Summary
Most tubes have “seams” – intercellular or autocellular junctions that seal membranes together into a tube – but “seamless” tubes also exist [1-3]. In Drosophila, stellate-shaped tracheal terminal cells make seamless tubes, with single branches running through each of dozens of cellular extensions. We find that mutations in braided impair terminal cell branching and cause formation of seamless tube cysts. We show that braided encodes Syntaxin7, and that cysts also form in cells deficient for other genes required either for membrane scission (shibire), or for early endosome formation (Rab5, Vps45, and Rabenosyn-5). These data define a requirement for early endocytosis in shaping seamless tube lumens. Importantly, apical proteins Crumbs and phospho-Moesin accumulate to aberrantly high levels in braided terminal cells. Overexpression of either Crumbs, or phospho-mimetic Moesin, induced lumenal cysts and decreased terminal branching. Conversely, the braided seamless tube cyst phenotype was suppressed by mutations in crumbs or Moesin. Indeed, mutations in Moesin dominantly suppressed seamless tube cyst formation and restored terminal branching. We propose that early endocytosis maintains normal steady-state levels of Crumbs, which recruits apical p-Moe, which in turn regulates seamless tube shape through modulation of cortical actin filaments.
Results
Terminal cells mutant for braided have lumenal cysts and fewer branches
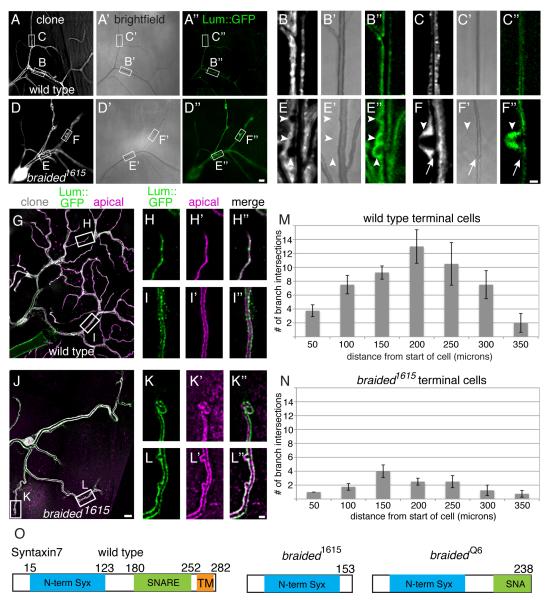
Detailed characterization of braided mutant terminal cells revealed the presence of small cysts in the seamless tube. Homozygous mutant terminal cells (positively labeled with DsRed or GFP) were generated by mitotic recombination in heterozygous animals, and their tubes were examined directly using brightfield microscopy to visualize gas-filled lumina, or indirectly by using fluorescence microscopy to reveal lumina as regions of fluorescent protein exclusion (Figure 1A-F). On occasion, partially gas-filled (Figure 1D’,E’,F’) cystic dilations were visible, but most often the fluorescent protein excluding regions were not gas-filled. To address more carefully whether the regions of exclusion were contiguous with the seamless tube lumen, we carried out two additional experiments. First, we examined the localization of secreted GFP in the mutant cells. In living and heat-killed wild type larvae, secreted GFP (lum-GFP; [4]) was detected in a few bright puncta and as a faintly visible haze surrounding the tube lumen (Figure 1A’’, B’’, C’’). We have previously shown that in animals with gas-filling defects, secreted GFP fills the tube lumen [4]; however, in braided terminal cell clones, lum-GFP brightly outlined gas-filled tubes and seemed to fill the cysts (Figure 1D’’, E’’, F’’). These data suggest that the cysts may contain fluid and matrix materials instead of gas. To strengthen the conclusion that the regions of fluorescent protein exclusion are cystic dilations of the seamless tube, 3rd instar larvae were filleted and co-stained with lumenal membrane markers. Staining with α-Wkdpep sera (Figure 1G - L), that we previously showed recognizes an unknown lumenal membrane-associated antigen [5], and other apical membrane markers such as actin and Whacked::mKate2 (an apical-localized Rab35GAP-mKate2 fusion [5]) (Figure S1A-D’’), clearly outlined the lum-GFP filled bulges, consistent with the hypothesis that these are cystic dilations of the seamless tube (Figure 1L and Figure S1D).
Figure 1. Terminal cells mutant for braided (Syntaxin7) have branching and lumen defects.
Homozygous terminal cells (DsRed, white) from heat-killed mosaic third instar larvae (wt A,B,C ; braided1615 D,E,F). The braided cells are less branched (A-A’’,D-D’’) and exclude DsRed from regions (E’,F’) adjacent to gas-filled tube (D,E,F) in contrast to wt (A,B,C). Brightfield images show gas-filled lumens of wt (A’,B’,C’) and braided terminal cells (D’,E’,F’). Secreted lum-GFP, barely detected in wt (A’’,B’’,C’’), outlines braided seamless tubes and adjacent DsRed-excluding structures. Boxes in (A-A’’ and D-D) magnified in (B-C’’and E-F’’). Arrowheads (E-F’’) indicate DsRed-excluding structures; arrow (F-F’’) indicates rare gas-filling. (G-L’’) Staining (cytoplasm – white; lum-GFP-green; and apical, αWkdpep-magenta) shows DsRed-excluding bodies are bounded by apical membrane. Lum-GFP in lumen does not survive fixation (JSR unpublished data). Boxed regions (G, J) are magnified in H-L’’. Tubes tips are disorganized (K-K’’) compared to wt (H-H’’) and apical membrane expansions are detected (I-I’’,L-L’’). (B-C’’, E-F’’, and H-L’’) oriented proximal down, distal up. Sholl analysis illustrates branching defects of braided1615 terminals cells (M,N – wt n-4, 1615 n=4 (p=0.003)). Error bars = +/− SEM. (O) Mapping of braided identified Syx7 as the gene affected in 1615 and Q6. Syntaxin7 is a 282 amino acid protein with an N-terminal Syntaxin domain, a central t-SNARE domain, and a C-terminal transmembrane domain. Nonsense mutations truncate Syx7 at aa 153 in braided1615 and at aa 238 in braidedQ6. Scale bars = 10 microns in D’’ (for A-A’’, D-D’’) and J (for G and J), 2 microns in F’’ (for B-C’’ and E-F’’) and L’’ (for H-I’’ and K-L’’). See also Figure S1.
Indeed, we observed apical membrane that surrounded an uninterrupted lumen and was continuous between liquid-filled bulges and gas-filled tubes. In addition, lumenal membrane staining revealed that the tips of the seamless tubes were aberrant in braided mutant terminal cells: in contrast to wild type seamless tubes that come to smoothly rounded blind-ends, approximately 70% of braided seamless tube termini were cystic and grossly irregular in shape (Figure 1H-H’’,K-K’’). The cysts could be detected as early as 2nd larval instar and were specific to terminal cell seamless tubes, as no other tracheal cells displayed tube defects when compromised for braided (Figure S1E-F’). As previously noted, braided terminal cells had fewer cellular extensions than wild type (Figure 1M,N) [4]; a large proportion of these branches were short as compared to wild type, although long branches were also present (compare peaks, Figures: 1M,N; S1J). This mosaic analysis demonstrated a cell-autonomous role for braided in tracheal terminal cell branching and seamless tube morphogenesis.
The early endosome SNARE, Syntaxin7, is encoded by braided
To determine the function of braided, we identified the affected gene. Prior to this study, there was a single extant allele of braided (braided1615) [4]; therefore we isolated an additional allele, braidedQ6 (Materials and Methods), for use in mapping the locus and characterizing the phenotype. The tracheal phenotype of the two alleles was indistinguishable (Figure S1 G-I’). We determined that braided encodes Syntaxin7 (Syx7), previously identified in Drosophila as avalanche (avl) [6]. Alleles of braided do not complement avl, and sequence analysis revealed single nucleotide changes in the Syx7 coding sequence of braided1615 and braidedQ6, each resulting in a nonsense mutation (Figure 1O). Terminal cells expressing Syx7 RNAi transgenes (Figure S1 L-L’’), and terminal cells homozygous for avl3, displayed the braided phenotype (Figure S1 M-M’’); hereafter, we refer to braided and avl alleles as Syx7brd or Syx7avl. The Syx7brdQ6, Syx7brd1615, and Syx7avl3 alleles are presumed null, as they truncate Syx7 more severely than the previously characterized null allele, Syx7avl1 [6], although it is possible that any protein produced might interfere with normal Syx7 function or that of Syx7 binding partners.
Syx7 is a SNARE protein that promotes fusion of endocytic vesicles to early endosomes[6]. Subsequent to vesicle scission the Vps45-Rabenosyn-5 (Rbsn-5) complex physically links Syx7 to the early endosome regulator, Rab5 [7], to promote early endosome maturation (Figure 2A). As expected, Syx7 was required for proper early endosome formation in terminal cells: large YFP::Rab5 puncta present in control terminal cells were largely absent from Syx7brd terminal cells, in which Rab5 puncta appeared markedly smaller, more numerous, and dispersed (Figure 2B-C’).
Figure 2. Terminal cells mutant for early endosome genes show Syx7-like defects.
(A) Shibire/Dynamin (Shi) is required for vesicle scission at the plasma membrane, whereas Syx7, Vps45, Rbsn-5, and Rab5 promote fusion of endocytic vesicles to early endosomes. Rbsn-5, a Rab5 effector, recruits Vps45, which in turn promotes Syx7 function [7]. YFP::Rab5 marks large, widely spaced early endosomes (B,B’-YFP in green, apical/⟨Wkdpep in magenta) in wt, and is disrupted in Syx7brd1615 cells (C,C’). Images in B-C’ oriented proximal left, distal right. In mosaic larvae, wt (D,D‘) terminal cells have smooth lumens, whereas Vps45 (E), Rbsn-5 (F), and Rab5 (G) cells display multiple cysts (clone, white). Terminal cells mutant for shi do not show clear exclusion of GFP (clone marker) from seamless tubes (H). Unlike in wt (I), apical membrane in Vps45 (J’), Rbsn-5 (K’), and Rab5 (L’) cells, and in shi RNAi (M’) cells, (apical/⟨Wkdpep, magenta) shows cystic dilations. Branch tips often showed disorganized apical membrane (I vs J,K,L,M,). All early endocytic mutant terminal cells showed reduced branching (N – wt n=4, Vps45 n=5 (p=0.08), Rbsn-5 n=3 (p=0.03), Rab5 n=5 (p=0.002), shi n=6 (p=0.0004)). Error bars = +/− SEM. Images in I-M’ oriented proximal down, distal up. Scale bars = 10 microns in H (for D-H), 2 microns in C’ (for B-C’), and M’ (for I-M’). See also Figure S2.
Defective endocytosis causes cyst formation and reduced branching in terminal cells
To test whether seamless tube defects in Syx7 cells result from interfering with endocytosis, or from an unappreciated role of Syx7 in another process, we analyzed terminal cells homozygous for Vps45, Rabenosyn-5 (Rbsn-5), Rab5, or shibire (shi)/Dynamin. Vps45, Rbsn-5, and Rab5 mutant cells displayed seamless tube cysts and disorganized tube termini; they also showed reduced terminal branching (Figure 2D-G,I-L’,N). We note that Vps45 and Rbsn-5 defects were milder than observed with mutations in the other genes, and that branching of Vps45 cells was not significantly altered (Figure 2 legend). In contrast, shi null clones exhibited more severe phenotypes; mutant cells were largely unbranched and contained clumps of grossly disorganized apical membrane (Figure 2H,N and Figure S2A-A’’). Similarly severe defects were detected in some Rab5 terminal cells, which also showed failure in gas-filling, increased tube diameter, and extreme disorganization of the apical membrane (Figure S2B-D’ and data not shown). Variability among these terminal cell phenotypes likely reflects differential contribution of maternally deposited gene product. To determine whether cystic tube defects could be induced by blocking membrane internalization, we examined cells less severely compromised for shi; partial knockdown of shi by RNAi produced terminal cell defects comparable to those seen for Syx7 (Figure 2M,M’). Since mutations compromising function of the Syx7-Vps45-Rbsn-5-Rab5 complex, as well as Shi activity, result in lumenal cysts, we conclude that early endocytic events are required for normal seamless tube morphology.
Elevated Crumbs and Moesin in Syx7 terminal cells cause seamless tube cysts
One explanation for the lumenal morphology defects of Syxbrd would be that altered membrane transport might lead to an accumulation of excess apical membrane, resulting in cysts. Alternatively, altered steady-state levels of specific apical proteins might be responsible for the observed defects. Consistent with the second model, previous work suggested that a block in early endocytosis causes an accumulation of the polarity protein Crumbs (Crb) on apical membrane [6]. In addition, Crb overexpression is sufficient to produce polarity and overgrowth defects associated with depletion of Syx7 and Rab5 in wing epithelia [6]. We showed previously that Crb localizes to distinct domains along the lumenal membrane of terminal cells [5]; therefore, we tested whether Crb accumulated ectopically in these cells when early endocytic events were compromised. Terminal cells mutant for Syx7brd exhibited dramatically higher Crb levels compared to neighboring heterozygous cells (Figure 3A-D’). Additionally, Crb interacting-protein dPatj co-localized with Crb at the lumenal membrane of seamless terminal tubes and also showed ectopic apical localization in Syx7brd clones (Figure S3A-G’’). Interestingly, Crb levels were not strongly altered in Syx7brd clones in other tracheal cell subtypes (data not shown). The actin-bundling protein Moesin (Moe) also physically interacts with Crb [8], and in wild type terminal cells, phosphorylated (active) Moe (p-Moe) shows a discrete localization to the lumenal membrane [9, 10] (Figure S3H-H’’). In Syx7brd cells (Figure 3E-G’) – and Rab5 cells (Figure S3K,K’) – we found p-Moe at the apical membrane was dramatically increased (patches of p-Moe were sometimes seen at the basal membrane; Figure 3G, arrowhead). To test whether the change in p-Moe reflected increased steady-state levels of Moe, we compared total Moe staining in mutant terminal cells with total Moe staining in neighboring heterozygous terminal cells of the same mosaic animals. We found that total Moe levels were unchanged at the level of resolution provided by antibody staining; however, more intense patches of Moe-staining were detected near the apical membrane of mutant cells (Figure S3I-J’,L,L’).
Figure 3. Crumbs and phospho-Moesin are elevated in Syx7 terminal cells, and are sufficient to induce cysts and decrease branching.
In mosaic Syx7brdQ6 animals, Syx7brdQ6 / brdQ6 (A-A’’’) (DsRed, white) and Syx7brdQ6 / + (wt) terminal cells (A’-A’’’) were imaged. Wt terminal branches were revealed by apical membrane staining (αWkdpep, magenta), while Syx7 brdQ6 / brdQ6 cells were additionally labeled by the clone marker (white). We note the dramatic difference in Crb staining (Crb::GFPA/α-GFP, green; A’’,A’’’) between wt (B’’,B’’’) and Syx7brdQ6 / brdQ6 cells (C’’,C’’’), quantified in D and D’ (11 total branches from 6 terminal cells were scored for each). Boxes in A-A’’’ are magnified (B-B’’’, C-C’’’) and reoriented with proximal left and distal right. Similar to Crb, p-Moe was enriched (quantified in E and E’; 17 total branches from 7 wt, and 15 total branches from 5 Syx7brd1615/ cells were scored) at the lumenal membrane in wt terminal cells (F,F’) and was elevated in neighboring Syx7brd1615 cells (G,G’). Error bars = +/− SEM. Arrowhead (G) indicates basolateral enrichment of pMoe. Images (F, G) depict cells from the same larva. Boxes (F, G) are magnified (F’, G’). Wt (H,H’), Crb overexpressing (I,I’), and phospho-mimetic Moe expressing (J,J’) cells are shown (cytoplasm/DsRed or GFP, white; apical/αWkdpep, magenta). Overexpression of full-length Crb reduced branch number and induced cyst formation (H,H’), as did expression of phospho-mimetic Moe (MoeT559D; J,J’). Boxes (H,I,J) magnified (H’,I’,J’ ) and reoriented proximal down, distal up. Arrowheads indicate cysts in I’ and J’. Scale bars = 10 microns in A’’’ (for A-A’’’), G (for F and G), and J (for H-J), 2 microns in C’’’ (for B-C’’’), G’ (for F’ and G’), and J’ (for H’-J’). See also Figure S3.
Since Crb, dPatj, and p-Moe showed elevated levels in Syx7 terminal cells, we tested whether increased levels of these proteins were sufficient to induce cysts in cells in which endocytosis had not been perturbed. Strikingly, MARCM clones over-expressing full-length Crb in terminal cells exhibited cysts (and decreased branch number) similar to those in Syx7 (Figure 3I,I’ and data not shown). Terminal cells expressing a phospho-mimetic isoform of Moe (MoeT559D), but not wild type Moe::myc or Moe::GFP, also exhibited Syx7-like dilations of the lumenal membrane and reduced branch number (Figure 3J,J’ and data not shown). In contrast, overexpression of dPatj did not affect terminal cell tubes or branching (data not shown), suggesting that the phenotypes exhibited in Syx7 mutants are likely due to increased activity of Moe, potentially through the ability of Crb to recruit p-Moe to the apical membrane.
Mutations in crumbs and Moe suppress Syx7 terminal cell defects
To determine whether ectopic Crb or Moe were necessary for seamless tube cyst formation in Syx7 terminal cells, we sought to suppress the Syx7 defects by reduction of crb or Moe. Indeed, elongation of multi-cellular tracheal tubes caused by mutations in yurt or coracle can be suppressed by heterozygosity for crb [11]. While no suppression of the Syx7 defect was observed in animals heterozygous for crb, we found that terminal cells homozygous mutant for both crb and Syx7 were almost completely rescued for cysts, although interestingly, defects in branch number were enhanced as compared to Syx7brd alone (Figure 4A-C,E,F). Terminal cells mutant for crumbs alone appeared wild type for branch number and lumen morphology (Figure S4A-B’). This is perhaps surprising, given the essential role of Crb in establishing polarity in most embryonic epithelia, including the trachea; however, it is has been established that Crb is not required for maintenance of polarity in several tissues in which it is expressed [12]. Strikingly, heterozygosity for a mutant allele of Moe dramatically rescued Syx7brd terminal cells: the number of cysts was reduced and terminal branching was greatly restored (Figure 4D-F). Importantly, heterozygosity for Moe did not appear to bypass the requirement for Syx7 in early endocytosis since Rab5 distribution remained disrupted (YFP::Rab5, Figure 4G-I’).
Figure 4. Mutations in crb and Moe suppress Syx7 defects.
Wt (A), Syx7brd1615 (B) Syx7brd1615, crb11A22 (C) and Moe+/−; Syx7brd1615 (D) cells (white) are shown. Lumenal staining (αWkdpep, magenta) was used to count cystic dilations. Terminal cell nuclei are marked in green. Whereas wt cells displayed 1.5 cysts/millimeter (mm) of lumenal membrane (n=12, E), Syx7brd1615 cells exhibited 156 cysts/mm of membrane (n=23, E). Cells mutant for both Syx7brd1615 and crb11A22 showed a dramatic reduction in cysts (31 cysts/mm, n=15, E), as well as a striking reduction in terminal branching (F). In animals heterozygous for Moe, Syx7brd1615 mutant cells showed more than a 60% reduction in the number of cysts (93 cysts/mm, n=16, E). In addition, Moe also dominantly suppressed the Syx7brd1615-induced decrease in terminal branching, as approximated by measuring the total length of all cellular extensions (F). Although apical membrane (αWkdpep-magenta) is largely rescued for dilations in Moe heterozygotes, YFP::Rab5-positive early endosomes (green) have the same dispersed appearance in Syx7brd1615 (G,G’) and Moe+/−; Syx7brd1615 (H,H’) - compared to wt (F,F’). (J-K’) p-Moe (green – DsRed expressing nuclei are also labeled in green) enrichment is lost in Syx7brd1615, crb double mutant terminal cells (GFP, white, J’; 15 total branches from 6 heterozygous neighboring cells and 11 total branches from 8 Syx7brd1615, crb cells in K,K’). Lifeact-ruby (green) and apical membrane (αWkdpep, magenta) are shown in wt (L,L’), Syx7brd1615 (M,M’; 15 total branches from 5 sibling control cells and 16 total branches from 5 Syx7brd1615 cells in P,Q), Crb overexpressing (N,N’; 15 total branches from 5 sibling control cells and 16 total branches from 6 Crb overexpressing cells in P,Q), and MoeT559D expressing (O,O’; 15 total branches from 5 sibling control cells and 15 total branches from 5 phospho-mimetic Moe expressing cells in P,Q) cells. Apical actin is enriched in all of these genetic backgrounds (M-Q) as compared to wt (L,P,Q). Asterisks in P and Q indicate a lack of statistical significance. Error bars = +/− SEM. Small cysts detected at the lumenal membrane (αWkdpep, magenta) of tsr mutant terminal cells (R). Scale bar = 10 microns in D (for A-D) and J’ (for J and J’) and 2 microns in I’ (for G-I’) and O’ (for L-O’) and R (for R).
We next sought to determine the order of action between Crb and Moe in seamless tube cyst formation. First, we tested whether p-Moe was elevated in Syx7brd, crb double mutant animals; it was not (Figure 4J-K’). Second, we tested whether expression of phospho-mimetic Moe was sufficient to induce formation of seamless tube cysts and to reduce terminal branching in cells mutant for crb. Although the overall phenotype of the cells appeared somewhat different than with expression of active Moe alone, Moe-induced dilations were present and branching was reduced (Figure S4C). We speculate that active Moe does not completely bypass a requirement for Crb because Crb plays a substantial role in the subcellular localization of active Moe; it is also possible that Crb-dependent recruitment of aPkc may play a role in activation of Moe via phosphorylation [13].
Taken together, these data suggest that p-Moe is critically regulated by endocytosis, and that seamless tube cysts in Syx7 terminal cells arise due to a stabilized and stiffened cortical actin cytoskeleton [14, 15]. In agreement with this hypothesis, we found that apical actin was significantly increased in terminal cells expressing lifeact-ruby and mutant for Syx7 or that expressed MoeT559D; however, the increased signal measured for Crb was not statistically significant (Figure 4L-Q). This may reflect poor sensitivity of our assay, or the variable expressivity of the Crb overexpression phenotype. To test whether stabilized actin is sufficient to cause seamless tube cysts, we examined cells mutant for twinstar, which encodes Drosophila Cofilin, a protein with actin filament severing activity. Indeed, twinstar cells displayed small cystic dilations along their seamless tubes (Figure 4R). Likewise, mutations in slingshot, a cofilin phosphatase that activates cofilin, also displayed seamless tube cysts (data not shown). Therefore, we propose that defects in endocytosis lead to higher steady-state levels of Crb, resulting in higher Moe activity at the apical membrane, which in turn leads to development of cysts as a consequence of a stabilized/stiffened actin cortex.
Discussion
Endocytosis plays a critical role in the maturation and morphogenesis of many multicellular tubes. In the embryonic Drosophila trachea, Shibire (dynamin), Clathrin, and Rab-5 are required for liquid clearance of dorsal trunk tubes [16], and likewise, endocytosis plays a role in liquid clearance of lung airway epithelia [17]. Tube length is also regulated by endocytosis: mutations in Drosophila clathrin heavy chain and wurst result in dorsal trunk elongation [18]. Mutations in Rab9 and the retromer complex, which mediate retrograde trafficking from the late endosome, likewise cause dorsal trunk elongation [19]. Additionally, regulation of tube diameter is linked to endocytosis, with mutations in shibire and Rab5 resulting in increased diameter of salivary gland tubes [20]. In these cases, increases in tube length or diameter are thought to be due to expansion of apical membrane as a consequence of disorganized apical extracellular matrix, or of failed endocytosis of junctional proteins. Here we provide the first genetic evidence of endocytosis shaping the lumen of seamless tubes, although pinocytosis has long been implicated in formation of seamless tubes in endothelial cells and in the excretory canal of C. elegans [21, 22]. In contrast to the defects in multicellular tubes with impaired endocytosis, in Syx7 terminal cells we see multiple distinct dilations rather than a uniform increase in tube length or isotropic diametric dilation (although we note that the most severely compromised cells – eg shibire null and select Rab5 mutant cells – show an overall increase in tube diameter in addition to cystic dilations). Since seamless tubes are not bounded by adherens junctions, improper turnover of junctional components should not contribute to localized apical membrane expansion.
Our data point to a critical role of endocytosis in regulation of cortical actin apposed to the apical membrane of seamless tubes. We propose this role of endocytosis is mediated by regulation of the steady-state level of Crb, a key apical polarity protein. Indeed, in addition to controlling turnover of junctional components, and the bulk flow of membrane between different compartments, endocytosis has been shown to regulate levels of transmembrane growth factor receptors and polarity pathway components. Previous studies from the Bilder lab, which established the role of Drosophila Syx7, Rab5, Rabenosyn-5, and Vps45 in early endosome formation, identified a requirement for the pathway in regulating Notch and Crb in wing disc and follicle cell epithelia [6, 7]. Similarly, studies examining Dynamin2 or Epsin depletion in mouse endothelial cells showed endocytosis regulates β-Integrin and Vegfr2 levels [23, 24]. Consistent with these results, we find that perturbation of early endocytosis resulted in elevated levels of Crb in tracheal terminal cells. While Lu and Bilder were unable to characterize Syx7avl, crb double mutant disk epithelia to definitively test whether the Syx7 defects were due to Crb elevation, we find that loss of crb strongly suppressed the tube dilation defect but not the branching defect of Syx7. Thus, Syx7-induced cysts depend upon elevated Crb, but regulation of cellular branching may be more complex. In contrast to the wing disc and follicular epithelia, which lose apical-basal polarity and become neoplastic [6], tracheal terminal cells maintain a restricted, if enlarged, apical domain. This raises the question of whether the tube-dilation defect associated with elevated Crb depends upon the function of Crb in apical polarity per se, or if it might reflect another role of Crb.
Our data suggest that Crb acts on seamless tube shape through regulation of apical p-Moe: when endocytosis is abrogated, Crb accumulates to high levels on the apical membrane, where it recruits or stabilizes p-Moe. In the absence of Crb, p-Moe enrichment in Syx7brd mutants is lost, and seamless tube cysts are not induced. Moe is the sole ERM-family protein in Drosophila; it organizes complex membrane domains through its ability to interact with and link phospholipids, membrane associated proteins, and the cytoskeleton [25]. Upon activation of Moe through phosphorylation of a conserved Threonine residue, the F-actin binding site is exposed; when levels of active Moe are increased, cortical rigidity is enhanced and the actin cytoskeleton is stabilized [14, 15, 26]. Medina et al demonstrated the existence of a Moe-interaction domain in the cytoplasmic tail of Crb (as predicted by Klebes and Knust [27]), and showed that the intracellular domain of Crb was sufficient to recruit or stabilize p-Moe in S2 cells and embryonic epithelia [8]. This relationship between Crb and ERM family proteins has been independently validated, for example, during invagination of Drosophila tracheal placodes, and appears to be evolutionarily conserved, as it has also been described in mouse intestinal epithelia and zebrafish neuroepithelia [28-30]. Our data suggests a novel, junction- and polarity-independent role of Crb in seamless tube morphogenesis, through the recruitment or stabilization of p-Moe.
Why does elevated p-Moe induce cystic dilation of seamless tubes? One possibility is that p-Moe may act to target delivery of apical membrane vesicles to the lumenal membrane. Indeed, such a role for Moe was recently proposed, in which Moe-targeting to apical membrane by the synaptotagmin-like protein, Bitesize, was suggested to be essential for seamless tube formation [9].
In the seamless tubes of the C. elegans excretory canal cell, ERM-1 (the nematode Moe ortholog) is required for lumenization [31], and the same may be true in tracheal terminal cells [9]. In the excretory canal, ERM-1 promotes actin coating of vesicles prior to their addition to the lumenal membrane, and, under conditions of osmotic stress, promotes tube elongation via recruitment of canalicular vesicles decorated with Aquaporin-8 (AQP-8) [31, 32]. Fluid flux through the water channel was proposed to expand seamless tube [31] in a manner analogous to hydrostatic pressure in endothelial tube lumenization [2, 33]. During naturally occurring periods of osmotic shock, AQP-8 becomes enriched in varicosities where it is thought to promote lumenal membrane addition via interaction with ERM-1/Moe [31, 32]; it is tempting to speculate that in tracheal terminal cells, the observed small patches of Crb on the lumenal membrane may serve as similar organizing sites for membrane addition through the recruitment of p-Moe. Although we cannot exclude a role for aquaporin proteins during this process in tracheal terminal cells, knockdown of individual aquaporin family members by RNAi have failed to produce terminal cell defects (ASG unpublished data).
How p-Moe modulation of the actin cytoskeleton impacts seamless tubulogenesis remains to be resolved. One possibility is that stabilized actin filaments promote enhanced exocytosis and apical membrane hypertrophy; if patches of apical Crb transiently enrich p-Moe locally, this may account for the focal nature of the cysts. This model would mesh nicely with a proposed role of Moe in apical vesicle targeting to the lumenal membrane [9]. If trafficking is altered by elevated p-Moe, lumenal matrix deposition may also be abnormal, which could account for the region of GFP exclusion present in the Syx7 seamless tube dilations that lack gas-filling; however, we were unable to detect defects in the chitinous apical extracellular matrix in Syx7 homozygous embryos, nor were we able to observe apical matrix irregularities with a tagged-TwdlD protein, or by UV illumination, in larval terminal cell cysts [J. S-R and ASG, data not shown).
Another possibility is that a stiffened actin cortex may hinder generation of new cellular extensions, and that in the absence of such outgrowths, budding tubes arrest with a cyst like morphology. Alternatively, increased cortical tension may impose physical constraints on apical membrane growth, arresting its outgrowth. Interestingly, we have found that under certain mutant conditions cellular extensions lacking lumens collapse back into the soma [34], potentially explaining the branching defect of the mutant terminal cells.
Supplementary Material
Highlights.
* disruption of early endosomes perturbs seamless tube shape
* disruption of early endosomes decreases terminal cell ramification
* Crumbs and Phospho-Moesin levels are negatively regulated by endocytosis
* Phospho-Moesin constrains seamless tube shape and terminal cell ramification
Acknowledgements
We would like to thank Steve DiNardo, Meera Sundarum, and the Ghabrial and DiNardo labs for discussions and comments on the manuscript. JS-R was supported by The American Heart Association Postdoctoral award 12POST12050009, and an NIH training grant 5-T32-HD007516-12 and postdoctoral fellowship (NRSA GM090438). ASG was supported by the University of Pennsylvania and the NIH (1R01GM089782). This work was supported in part by Basil O’Connor Starter Scholar Research Award Grant No.5-FY09-43 from the March of Dimes Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bar T, Guldner FH, Wolff JR. “Seamless” endothelial cells of blood capillaries. Cell Tissue Res. 1984;235:99–106. doi: 10.1007/BF00213729. [DOI] [PubMed] [Google Scholar]
- 2.Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, Affolter M. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 4.Ghabrial AS, Levi BP, Krasnow MA. A systematic screen for tube morphogenesis and branching genes in the Drosophila tracheal system. PLoS Genet. 2011;7:e1002087. doi: 10.1371/journal.pgen.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schottenfeld-Roames J, Ghabrial AS. Whacked and Rab35 polarize dynein-motor-complex-dependent seamless tube growth. Nat Cell Biol. 2012;14:386–393. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 7.Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;19:4167–4176. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol. 2002;158:941–951. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JayaNandanan N, Mathew R, Leptin M. Guidance of subcellular tubulogenesis by actin under the control of a synaptotagmin-like protein and Moesin. Nat Commun. 2014;5:3036. doi: 10.1038/ncomms4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, Hayashi S. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16:1531–1537. doi: 10.1016/j.cub.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Laprise P, Paul SM, Boulanger J, Robbins RM, Beitel GJ, Tepass U. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr Biol. 2010;20:55–61. doi: 10.1016/j.cub.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Wu Z, Shi X, Li W, Liu C, Wang D, Ye X, Liu L, Na J, Cheng H, et al. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates Ezrin during eight-cell embryocompaction. Dev Biol. 2013;375:13–22. doi: 10.1016/j.ydbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Cheshire AM, Kerman BE, Zipfel WR, Spector AA, Andrew DJ. Kinetic and mechanical analysis of live tube morphogenesis. Dev Dyn. 2008;237:2874–2888. doi: 10.1002/dvdy.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13:214–225. doi: 10.1016/j.devcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells. Channels (Austin) 2010;4:150–154. doi: 10.4161/chan.4.3.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behr M, Wingen C, Wolf C, Schuh R, Hoch M. Wurst is essential for airway clearance and respiratory-tube size control. Nat Cell Biol. 2007;9:847–853. doi: 10.1038/ncb1611. [DOI] [PubMed] [Google Scholar]
- 19.Dong B, Kakihara K, Otani T, Wada H, Hayashi S. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat Commun. 2013;4:1358. doi: 10.1038/ncomms2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirraglia C, Walters J, Myat MM. Pak1 control of E-cadherin endocytosis regulates salivary gland lumen size and shape. Development. 2010;137:4177–4189. doi: 10.1242/dev.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K. 1980;100:346–353. [PubMed] [Google Scholar]
- 23.Lee MY, Skoura A, Park EJ, Landskroner-Eiger S, Jozsef L, Luciano AK, Murata T, Pasula S, Dong Y, Bouaouina M, et al. Dynamin 2 regulation of integrin endocytosis, but not VEGF signaling, is crucial for developmental angiogenesis. Development. 2014;141:1465–1472. doi: 10.1242/dev.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, et al. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. J Clin Invest. 2012;122:4424–4438. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klebes A, Knust E. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr Biol. 2000;10:76–85. doi: 10.1016/s0960-9822(99)00277-8. [DOI] [PubMed] [Google Scholar]
- 28.Letizia A, Sotillos S, Campuzano S, Llimargas M. Regulated Crb accumulation controls apical constriction and invagination in Drosophila tracheal cells. J Cell Sci. 2010;124:240–251. doi: 10.1242/jcs.073601. [DOI] [PubMed] [Google Scholar]
- 29.Whiteman EL, Fan S, Harder JL, Walton KD, Liu CJ, Soofi A, Fogg VC, Hershenson MB, Dressler GR, Deutsch GH, et al. Crumbs3 is essential for proper epithelial development and viability. Mol Cell Biol. 2014;34:43–56. doi: 10.1128/MCB.00999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohata S, Aoki R, Kinoshita S, Yamaguchi M, Tsuruoka-Kinoshita S, Tanaka H, Wada H, Watabe S, Tsuboi T, Masai I, et al. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 69:215–230. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DH, Gobel V. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Biol. 2013;15:143–156. doi: 10.1038/ncb2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat Cell Biol. 2013;15:157–168. doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- 33.Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, Belting HG, Affolter M. In Vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell. 2013;25:492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Levi BP, Ghabrial AS, Krasnow MA. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development. 2006;133:2383–2393. doi: 10.1242/dev.02404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.