To the editor
GATA2 encodes a transcription factor that regulates stem cell homeostasis. Mutations in GATA2 result in a diverse clinical phenotype that includes myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), decreased monocytes, B cells, and NK cells, opportunistic infections, and lymphedema.2 Previous studies have noted normal immunoglobulin levels and detectable bone marrow plasma cells in patients with GATA2 mutations.1,3–6 We report 2 patients with hypogammaglobulinemia and defective antibody responses associated with an autosomal dominant mutation in GATA2.
Patient 1 presented at 3 years of age with recurrent otitis media and sinusitis. He had a normal lymphocyte and monocyte counts, platelet number, and hematocrit. He had IgG2 and IgA deficiency, absent tetanus and low PRP (52 ng/mL, protective >1000 ng/ml) antibody titers. Tetanus and Hib vaccine boosters resulted in protective tetanus (0.4 IU/mL) and PRP (>1200 ng/mL) titers. Two years later, his tetanus titer was undetectable, his PRP decreased to 185 ng/mL, and his IgG level was decreased at 444 mg/dL (normal 600 – 1500 ng/mL), prompting IVIG initiation. At 10 years of age, IVIG was discontinued to reassess his antibody response. His tetanus titer increased from 0.14 to 0.27 IU/mL after a booster, but waned within one year to 0.03 IU/mL. At 14 years of age, his PRP titer increased significantly after the Hib vaccine, but he failed to respond to repeated vaccinations of tetanus, Prevnar, and Pneumovax; additionally, his IgG was low and IgA was undetectable (Table 1). He developed pneumonia and pan-sinusitis in the setting of absent B cells and CD4+ T cell lymphopenia (Table I); therefore, IVIG was restarted. He had absent tonsils, which had been barely visible on previous exams. No mutations were found in TACI, PNP, ADA, BTK, or SH2D1A. At 16 years of age, he developed persistent warts on his hands and severe bronchiectasis. Sputum cultures were positive for Mycobacterium kansasii. Lymphocyte proliferation to mitogens and antigens was normal, but the counts of NK cells, monocytes, and platelets were low (Table I). Progressive respiratory decline led to his death at 22 years of age.
Table I.
Immune profiles
| Patient 1 | Patient 2 | |||
|---|---|---|---|---|
| 14 years | 17 years | 48 years | 50 years | |
| Immunoglobulins (mg/dL)1 | ||||
| IgG (639 – 1344) | 260 | 886 | 560 | 1122 on IVIG |
| IgG1 (240 – 1118) | ND | ND | 329 | ND |
| IgG2 (124 – 549) | ND | ND | 82 | ND |
| IgG3 (21 – 134) | ND | ND | 64 | ND |
| IgG4 (7 – 89) | ND | ND | <1 | ND |
| IgA (70 – 312) | <7 | <5 | 104 | 89 |
| IgM (34 – 210) | 12 | <4 | 220 | 195 |
| Vaccine titers (normal range) | ||||
| Pneumococcal IgG (Positive: > 1µg/mL, normal > +7/14 serotypes) | Absent; +1/14 after booster | ND | +3/14 | ND |
| Tetanus IgG, IU/ml (0.15 – 7.0 IU/mL) | 0.03; 0.02 after booster | ND | 2.75 | ND |
| Polysaccharide ribose phosphate, ng/mL (>1000 ng/mL) | 131; >9000 after booster | ND | 460 | ND |
| Lymphocytes, cells/mL (normal range)2 | ||||
| CD3+ (1000 –2600) | 770 | 871 | 1139 | 1257 |
| CD3+CD4+ (530 – 1500) | 237 | 299 | 330 | 428 |
| CD3+CD8+ (330 – 1100) | 494 | 547 | 742 | 801 |
| CD4+/CD8+ ratio (0.9 – 3.7) | 0.48 | 0.52 | 0.44 | 0.53 |
| CD19+ (110 – 570) | 0 | 0 | 39 | 59 |
| IgD−CD27+ (5 – 21%) | ND | ND | 9.7 | ND |
| IgD+CD27+ (8.7 – 18.7%) | ND | ND | 81 | ND |
| IgD+CD27− (57.7 – 79.7 %) | ND | ND | 4.6 | ND |
| CD16+/CD56+ (90 – 600) | 104 | 20 | 2 | 2 |
| Hemogram | ||||
| Monocytes, cells/mL (200 – 900) | 252 | 41 | 90 | 75 |
| Neutrophils, cells/mL (2,730 – 6,680) | 10,970 | 3,050 | 1,470 | 1,110 |
| Platelets, cells/mL (168,000 – 339,000) | 256,000 | 162,000 | 144,000 | 141,000 |
| Proliferation, cpm (normal control on test day) | ||||
| Phytohemagglutinin | ND | 255,173 (227,256) | ND | 174,994 (112,915) |
| Concanavalin A | ND | 127,955 (127,472) | ND | 127,538 (57,266) |
| Pokeweed mitogen | ND | 66,517 (112,473) | ND | 35,255 (77,049) |
| Background | ND | 462 (1,430) | ND | 342 (231) |
| Tetanus | ND | 37,464 (71,042) | ND | 5,285 (90,686) |
| Diptheria | ND | 5,769 (6,687) | ND | 2446 (68,326) |
| Background | ND | 166 (2,594) | ND | 561 (647) |
Jolliff CR Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, Fijan KJ, Fletcher LL, Shriner HC. Clin Chem. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. 1982; 28:126–128.
Comans-Bitter WM, de Groot R, van der Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 1997; 130(3):388–393
ND: Not done
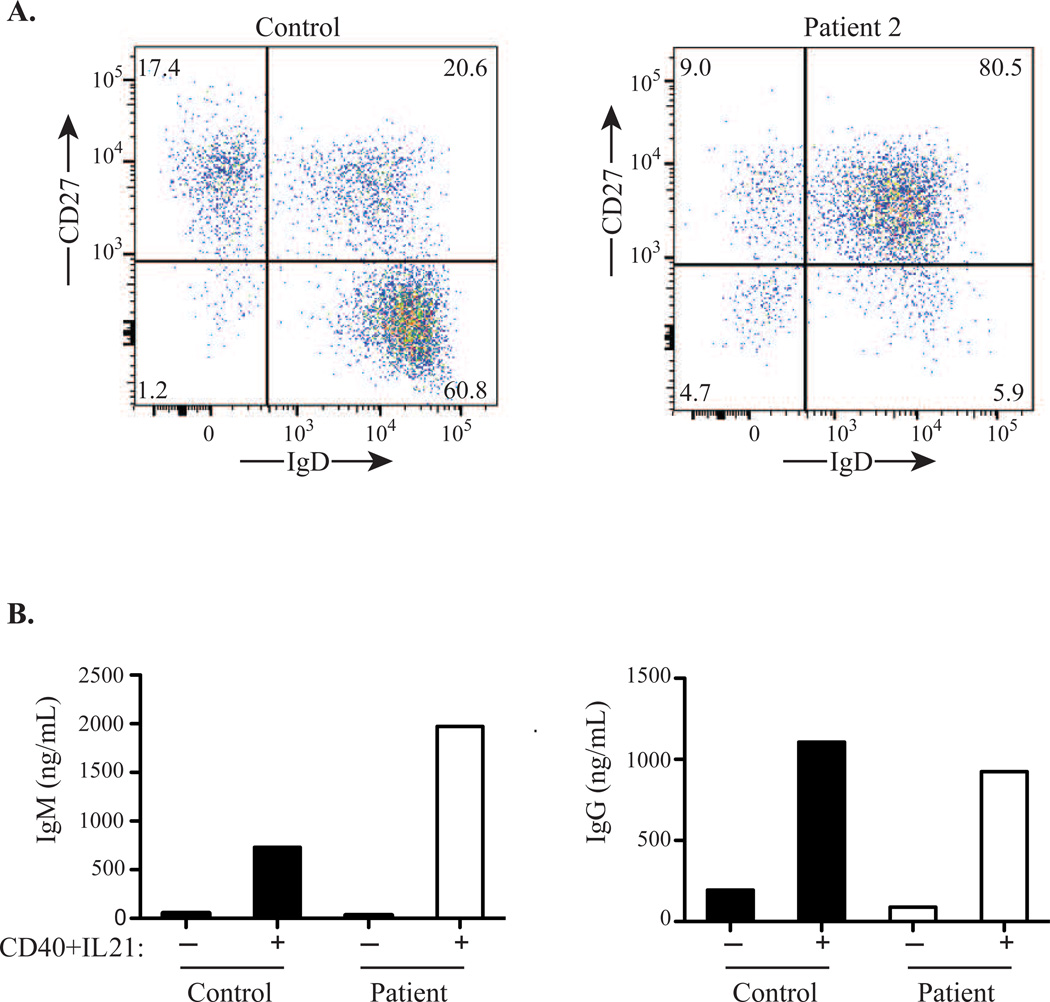
Patient 2, the mother of Patient 1, was well until 48 years of age, when her son was 18 years old. She developed diarrhea, anemia, and leukopenia, attributed to a viral illness causing bone marrow suppression. Although the anemia resolved, she had persistent neutropenia, monocytopenia, and thrombocytopenia. Immune evaluation revealed CD4+ T and B cell lymphopenia, nearly absent NK cells, and monocytopenia (Table I). She had low IgG, normal IgA and IgM, low pneumococcal and PRP titers, and a normal tetanus titer (Table I). Pneumovax and Hib vaccinations caused no significant increase in pneumococcal titers, but her PRP titer normalized to >9000 ng/mL. IVIG was started; since then, she has had no significant infections. Lymphocyte proliferation two years later was normal to mitogens and present to antigens (Table I). Analysis of B cells revealed a deficiency of IgD+CD27− naïve B cells, markedly increased IgD+CD27+ marginal zone (MZ)-like B cells, and a normal percentage of switched IgD−CD27+ memory B cells (Fig. 1A), suggesting skewed differentiation of transitional B cells toward MZ-like B cells or/and impairment of naïve B cell survival. Stimulation of sorted CD19+ B cells with anti-CD40+IL-21 resulted in IgM and IgG secretion comparable to a control (Fig. 1B), indicating that class-switching downstream of CD40 was intact.
Figure 1.
(A) B cell subpopulations in Patient 2 and a control. (B) IgM and IgG production from sorted CD19+ B cells isolated from Patient 2 and a control stimulated with anti-CD40+IL21.
Whole exome sequencing on both patients identified a heterozygous mutation in GATA2 (c. C1061T) that was confirmed by Sanger sequencing. The mutation results in a.a. change from threonine to methionine at position 354 (T354M) in the second zinc finger domain7 and is predicted to be damaging to protein function by both Polyphen (score 0.997) and SIFT (score 0). The T354M mutation does not affect GATA2 expression or nuclear localization, but significantly impairs GATA2 binding to DNA and to the transcription factor PU.1, resulting in a dominant negative effect on transcriptional activation.7 The phenotypes associated with the T354M mutation include autosomal dominant MDS/AML, MDS with pancytopenia, multilineage cytopenias, and opportunistic infections.2, 7–9 One patient with this mutation had one episode of parainfluenza and mycoplasma with normal immunoglobulins,6 another had pneumonias limited to childhood 6, and three others had mycobacterial and viral infections.2, 3, 8 Four individuals were healthy into adulthood.7 Patient 1 presented with IgG2 and IgA deficiency and an abnormal vaccine response, which has not been previously reported in patients with GATA2 mutations.
No additional mutations were found through whole exome sequencing that would account for the hypogammaglobulinemia seen in both patients. The normal immunoglobulin levels in patients with GATA2 mutations have been attributed to the presence of plasma cells. However, atypical plasma cell morphology has been reported in patients with different mutations in GATA2.2, 5, 8 GATA2 may therefore be important for a normal plasma cell population. Our patients illustrate the broad spectrum of clinical presentation inherent in this disease. Patient 1 had an initially mild clinical presentation, with recurrent otitis media and sinusitis, but eventually developed warts and mycobacterial infections despite normal proliferation to mitogens and antigens (Table I). Patient 2 had no history of recurrent infections despite her impaired lymphocyte proliferation to antigens. Thus in our patients, as in others,6 the same GATA2 mutation can result in different phenotypes. This may be due to differences in modifier genes, environmental exposures, and epigenetic factors.
This report highlights the importance of considering mutations in GATA2 in patients with hypogammaglobulinemia particularly in the setting of abnormal lymphocyte subsets and monocyte counts.
Acknowledgments
Supported by NIH (AI-076210 and AI094017 (RSG), The Manton Foundation (LDN), and a Manton Foundation Pilot Award (JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camargo JF, Lobo SA, Hsu AP, Zerbe CS, Wormser GP, Holland SM. MonoMAC Syndrome in a Patient With a GATA2 Mutation: Case Report and Review of the Literature. Clinical Infectious Diseases. 2013 doi: 10.1093/cid/cit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishida H, Imai K, Honma K, Tamura S, Imamura T, Ito M, et al. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. European Journal of Pediatrics. 2012;171:1273–1276. doi: 10.1007/s00431-012-1715-7. [DOI] [PubMed] [Google Scholar]
- 5.Calvo KR, Vinh DC, Maric I, Wang W, Noel P, Stetler-Stevenson M, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96:1221–1225. doi: 10.3324/haematol.2011.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119:1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nature Genetics. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121:2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]