Abstract
Cognitive decline is a frequent complaint during the menopause transition and among post-menopausal women. Changes in memory correspond with diminished estrogen production. Further, many peri- and post-menopausal women report sleep concerns, depression, and hot flashes, and these factors may contribute to cognitive decline. Hormone therapy can increase estrogen but is contraindicated for many women. Mind–body medicine has been shown to have beneficial effects on sleep, mood, and hot flashes, among post-menopausal women. Further, mind–body medicine holds potential in addressing symptoms of cognitive decline post-menopause. This study proposes an initial framework for how mind–body interventions may improve cognitive performance and inform future research seeking to identify the common and specific factors associated with mind–body medicine for addressing memory decline in peri- and post-menopausal women. It is our hope that this article will eventually lead to a more holistic and integrative approach to the treatment of cognitive deficits in peri- and post-menopausal women.
Keywords: hormone therapy, estrogen, cognition, memory, mind–body medicine
The menopausal transition is associated with numerous problematic symptoms including weight gain,1 anxiety,2 depression,3 sexual dysfunction,4 vasomotor irregularities,5 and sleep disturbance.6 Some researchers have suggested that declines in estrogen level may lead to deficits in the cognitive ability of post-menopausal women.7 However, additional factors are likely to include stress, anxiety, depression, sleep concerns, and hot flashes that occur during the menopause transition.8 Hormone therapy (HT) can increase estrogen levels, but has been associated with an increased risk of breast cancer and cardiovascular disease for some women.9 Therefore, alternatives are needed to alleviate the patterns of cognitive decline that occur for many women post-menopause. This article offers a review of the currently available research linking cognitive performance to the menopause transition and provides an initial conceptual framework for how mind–body interventions may improve cognitive performance. In addition, it is hoped that the framework may inform future research seeking to identify the common and specific factors associated with mind–body medicine to alleviate memory decline in post-menopausal women.
Menopause and Cognitive Decline
Results from multiple large-scale survey studies indicate that peri- and post-menopausal women report a significantly greater number of memory problems than do premenopausal women. For example, Mitchell and Woods10 reported that 62% of the women in the Seattle Midlife Women’s Health Study (SMWHS, n = 230, mean age = 46.7 years) reported noticeable declines in cognitive performance following menopause. The most common complaints were difficulty recalling words or numbers, needing memory aids, and forgetting why one was involved in a certain behavior.
Similarly, in a survey study designed to assess attitudes toward menopause in a sample of 88 post-menopausal Italian women,11 results indicated that 70% of participants reported deficits in memory. In an additional study that utilized a sample of 151 female faculty members between the ages of 30 and 60 years who worked for a large New York school district,12 results indicated that peri- and post-menopausal women were over three times more likely to report memory complaints than were premenopausal women. Finally, in a study conducted by Schaafsma et al.,13 cognitive performance and memory complaints were assessed in a sample of 120 Australian women between the ages of 45 and 60 years. Eighty-two percent of the women in the sample reported memory complaints, and peri- and post-menopausal women were significantly more likely to report memory complaints than were premenopausal women. Multivariate analysis indicated that subjective memory complaints were significantly associated with impairments in attention, reaction time, and verbal memory. When examined collectively, these results suggest that declines in cognitive ability result from changes occurring during menopause.
Estrogen and Cognitive Performance
Although the survey studies described above indicate that peri- and post-menopausal women express dissatisfaction with their cognitive abilities more frequently than do premenopausal women, survey studies are not designed to determine whether impairments in cognitive performance are due solely to declines in circulating estrogen, or whether other factors such as diminished sleep quality, increased depression, and the onset of hot flashes also contribute. However, multiple clinical trials, during which the circulating estrogen of post-menopausal women was returned to premenopausal levels through HT, have been conducted. Results have indicated that HT may not prove beneficial for all women, and that the age during which women begin HT may be of critical importance.
For example, in the largest clinical trial conducted thus far, the Women’s Health Initiative Memory Study (WHIMS),14 6,998 post-menopausal women between the ages of 65 and 79 years (mean = 76) were randomized to a treatment group that received 0.625 mg of conjugated equine estrogens (CEE) daily for up to five years, or to a control group that received a placebo. The results of this study have been published in several journals; however, in a synopsis of study outcomes, it was reported that women who received HT were actually 1.6 times more likely than control group participants to be at risk for dementia.14
In addition to the WHIMS data, Henderson and Sherwin15 have conducted an excellent systematic literature review during which they examined the evidence for the treatment utility of HT from 20 well-designed research trials. Inclusion criteria for the studies included treatment duration of at least one month, a placebo control condition, and an objective measure of verbal and nonverbal episodic memory. The review concluded that HT was not effective at enhancing cognitive performance in nine of these studies, all of which included samples consisting mainly of older women (mean age range = 64–81). These findings were replicated in nine studies, whose samples comprised midlife women (mean age range 51–58). Therefore, it is likely that factors other than estrogen decline also impact the cognitive abilities of peri- and postmenopausal women.
Sleep Impairment and Cognitive Decline
Although memory complaints are known to exist among peri- and post-menopausal women,16 sleep loss, mood disorders, and hot flashes represent more common symptomatology. Although these symptoms are difficult and uncomfortable in their own right, we suggest that these symptoms are also problematic, because they can lead to more severe cognitive impairment.
Multiple studies indicate that women report significantly diminished sleep quality following menopause.17–19 Common symptoms include difficulty falling asleep, frequent awakenings, and less restorative sleep.20 These problems tend to get progressively worse as time goes by21 and may eventually result in insomnia.22 In fact, estimates suggest that while the prevalence of insomnia in the general population is somewhere between 9% and 15%,23 as many as 63% of post-menopausal women may suffer from insomnia.24 Furthermore, Hsu et al.25 have suggested that attempts to relieve insomnia tend to be so unsuccessful that many post-menopausal women come to accept the disorder. Apart from declines in estrogen levels, key factors influencing sleep quality in post-menopausal women include hot flashes, stress, weight gain, mood disorders, pain, circadian rhythm abnormalities, and the development of primary sleep disorders such as sleep-disordered breathing.19,21
Associations between poor sleep quality and cognitive decline have been well documented elsewhere.26 However, we provide two representative examples, which specifically examine the nature of cognitive decline in post-menopausal women stemming from poor sleep quality. In the first of these studies,27 1,844 elderly women aged between 70 and 81 years were asked to complete annual evaluations of cognitive performance as well as a self-report measure of sleep quality and duration. Results indicated that women who slept for five or fewer hours each night performed significantly worse on tests of verbal memory, attention, and general cognition than did women who received at least seven hours of sleep per night. Furthermore, participants who reported having difficulty falling asleep and staying asleep performed significantly worse on these cognitive tasks than did women who reported having few or no sleep problems.
An additional study examined whether cognitive deficits that are commonly linked to genetic factors could be better explained by deficits in sleep quality.28 During this study, 698 elderly participants, the majority of whom were female (77%), who either were or were not carriers of the apolipoprotein E ε4 allele underwent annual evaluations for Alzheimer’s disease (AD) for a period of up to six years.28 In addition to an AD screening, participant had their sleep quality and quantity measured for up to 10 consecutive days each year via wrist actigraphy. Participants also agreed to have an autopsy performed in the event of their death.
Results indicated that carriers of the ε4 allele were significantly more likely than non-carriers to develop AD over the course of the study.28 Furthermore, annual rates of cognitive decline were also significantly worse for allele carriers. However, most importantly, a series of linear mixed effects models indicated that the effects of the ε4 allele were attenuated by participants’ sleep quality. Additionally, when autopsies were performed on the 201 participants who passed away during the study, results indicated that the effect of the ε4 allele on the density of neurofibrillary tangles was also attenuated by sleep quality. These results demonstrate the severity of the effects that poor sleep quality can have on cognition, and highlight the importance of accounting for sleep quality when attempting to establish a treatment program aimed at preserving cognitive function in post-menopausal women.
Depression and Cognitive Impairment
Another problematic symptom that likely hinders both peri- and post-menopausal women’s ability to store and process information effectively is an increase in depressive symptomatology. According to the National Comorbidity Survey,29 women aged between 45 and 55 years are more likely to meet diagnostic criteria for clinical depression than are women belonging to any other age group. Furthermore, while the prevalence rate for depression is around 7% for the general population,30 studies have indicated that prevalence rates range from 12% to 36% percent among peri- and post-menopausal women.31–33 Additionally, Hay et al.34 reported that prevalence rates may be as high as 45% for women receiving outpatient menopausal services. Finally, when 436 women were interviewed for the Penn Ovarian Aging Study (POAS),35 results indicated that even though they may not meet criteria for clinical depression, women are still three times more likely to report depressive symptomatology during the menopausal transition than they were previously.
Although multiple factors, such as decreased motivation for sustained attention and the inability to find enjoyment in learning are likely to explain how depression hinders immediate cognitive performance, it has also been suggested that depression may lead to sustained cognitive decline. For example, Goveas et al.36 suggested that increased cortisol availability during depressive episodes may lead to atrophy of the hippocampus, a brain structure critical for sustaining episodic memories.
Although research on the relationship between depression and cognitive impairment in peri- and post-menopausal women represents a growing area of investigation, empirical evidence does support a link between the two variables. For example, in a subsample of 1,903 post-menopausal women who participated in the Study of Women’s Health Across the Nation (SWAN), results indicated that participant depression levels as assessed by the Center for Epidemiological Studies Depression Scale (CES-D)37 were significantly correlated with deficits in processing speed, verbal memory, and working memory.38 Furthermore, the correlation between processing speed and depression remained significant after controlling various demographic variables, such as age, ethnicity, and education level. An additional study involving 6,376 women enrolled in the WHIMS found that participants who met diagnostic criteria for depression at baseline were nearly two times more likely to be diagnosed with either mild cognitive impairment or probable dementia within the next six years.36 While these are only preliminary findings, the vast number of publications that have found links between depression and cognitive performance in other populations39–41 suggest that the potential relationship between depression and cognitive performance in post-menopausal women is worth addressing.
Hot Flashes and Cognitive Impairment
Treatment programs designed to improve cognitive performance in peri- and post-menopausal women should also attempt to diminish the frequency and severity of hot flashes. Hot flashes are the most common symptom reported by peri- and post-menopausal women.16 Although as many as 75% of women may express concerns over the ways in which hot flashes negatively impact their quality of life, research comparing the subjective reporting of hot flashes to the objective experience indicates that women actually underreport the frequency with which hot flashes occur.42 This problem is particularly characteristic of patients being treated for breast cancer, who report less than half of the hot flashes they experience.43
Research directly addressing the relationship between hot flash occurrence and cognitive performance is scant. However, results from a study, which utilized a sample of 29 peri- and post-menopausal women (mean age = 53 years) indicated that there was a significant negative correlation between the total number of objective hot flashes experienced by participants and their immediate and delayed paragraph recall performance.42 In a related study involving 68 midlife women (mean age = 53 years), results indicated that hot flash frequency was significantly related to performance on a test of episodic memory.44
Several theories have been put forth to explain why hot flashes may lead to declines in cognitive performance. For example, Maki and colleagues42 have suggested that frequent hot flash occurrence may cause a change in the rate of blood flow through the temporal lobe, thus causing a disruption in one’s ability to process logical units of information. It is also possible that an increase in cortisol release following hot flashes eventually results in significant damage to important brain structures such as the hippocampus.45,46 Disrupted slow wave sleep resulting from frequent awakenings after hot flashes occurring during the early portions of the evening may also interfere with memory consolidation.42,47,48 Finally, it is also likely that the diminished quality of life experienced by women suffering from frequent and severe hot flashes causes an increase in depression, thus indirectly impacting cognitive performance.49
Collectively, this section has examined factors other than decreased cortisol availability that may lead to a decline in the cognitive performance levels of peri- and post- menopausal women. Specifically, we suggest that sleep disruption, depression, and hot flashes are key symptoms that can both directly and indirectly lead to cognitive impairment. A model addressing how these symptoms effect cognitive impairment is shown in Figure 1. While other factors such as anxiety and poor self-image may also play a role, significant improvement in these areas is likely to be obtained if our key symptoms are successfully addressed. Therefore, we have chosen to focus on these symptoms because of their relative severity and also the frequency with which they are reported by peri- and post-menopausal women.
Figure 1.
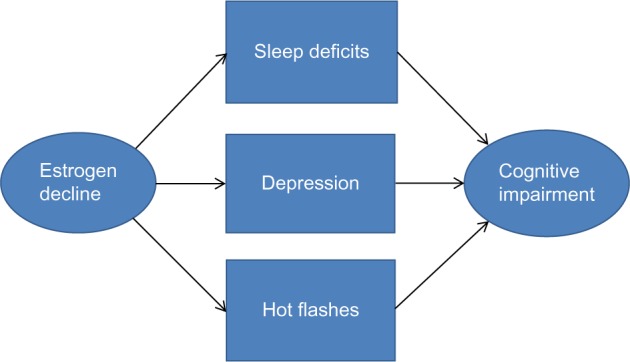
Factors influencing cognitive impairment in peri- and postmenopausal women.
Although HT can boost estrogen levels, restoring estrogen to premenopausal levels is unlikely to result in the complete alleviation of these symptoms, as other factors, such as weight gain, stress, and normal aging also impact sleep, depression, and, to a lesser extent, hot flash frequency and severity. Furthermore, HT may be contraindicated for some women. Therefore, alternative therapies, which can be used to alleviate the severity of multiple symptoms are needed. Several potential candidates falling under the larger category of mind–body medicine have proven effective for improving sleep quality, depression, and hot flash occurrence and severity. Their potential for improving the cognitive performance of peri- and post-menopausal women is detailed in the next section.
Enhancing Cognitive Performance Through Mind–Body Medicine
Although HT is often recommended to peri- and post- menopausal women who report memory complaints, attempts to provide empirical support for this practice suggest that HT alone may not always be adequate for preventing declines in cognitive performance and may actually be detrimental for some women.9 Therefore, alternative treatment options are needed. One potentially fruitful area is mind–body medicine. Mind–body medicine involves a variety of different treatment options that account for the ways in which mental processes impact health. Different mind–body therapies include meditation, mindfulness, hypnosis, and yoga, among others. What these techniques have in common is that they address the person as a whole and seek to improve health without introducing an exogenous substance into the body.
Peri- and post-menopausal women may find mind–body medicine attractive for many reasons. First of all, because mind–body therapies do not rely on medication, they have no troubling side-effects. Furthermore, patients do not need to be concerned with any potentially dangerous drug interactions. Therefore, mind–body therapies are appropriate for women for whom hormone replacement therapy is not an option, and for those who, although they may currently be on an HT regimen, are seeking greater relief from their symptoms. Furthermore, because mind–body therapies are aimed at treating the person as a whole, they are often effective at treating more than one symptom cluster.50–52 Finally, once learned, patients are able to utilize mind–body therapies whenever they feel the need for extra treatment, thus increasing self-reliance.
Past research has provided initial support for the efficacy of mind–body therapies for improving post-menopausal women’s distress resulting from sleep disturbance, depression, and hot flashes. Emerging evidence suggests that mind–body therapies may be effective at directly targeting memory complaints in peri- and post-menopausal women as well.
For example, in a small, uncontrolled pilot study, 14 menopausal women (mean age = 55 years) were asked to complete two, 90 minute, weekly mediation sessions over an eight week period.53 Mediation sessions included guided imagery, self-affirmation, and silent reflection. Results indicated that the frequency of self-reported hot flashes was reduced by 67% from baseline to post-treatment. Depression scores were also reduced by nearly 70% across the eight week study. Despite the small sample size, both of these decreases represent statistically significant reductions in problematic symptoms. Unfortunately, participant sleep quality and quantity was not assessed.
In a more recent study, 110 women (mean age = 53 years), who were either in the late stages of perimenopause or in the early stages of post-menopause, were randomized to either an eight week mindfulness-based stress reduction (MBSR) intervention (n = 57) or a wait list control group (n = 53).54 Participants assigned to the MBSR condition were asked to attend weekly, 2.5 hour, mindfulness sessions that included supine body scans, seated meditation, mindful stretching, and education. During days on which participants did not attend formal sessions, they were asked to complete 45 minutes of compact disk (CD)-guided mindfulness training. Finally, an all-day retreat was held during the sixth week of the intervention.
The main outcome of this study was the degree to which participants reported being bothered by their hot flashes over the past 24 hours. Bother was measured on a four-point scale, with a score of 1 indicating that the participant was not at all bothered by hot flashes, and a score of 4 indicating that the participant was extremely bothered. Results indicated that the degree of bother decreased by 14.77% from baseline to endpoint for participants assigned to the MSBR condition and continued to decrease to an overall improvement of 21.62% at 20 weeks follow-up.54 Conversely, control participants only reported a 6.79% improvement from baseline to endpoint, and a total improvement of 10.50% at follow-up. Additional analysis of secondary outcomes indicated that MBSR participants also reported a greater degree of improvement in the intensity of their hot flashes over the course of the study and at follow-up than did control participants (32.25% and 44.56% vs. 20.69% and 26.97%, respectively). Significant between-group differences were also seen in degree of improvement in participants’ overall quality of life, as measured by the Menopause-Related Quality of Life Scale,55 and sleep quality, as measured by the Women’s Health Initiative Insomnia Rating Scale.56
Hypnosis has also shown promise for treating menopausal symptoms that may be linked to cognitive impairment. In a large randomized controlled trial, 187 post-menopausal women (mean age = 54.6 years) were randomized to either a five week clinical hypnosis intervention or a structured attention control group.50 Participants receiving hypnosis (n = 93) attended weekly 45 minute sessions that included a hypnotic induction and suggestions for coolness and relaxation. They were also taught self-hypnosis and were asked to practice daily. Meanwhile, participants receiving structured attention (n = 94) also attended weekly sessions with a therapist. Sessions followed a standardized manual and included monitoring, attentive listening, and discussion of symptoms.
Results indicated that the number of hot flashes experienced by participants assigned to the hypnosis intervention decreased by over 63% from baseline to endpoint, and continued to decrease by an additional 10% at 12 weeks follow-up.50 Meanwhile, hot flash frequency was only reduced by 17% for participants who had received structured attention. Analysis of secondary outcomes also indicated that participants who had received the hypnosis intervention reported a significantly greater reduction in the extent to which hot flashes interfered with their daily lives (69%) than did control participants (18%), as measured by the Hot Flash Related Daily Interference Scale (HFRDIS).57 Hypnosis participants also reported a significantly greater improvement in their overall sleep quality as measured by the PSQI (53.63%) than did participants assigned to the control condition (10.34%).
Finally, yoga is the most frequently utilized mind–body intervention for the treatment of menopausal symptoms.58 However, a relatively recent systematic review and meta-analysis of the literature was only able to identify five well-designed randomized controlled trials that met Cochrane criteria for lack of bias.59 The results of the meta-analysis revealed that yoga leads to small, but significant reductions in the number of psychological and vasomotor symptoms experienced by peri- and post-menopausal women. Therefore, yoga may hold promise for treating sleep, depression, and hot flashes, thus leading to improved cognitive function.
Additionally, in the only study, which to our knowledge, has attempted to directly assess the utility of a mind–body intervention for improving the cognitive performance of women during the menopausal transition,60 108 perimenopausal women were randomly assigned to an eight week integrated yoga intervention (n = 54) or an exercise control group (n = 54). Memory performance was assessed before and after treatment with the Punit Govil Intelligence Memory Scale (PGIMS).61 The PGIMS is composed of 10 different subscales, which measure various cognitive abilities, such as long-term and short-term memory, attention, and concentration. Results indicated that while participants assigned to the control condition experienced significant improvements on 6 of the 10 PGIMS subscales, participants assigned to the yoga condition scored significantly better following treatment on 8 of the 10 subscales.60 Furthermore, participants assigned to the yoga group performed significantly better than control participants on 7 of the 10 PGIMS subscales following treatment.
To summarize, the currently available research suggests that mind–body interventions may be effective for treating menopausal symptoms and reducing cognitive impairment. We put forth a potential framework for addressing the problem of cognitive impairment in peri- and post-menopausal women that many researchers may not have considered previously. Our hope is that consideration of this framework will lead to new research, which may hold promise for the eventual utilization of a holistic approach to helping women cope with concerns about their cognitive functioning during menopause. Numerous studies involving larger samples and greater control will need to be conducted before an assessment of the utility of this framework can be made.
Conclusions
This paper has provided a conceptual framework to bridge the gap between the frequency of memory complaints in post-menopausal women and the development of a more holistic treatment approach. We have provided estimates of the relative frequency with which peri- and post-menopausal women report memory problems. It is likely that cognitive deficits are not solely caused by declines in estrogen production, but are also likely to stem from reductions in sleep quality, increased depression levels, and the onset of hot flashes. We suggest that mind–body interventions may hold promise for treating cognitive declines associated with the menopausal transition, and we have discussed how these therapies may be used alone or in combination with HT depending on the preferences of the patient and her medical provider. Further investigation is warranted to determine whether or not mind–body therapies have real efficacy for improving cognitive abilities in this population.
Acknowledgments
The authors acknowledge Vicki Patterson for her assistance in obtaining articles for this manuscript.
Footnotes
Author Contributions
JS wrote the first draft of the manuscript. JS, AJ, and GE contributed to the writing of the manuscript. JS, AJ, and GE agree with manuscript results and conclusions. JS, AJ, and GE made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Christopher Chang, Editor in Chief
FUNDING: GE was supported by grant # U01AT004634 from the National Center for Complementary and Alternative Medicine of the National Institutes of Health.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Schroeder J. Weight gain during menopause. Am Fitness. 2012;30:58–9. [Google Scholar]
- 2.Bryant C, Judd FK, Hickey M. Anxiety during the menopausal transition: a systematic review. J Affect Disord. 2012;139:141–8. doi: 10.1016/j.jad.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 3.Freeman E. Associations of depression with the transition to menopause. Menopause. 2010;17:823–7. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 4.Schnatz P, Whitehurst S, O’Sullivan D. Sexual dysfunction, depression, and anxiety among patients of an inner-city menopause clinic. J Women Health (Larchmt) 2010;19:1843–9. doi: 10.1089/jwh.2009.1800. [DOI] [PubMed] [Google Scholar]
- 5.Thurston R, Joffe H. Vasomotor symptoms and menopause: findings from the study of women’s health across the nation. Obstet Gynecol Clin North Am. 2011;38:489–501. doi: 10.1016/j.ogc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Thurston RC, Matthews KA, et al. Are hot flashes associated with sleep disturbance during midlife? Results from the STRIDE cohort study. Maturitas. 2012;71:34–8. doi: 10.1016/j.maturitas.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 8.Goveas JS, Hogan PE, Kotchen JM, et al. Depressive symptoms, antidepressant use, and future cognitive health in postmenopausal women: the Women’s Health Initiative Memory Study. Int Psychogeriatr. 2012;24:1252–64. doi: 10.1017/S1041610211002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principle results from the Women’s Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell SE, Woods FN. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Women Health Gend Based Med. 2001;10:351–62. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- 11.Betti S, Orsini MR, Sciaky R, Cristini C, Cesa-Bianchi G, Zandonini GF. Attitudes towards menopause in a group of women followed in a public service for menopause counseling. Aging. 2001;13:331–8. doi: 10.1007/BF03353429. [DOI] [PubMed] [Google Scholar]
- 12.Devi G, Hahn K, Massimi S, Zhivotovskaya E. Prevalence of memory loss complaints and other symptoms associated with the menopause transition: a community survey. Gend Med. 2005;2:255–64. doi: 10.1016/s1550-8579(05)80055-5. [DOI] [PubMed] [Google Scholar]
- 13.Schaafsma M, Homewood J, Taylor A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric. 2010;13:84–98. doi: 10.3109/13697130903009187. [DOI] [PubMed] [Google Scholar]
- 14.Coker LH, Espeland MA, Rapp SR. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118:304–10. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–9. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 16.Asadi M, Jouyandeh Z, Nayebzadeh F. Prevalence of menopause symptoms among Iranian women. J Fam Plan Reprod Health. 2012;6:1–3. [Google Scholar]
- 17.Ashrafi M, Kazemi Ashtiani S, Shabani F, et al. Symptoms of natural menopause among Iranian women living in Tehran, Iran. Iran J Reprod Med. 2010;8:29–32. [Google Scholar]
- 18.Danby FW. Management of menopause-related symptoms. Ann Intern Med. 2005;143:845–6. doi: 10.7326/0003-4819-143-11-200512060-00021. [DOI] [PubMed] [Google Scholar]
- 19.Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68:224–32. doi: 10.1016/j.maturitas.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 21.Hachul H, Bittencourt L, Soares J, Tufik S, Baracat E. Sleep in post-menopausal women: differences between early and late post-menopause. Eur J Obstet Gynecol Reprod Biol. 2009;145:81–4. doi: 10.1016/j.ejogrb.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Shin C, Lee S, Lee T, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59:395–402. doi: 10.1111/j.1440-1819.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- 23.Phillips B, Collop N, Drake C, Consens F, Vgontzas A, Weaver T. Sleep disorders and medical conditions in women; Proceedings of the women & sleep workshop, national sleep foundation; Washington, DC. March 5–6, 2007; J Womens Health (Larchmt). 2008;17:1191–9. [DOI] [PubMed] [Google Scholar]
- 24.Kuh DL, Wadworth M, Hardy R. Women’s health in midlife: the influence of menopause social factors and health in earlier life. Br J Obstet Gynaecol. 1997;104:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu H, Chen N, Jou H, An C, Tsao L. Sleep disturbance experiences among perimenopausal women in Taiwan. J Clin Nurs. 2009;18:2116–24. doi: 10.1111/j.1365-2702.2008.02665.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Liu J, Xu J. Progress of effects of sleep deprivation on cognitive function. Prog Mod Biomed. 2013;13:791–4. [Google Scholar]
- 27.Tworoger S, Lee S, Schernhammer E, Grodstein F. Association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alz Dis Assoc Dis. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 28.Lim A, Yu L, Kowgier M, Schneider J, Buchman A, Bennett D. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer Disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–51. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler R, McGonagle K, Swartz M, Blazer D, Nelson C. Sex and depression in the National Comorbidity Survey I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993:2–3. 85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 31.Lee SK, Kuo BJ. Relationships of climacteric attitude and health behavior among middle-aged women and nurse. Chung Shan Med J. 2002;13:101–20. [Google Scholar]
- 32.Lee P, Lee J, Huang C, Lee C. The relationship between menopause attitude, climacteric symptoms and depression in women undergoing menopause. Evid Based Nurs. 2006;2:156–65. [Google Scholar]
- 33.Pan H, Wu M, Hsu C, Yao B, Huang K. The perception of menopause among women in Taiwan. Maturitas. 2002;41:269–74. doi: 10.1016/s0378-5122(01)00279-1. [DOI] [PubMed] [Google Scholar]
- 34.Hay A, Bancroft J, Johnstone E. Affective symptoms in women attending a menopause clinic. Br J Psychiatry. 1994;164:513–6. doi: 10.1192/bjp.164.4.513. [DOI] [PubMed] [Google Scholar]
- 35.Freeman E, Sammel M, Liu L, Gracia C, Nelson D, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 36.Goveas J, Espeland M, Woods N, Wassertheil-Smoller S, Kotchen J. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 2011;59:57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carleton RN, Thibodeau MA, Teale MJ, et al. The Center for Epidemiologic Studies Depression Scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS One. 2013;8:e58067. doi: 10.1371/journal.pone.0058067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171:1214–24. doi: 10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bangen KJ, Delano-Wood L, Wierenga CE, et al. Associations between stroke risk and cognition in normal aging and Alzheimer’s disease with and without depression. Int J Geriat Psychiatry. 2010;25:175–82. doi: 10.1002/gps.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franz CE, Lyons MJ, O’Brien R, et al. A 35-year longitudinal assessment of cognition and midlife depression symptoms: the Vietnam Era Twin Study of Aging. Am J Geriat Psychiatry. 2011;19:559–70. doi: 10.1097/JGP.0b013e3181ef79f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahodne L, Tremont G. Unique effects of apathy and depression signs on cognition and function in amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2013;28:50–6. doi: 10.1002/gps.3789. [DOI] [PubMed] [Google Scholar]
- 42.Maki P, Drogos L, Rubin L, Banuvar S, Shulman L, Geller S. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–56. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter J, Monahan P, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–6. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 44.Drogos L, Rubin L, Geller S, Banuvar S, Shulman L, Maki P. Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause. 2013;20:1236–42. doi: 10.1097/GME.0b013e318291f5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meldrum DR, Defazio JD, Erlik Y, et al. Pituitary hormones during the menopause hot flash. Obstet Gynecol. 1984;64:752–6. [PubMed] [Google Scholar]
- 46.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopause transition. Menopause. 2006;13:212–21. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 47.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 48.Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741–7. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 49.Lu S, Tseng H, Lin L, Luh W, Shu B. Factors related to depression during menopause: a study in southern Taiwan. J Nurs Res. 2009;17:128–35. doi: 10.1097/JNR.0b013e3181a53f82. [DOI] [PubMed] [Google Scholar]
- 50.Elkins G, Fisher W, Johnson A, Carpenter J, Keith T. Clinical hypnosis in the treatment of postmenopausal hot flashes: a randomized controlled trial. Menopause. 2013;20:291–8. doi: 10.1097/GME.0b013e31826ce3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalsa SB. Treatment of chronic insomnia with yoga: a preliminary study with sleep wake diaries. App Biophys Biofeed. 2004;29:269–78. doi: 10.1007/s10484-004-0387-0. [DOI] [PubMed] [Google Scholar]
- 52.Kim YH, Kim HJ, Ahn SD, Se YJ, Kim SH. Effects of meditation on anxiety, depression, fatigue, and quality of life of women undergoing radiation therapy for breast cancer. Complement Ther Med. 2013;21:379–87. doi: 10.1016/j.ctim.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Manocha R, Semmar B, Black D. A pilot study of a mental silence form of meditation for women in perimenopause. J Clin Psychol Med Settings. 2007;14:266–73. [Google Scholar]
- 54.Carmody J, Crawford S, Salmoirago-Blotcher E, Leung K, Churchill L, Olendzki N. Mindfulness training for coping with hot flashes: results of a randomized trial. Menopause. 2011;18:611–20. doi: 10.1097/gme.0b013e318204a05c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coronado P, Sánchez-Borrego R, Palacios S, Ruiz M, Rejas J. Structural validity of a 14-item abridged version of the Menopause Cervantes Health-Related-Quality-Of-Life Scale. Value Health. 2013;16:A338. doi: 10.1097/GME.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 56.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale (WHIIRS) Psychol Assess. 2003;15:123–36. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter JS. The hot flash related daily interference scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–89. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 58.Newton KM, Buist DSM, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100:18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- 59.Cramer H, Lauche R, Langhorst J, Dobos G. Effectiveness of yoga for menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:A863905. doi: 10.1155/2012/863905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chattha R, Nagarathna R, Padmalatha V, Nagendra HR. Effect of yoga on cognitive functions in climacteric syndrome: a randomised control study. BJOG. 2008;115:991–1000. doi: 10.1111/j.1471-0528.2008.01749.x. [DOI] [PubMed] [Google Scholar]
- 61.Pershad D, Wig N. PGI memory scale: a normative study on elderly subjects. Indian J Clin Psychol. 1977;4:6–8. [Google Scholar]


