responses to primary HIV-1 infection in superinfected individuals, support the hypothesis that these superinfected individuals have compromised HIV-specific humoral responses prior to superinfection, which may increase their susceptibility to HIV-1 reinfection. Despite this, the HIV-1 superinfected individuals studied here are able to generate and maintain similar levels of binding antibodies to a common viral pathogen (CMV) during early infection compared to non-superinfected controls, suggesting that the potential humoral defect observed in superinfected individuals is specific to their primary HIV-1 immune response. These data lend hope for HIV-1 vaccine studies by supporting the idea that it may be possible to develop a primary protective immune response to HIV-1 infection, at least in situations where only a single HIV-1 subtype predominates.
Highlights.
Plasma associated ADCC activity is low or absent prior to HIV-1 superinfection
ADCC activity correlates with gp120 binding and virus neutralizing antibody titers
HIV superinfected subjects have similar titers of anti CMV antibodies to controls
Acknowledgements
We respectfully recognize the Rwanda-Zambia HIV Research Group (RZHRG) and Zambia-Emory HIV Research Project (ZEHRP) staff and participants for their invaluable contributions to this study. The Emory University Institutional Review Board and the University of Zambia Research Ethics Committee approved these studies. This research was supported by grants from the National Institutes of Health (R37 AI-51235 (EH); R01 AI-58706 (CAD); P01-AI096187 (EH and CAD))and the International AIDS Vaccine Initiative (SA). A Fogarty AITRP grant FIC 2D43 TW001042 supported WK. This project was also funded in part by the National Center for Research Resources P51RR165 and the Office of Research Infrastructure Programs/OD P51OD11132 for the Yerkes National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraft CS, Basu D, Hawkins PA, Hraber PT, Chomba E, Mulenga J, Kilembe W, Khu NH, Derdeyn CA, Allen SA, Manigart O, Hunter E. Timing and source of subtype-C HIV-1 superinfection in the newly infected partner of Zambian couples with disparate viruses. Retrovirology. 2012;9:22. doi: 10.1186/1742-4690-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu D, Kraft CS, Murphy MK, Campbell PJ, Yu T, Hraber PT, Irene C, Pinter A, Chomba E, Mulenga J, Kilembe W, Allen SA, Derdeyn CA, Hunter E. HIV-1 subtype C superinfected individuals mount low autologous neutralizing antibody responses prior to intrasubtype superinfection. Retrovirology. 2012;9:76. doi: 10.1186/1742-4690-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen S, Meinzen-Derr L, Kautzman M, Zulu I, Trask S, Fideli U, Musonda R, Kasolo F, Gao F, Haworth A. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 4.Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, Liu Y, Petropolous CJ, Daar ES, Little SJ, Richman DD. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355:1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Chaillon A, Wagner GA, Hepler NL, Little SJ, Kosakovsky Pond SL, Caballero G, Pacold ME, Phung P, Wrin T, Richman DD, Wertheim JO, Smith DM. Dynamics of viral evolution and neutralizing antibody response after HIV-1 superinfection. J Virol. 2013;87:12737–12744. doi: 10.1128/JVI.02260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blish CA, Dogan OC, Derby NR, Nguyen MA, Chohan B, Richardson BA, Overbaugh J. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82:12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronen K, McCoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, Jaoko W, Mandaliya K, McClelland RS, Richardson BA, Overbaugh J. HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women. PLoS Pathog. 2013;9:e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 11.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccineinduced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 15.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV AIDS. 2013;8:392–400. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. Journal of immunological methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Kramski M, Schorcht A, Johnston AP, Lichtfuss GF, Jegaskanda S, De Rose R, Stratov I, Kelleher AD, French MA, Center RJ, Jaworowski A, Kent SJ. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. Journal of immunological methods. 2012;384:51–61. doi: 10.1016/j.jim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Tudor D, Bomsel M. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRIdependent manner. AIDS. 2011;25:751–759. doi: 10.1097/QAD.0b013e32834507bd. [DOI] [PubMed] [Google Scholar]
- 19.Gompels UA, Larke N, Sanz-Ramos M, Bates M, Musonda K, Manno D, Siame J, Monze M, Filteau S. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:434–442. doi: 10.1093/cid/cir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forthal DN, Landucci G, Chohan B, Richardson BA, McClelland RS, Jaoko W, Blish C, Overbaugh J. Antibody-dependent cell-mediated virus inhibition antibody activity does not correlate with risk of HIV-1 superinfection. J Acquir Immune Defic Syndr. 2013;63:31–33. doi: 10.1097/QAI.0b013e3182874d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redd AD, Quinn TC, Tobian AA. Frequency and implications of HIV superinfection. The Lancet infectious diseases. 2013;13:622–628. doi: 10.1016/S1473-3099(13)70066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, Greenberg L, Allen S. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]