Abstract
Interstitial bone fluid flow (IBFF) is suggested as a communication media that bridges external physical signals and internal cellular activities in the bone, which thus regulates bone remodeling. Intramedullary pressure (ImP) is one main regulatory factor of IBFF and bone adaptation related mechanotransduction. Our group has recently observed that dynamic hydraulic stimulation (DHS), as an external oscillatory muscle coupling, was able to induce local ImP with minimal bone strain as well as to mitigate disuse bone loss. The current study aimed to evaluate the dose dependent relationship between DHS’s amplitude, i.e., 15 and 30 mmHg, and in vivo ImP induction, as well as this correlation on bone’s phenotypic change. Simultaneous measurements of ImP and DHS cuff pressures were obtained from rats under DHS with various magnitudes and a constant frequency of 2Hz. ImP inductions and cuff pressures upon DHS loading showed a positively proportional response over the amplitude sweep. The relationship between ImP and DHS cuff pressure was evaluated and shown to be proportional, in which ImP was raised with increases of DHS cuff pressure amplitudes (R2=0.98). 4-week in vivo experiment using a rat hindlimb suspension model demonstrated that the mitigation effect of DHS on disuse trabecular bone was highly dose dependent and related to DHS’s amplitude, where a higher ImP led to a higher bone volume. This study suggested that sufficient physiological DHS is needed to generate ImP. Oscillatory DHS, potentially induced local fluid flow, has shown dose dependence in attenuation of disuse osteopenia.
Keywords: intramedullary pressure, bone fluid flow, hydraulic fluid stimulation, bone remodeling, mechanical loading, loading magnitude
1. Introduction
Current research of mechanobiology and development of mechanical loading modalities aim to delineate the underlying mechanotransductive mechanisms in bone adaptation. Interstitial bone fluid flow (IBFF) has been suggested as a communication media that bridges external loading signals and internal cellular activities in the bone, in which changes in its velocity or pressure regulate the bone remodeling process [1–6]. As one of the physical regulators resulting in bone growth, intramedullary pressure (ImP) is suggested to initiate IBFF that further influences the osteogenic signals within the bone [7]. Therefore, based on the ImP-IBFF phenomenon, novel and non-invasive mechanical loading modalities can be developed as countermeasures for bone degenerative diseases such as osteoporosis.
Operated in vivo studies using turkey ulna and mouse femur models have shown that ImP, with no bone deformation, can induce potent osteogenic and adaptive responses in the bone [7, 8]. Oscillatory electrical muscle stimulation (MS) in a functional disuse rat model demonstrated its mitigation effect on disuse osteopenia by induced ImP and bone strain [9–11], as well as the interrelationship between vasculature adaptation and ImP alteration [12]. In order to non-invasively distinguish the ImP factor in an in vivo setting and to establish the translational potential of ImP, our group has developed a dynamic hydraulic stimulation (DHS) that meant to directly couple an external load and internal IBFF. Via a 4-week hindlimb suspension (HLS) rat study, DHS was shown to be able to mitigate disuse trabecular [13] and cortical bone loss [14]. Furthermore, a recent surgical study indicated that DHS can generate local ImP within the bone with simultaneous minimal bone strain, which makes DHS a novel and non-invasive method to be applied to an in vivo rodent model to isolate the ImP and bone strain factors [15].
Optimized stimulation regimen by identifying the roles of specific loading parameters, such as loading frequency and magnitude, helps to maximize the beneficial osteogenic responses in the bone. Direct ImP measurements in rats under DHS over a frequency spectrum indicated that its optimal loading at 2Hz can generate maximum ImP of 14.48±3.10mmHg [15]. On the other hand, dynamic components with large loading amplitudes typically result in pronounced osteogenic responses [16]. Therefore, in the current study, we aimed to 1) compare different loading magnitudes of DHS, 2) evaluate its interrelationship between the external oscillatory muscle coupling amplitude and in vivo ImP, and 3) determine this correlation in bone’s phenotypic change. It was hypothesized that non-invasive DHS can induce local ImP to mitigate disuse trabecular bone loss with magnitude dependency.
2. Materials and Methods
2.1. ImP and Cuff Pressure Measurements during DHS Loading
With the approval from Stony Brook University IACUC, surgical experiments were performed on three 15-month-old female Sprague Dawley virgin rats (Charles River, MA; body mass 415±19g) to measure the ImP induction during DHS loading. Rats at 15 months were used for assessment into the intramedullary cavity due to the cavity size. Under standard isoflurane inhalation, each animal received an operation on the right anterior knee to expose the proximal tibia. Similar to previous study, a micro-cardiovascular pressure transducer (Millar Instruments, SPR-524, Houston, TX) was carefully inserted into the tibial marrow cavity through a 1mm drilled hole, and was then tightly sealed within the drilled hole [15].
The setup of DHS was kept the same as previous [15]. DHS was applied with a static preload of 40mmHg and the dynamic pressures controlled by a programmable function generator (HP33120A, Hewlett-Packard, Palo Alto, CA). With a constant frequency of 2Hz, DHS was applied to the operated tibia over amplitudes derived from 0V to 2.4V. Balanced cuff pressures in contact to the loaded tibia were constantly monitored using a pressure sensor throughout the experiment. Measurements of ImP and cuff pressures of the stimulated tibia were recorded simultaneously. At least three repetitive recordings were obtained from each animal.
2.2. 4-Week In Vivo Study and microCT Analysis
All experimental procedures were approved by the IACUC at Stony Brook University. Thirty-eight 5-month-old female Sprague-Dawley virgin rats (Charles River, MA) were randomly assigned into four groups: 1) age-matched control (n=12), 2) hindlimb suspended (HLS, n=10), 3) HLS+15mmHg DHS (n=9), and 4) HLS+30mmHg DHS (n=7). Rats at 5 months represent adulthood that reaches musculoskeletal maturity. Young adult rats are physiologically more adaptive to hindlimb suspension and growth process compared to older rats [17]. The HLS procedure was used to introduce functional disuse in the rat hindlimbs, with a similar previous experimental setup [13]. Throughout the entire study, animals’ overall health was carefully monitored.
For the 4-week in vivo study, daily DHS was applied in conjunction with HLS to the right tibiae of the stimulated rats. Detailed setup of DHS was described previously [13]. The magnitudes of the pressure stimulation were 30mmHg static preload with either 15mmHg (p-p) dynamic pressure (Group 3) or 30mmHg (p-p) dynamic pressure (Group 4). The stimulation frequency was 2Hz. Daily stimulation of 20min with a 5min break was applied to each stimulated animal while under isoflurane inhalation for 5 days/week, for a total of 4 weeks. The rats were euthanized at the end of the study, and the stimulated tibiae were preserved in 70% Ethanol and stored at −20°C.
Using a high resolution microCT scanner (microCT-40, SCANCO Medical AG, Bassersdorf, Switzerland), the proximal metaphysis of the control and stimulated tibiae were scanned for trabecular bone morphology with a spatial resolution of 30μm. All images were evaluated using a Gaussian filter, with specific sigma of 0.3, support of 1, and threshold of 320. Starting from 1800μm away from the growth plate, a 1500μm thick region of trabecular bone was analyzed in the proximal metaphysis. Each metaphyseal trabecular region was evaluated using values for bone volume fraction (BV/TV, %), connectivity density (Conn.D, 1/mm3), trabecular number (Tb.N, 1/mm), and trabecular separation (Tb.Sp, mm).
2.3. Data Analysis
The ImP and cuff pressure recordings were processed using Fast Fourier Transform (FFT) in MatLab to remove the random and DC noise in the data. Measurements within each amplitude window were then divided into twenty intervals, and the difference between the maximum and minimum values were obtained for each interval. These values were averaged and then taken as the peak-to-peak measurement for the according amplitude. All results with statistical analysis were reported as mean±SD. Differences between groups for the calculated ImP and cuff pressure values as well as bone structural analysis were determined by One-way ANOVA on Tukey’s post-hoc test using GraphPad Prism 4.0 Software (GraphPad Software InC., La Jolla, CA). The level of significance was established at p<0.05.
3. Results
3.1. ImP in Function with DHS Cuff Pressure
At baseline, normal heart beats generated approximately 1mmHg tibial ImP within the marrow cavity. Additional fluid pressure was generated upon oscillatory DHS with various magnitudes over the tibial region. On the other hand, the measured cuff pressures over the range of the loading amplitudes increased proportionally with DHS’s magnitude. The interrelationship between ImP and DHS cuff pressure were plotted and shown in Figure 1. ImP was observed to be positively proportional to the DHS cuff pressure, which was induced by the external muscle coupling amplitude. The observed responding trend of the ImP values against DHS cuff pressure showed a linear proportional response during DHS loading at limited range. The equation of the fitted trendline was y = 0.3728x - 1.1247. Based on this equation, ImP was induced to generate approximately 5mmHg and 10mmHg by 15mmHg of DHS and 30mmHg of DHS, respectively.
Figure 1.
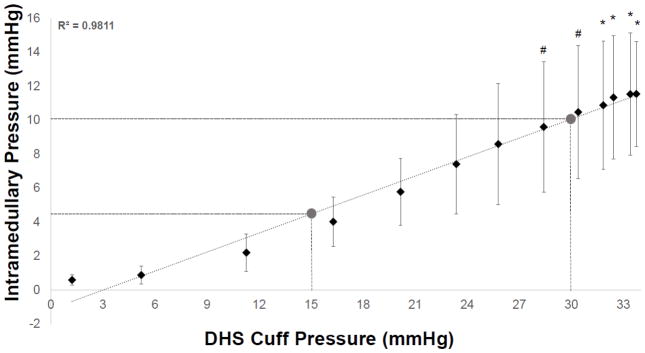
Interrelationship of DHS cuff pressure and ImP induction. Graph shows mean±SD values of ImP measurements vs. cuff pressures. A linear fit was shown between the observed responding trend of the ImP values against DHS cuff pressure. The equation of the fitted trendline was y = 0.3728x - 1.1247. Based on this equation, ImP was induced to about 5mmHg by 15mmHg of DHS and to about 10mmHg by 30mmHg of DHS. #p<0.05 vs. baseline ImP; *p<0.01 vs. baseline ImP.
3.2. microCT – Trabecular Bone
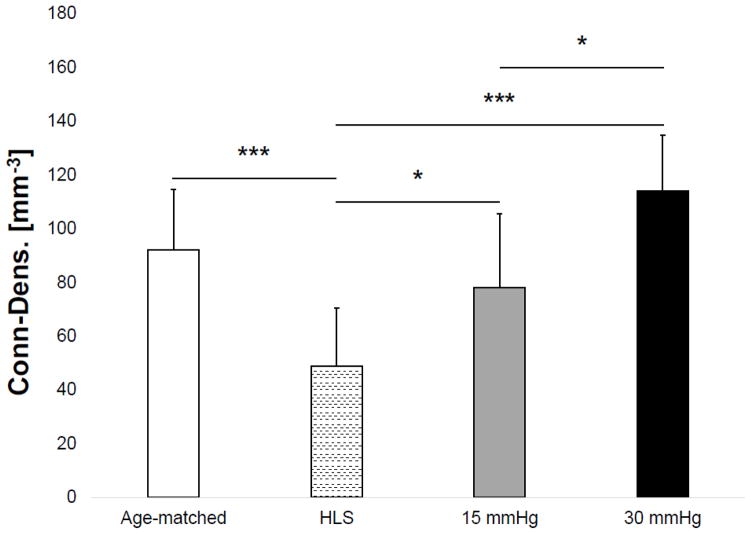
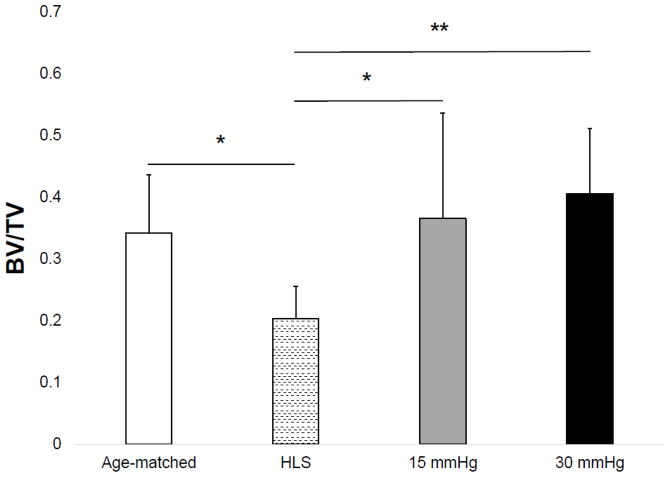
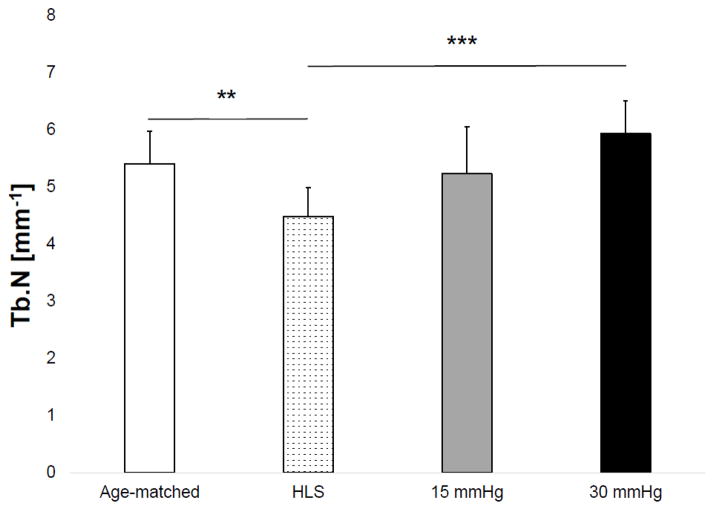
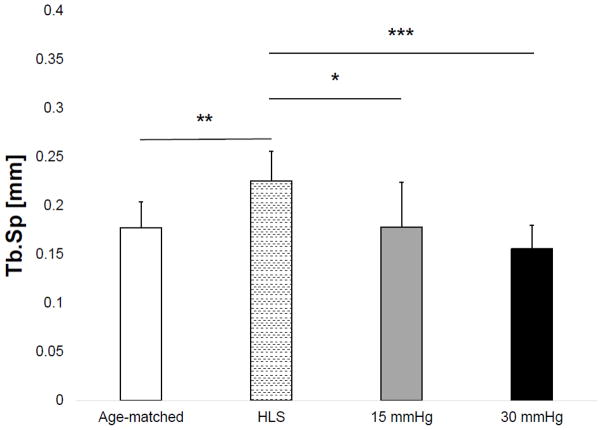
Results of the microCT analysis are shown in Figures 3–6 for the tibial metaphyseal trabecular microarchitecture. The lack of weight-bearing activity over 4 weeks significantly reduced trabecular bone quantity and quality, demonstrated by a 40.74% decrease in BV/TV (p<0.05) (Figure 2), a 46.86% decrease in Conn.D (p<0.001) (Figure 3), a 17.07% decrease in Tb.N (p<0.01) (Figure 4), and a 27.24% increase in Tb.Sp (p<0.01) (Figure 5) compared to age-matched. Trabecular BV/TV in DHS treated animals was significantly greater than that of HLS, and was loading dose dependent. Animals with 15mmHg DHS showed an increase in BV/TV by 80.20% (p<0.05) while 30mmHg DHS showed an increase in BV/TV by 100.30% (p<0.01) (Figure 2). Measures of Conn.D, Tb.N, and Tb.Sp were also significantly affected by DHS treatments with amplitude dependency. There were up to 59.41% (p<0.05) and 132.99% (p<0.05) increases for Conn.D respected to 15mmHg and 30mmHg of DHS (Figure 3); up to 16.61% (p>0.05) and 32.33% (p<0.001) increases for Tb.N respected to 15mmHg and 30mmHg of DHS (Figure 4); and up to 21% (p<0.05) and 30.99% (p<0.001) decreases for Tb.Sp respected to 15mmHg and 30mmHg of DHS (Figure 5).
Figure 3.
Values show mean ± SD for connectivity density (Conn.D, 1/mm3). 15mmHg and 30mmHg of DHS produced significant loading dose dependent changes compared to values obtained in 4-week HLS. *p<0.05, ***p<0.001.
Figure 2.
Values show mean±SD for bone volume fraction (BV/TV, %). 15mmHg and 30mmHg of DHS produced significant loading dose dependent changes compared to values obtained in 4-week HLS. *p<0.05, **p<0.01.
Figure 4.
Values show mean±SD for trabecular number (Tb.N, 1/mm). 15mmHg and 30mmHg of DHS produced significant loading dose dependent changes compared to values obtained in 4-week HLS. **p<0.01, ***p<0.001.
Figure 5.
Values show mean±SD for trabecular separation (Tb.Sp, mm). 15mmHg and 30mmHg of DHS produced significant loading dose dependent changes compared to values obtained in 4-week HLS. *p<0.05, **p<0.01, ***p<0.001.
4. Discussion
Results from this study demonstrated a positively proportional interrelationship between ImP and DHS cuff pressure, in which ImP was raised with increases of DHS cuff pressure amplitudes. In addition, bone adaptation related to ImP-IBFF was further demonstrated by DHS in a rat functional disuse model with a loading dose dependent manner. Loaded with 2Hz constant frequency and an amplitude sweep between 1.4V and 2.4V, which was found equivalent to the cuff pressures between 28.41±10.50 mmHg and 33.75±10.69 mmHg, respectively, DHS significantly induced ImP compared to the baseline level. These results suggest a strong regulatory potential of DHS on fluid pressure within the local marrow cavity, if loaded at proper frequency and amplitude, which can influence IBFF and ultimately attenuate disuse bone loss.
Optimal stimulation regimen of mechanical loading helps maximize the osteogenic responses in the bone. Various studies have shown positive correlation between loading frequency and the improved bone quality [18–20] but present dissimilar effective loading frequencies [18, 19, 21, 22]. Related to ImP-induced osteogenic response, direct ImP measurements under DHS over a frequency spectrum in rats indicated that its optimal loading at 2Hz can generate maximum ImP of 14.48±3.10 mmHg [15]. Further, based on different orientations of physical contacts between diverse mechanical loaders and the loaded tissue, as well as the different material densities and viscosities within the loaded tissues, the relationship between loading magnitude and bone adaptation seem to differ among various loading modalities and conditions. For example, external axial loading with a constant loading rate and a range of magnitude from 0 N and 14 N in mice resulted an essentially linear relationship between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition [23]. Axial loading applied between 5N and 13N on mouse tibiae showed significant load magnitude-related increases in cortical bone formation [24]. However, it is with interest to observe that no magnitude dependence on trabecular bone adaptation was found in a cyclic compressive loading study in a rabbit model [25]. In the current study, the observed ImP inductions were found to be positively proportional to DHS cuff pressure, namely, which indicates promising potential of DHS loading dose dependency on ImP related bone adaptation.
Results from this study showed that DHS loading at 2Hz between 28.41±10.50 mmHg and 33.75±10.69 mmHg cuff pressures can induce significant ImP increases. Coincided with these observations, our 4-week in vivo study showed that DHS loading at 2Hz with 15 mmHg and 30 mmHg dynamic pressure was able to mitigate disuse trabecular bone loss in a rat functional disuse model. These findings suggest that trabecular surface may sense the rapid changes in fluid pressure, and by means of direct relationship of ImP-induced IBFF bone adaptation, leading to the pronounced phenotypic change with loading dose dependency of DHS. In addition, daily DHS treatment on hindlimb suspended rats has also been found effective in mitigating disuse related reductions in cortical bone volume and thickness of the loaded bone. Both microCT and histological analysis revealed a strong trend of DHS’s positive effect on the endosteal surface of the cortical mid-shaft that helped thicken the cortical bone [14]. Circular compressions from DHS provide direct muscle coupling that mimics the condition when skeletal muscle contracts and temporarily occludes the within blood vessels to the bone, which generates an arteriovenous pressure gradient that increases the ImP and further drives IBFF [26–29]. At the cellular level, longitudinal quantifications of in vivo bone marrow mesenchymal stem cells (MSCs) indicated the positive influence of DHS-derived mechanical signals on time-sensitive cell growth [30]. At the molecular level, changes of longitudinal osteogenesis related gene expressions was further demonstrated in animals treated by DHS [14]. Changes of MSC populations and osteogenic gene expressions in response to DHS may bias osteogenic differentiation that can ultimately form bone even under disuse conditions [13, 14].
In summary, oscillatory DHS, if applied with proper frequencies and amplitudes, can generate local ImP that may subsequently enhance IBFF. Future study should point to further optimization of DHS loading regimes by varies combinations of the enhanced loading conditions, including loading durations and rest insertions. Results from this study provide a great support for the development of DHS as a non-invasive, biomechanical based intervention for osteoporosis prevention and treatment.
Highlights.
ImP inductions under dynamic hydraulic stimulation (DHS) were positively proportional to the loading amplitudes.
The mitigation effect of DHS on disuse trabecular bone was highly related to the loading amplitudes.
This study provides valuable information for DHS loading optimization, and confirms the regulatory effect of DHS on local fluid dynamics.
Acknowledgments
The authors greatly appreciate the kind support from National Institute of Health (R01 AR52379 and AR61821), US Army Medical Research and Materiel Command, and National Space Biomedical Research Institute through NASA Cooperative Agreement NCC 9-58.
Footnotes
Conflict of Interest Statement
All authors state that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollack SR, Korostoff E, Sternberg ME, Koh J. Stress-generated potentials in bone: effects of collagen modifications. J Biomed Mater Res. 1977;11:677–70. doi: 10.1002/jbm.820110505. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RJ, Sutker BD, Bronk JT, Smith SR, Kelly PJ. Interstitial fluid flow in cortical bone. Microvasc Res. 1988;35:295–307. doi: 10.1016/0026-2862(88)90084-2. [DOI] [PubMed] [Google Scholar]
- 3.Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–4. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 4.Rubin J, Biskobing D, Fan X, Rubin C, McLeod K, Taylor WR. Pressure regulates osteoclast formation and MCSF expression in marrow culture. J Cell Physiol. 1997;170:81–7. doi: 10.1002/(SICI)1097-4652(199701)170:1<81::AID-JCP9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–76. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB J. 1999;13 (Suppl):S101–12. [PubMed] [Google Scholar]
- 7.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–37. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 8.Kwon RY, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010;25:1798–807. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam H, Qin YX. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009;42:140–5. doi: 10.1016/j.jbiomech.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam H, Hu M, Qin YX. Alteration of contraction-to-rest ratio to optimize trabecular bone adaptation induced by dynamic muscle stimulation. Bone. 2011;48:399–405. doi: 10.1016/j.bone.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam H, Brink P, Qin YX. Skeletal nutrient vascular adaptation induced by external oscillatory intramedullary fluid pressure intervention. J Orthop Surg Res. 2010;5:18. doi: 10.1186/1749-799X-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu M, Cheng J, Qin YX. Dynamic hydraulic flow stimulation on mitigation of trabecular bone loss in a rat functional disuse model. Bone. 2012;51:819–25. doi: 10.1016/j.bone.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu M, Qin YX. Dynamic fluid flow stimulation on cortical bone and alterations of the gene expressions of osteogenic growth factors and transcription factors in a rat functional disuse model. Arch Biochem Biophys. 2014;545:154–61. doi: 10.1016/j.abb.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu M, Serra-Hsu F, Bethel N, Lin L, Ferreri S, Cheng J, Qin YX. Dynamic hydraulic fluid stimulation regulated intramedullary pressure. Bone. 2013;57:137–141. doi: 10.1016/j.bone.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo AB, Alam I, Tanaka SM, Levenda J, Li J, Warden SJ, Turner CH. Low-amplitude, broad-frequency vibration effects on cortical bone formation in mice. Bone. 2006;39:1087–96. doi: 10.1016/j.bone.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 18.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol. 1995;269:E438–42. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16:918–24. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 20.Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res. 1994:165–74. [PubMed] [Google Scholar]
- 21.Zhang P, Tanaka SM, Sun Q, Turner CH, Yokota H. Frequency-dependent enhancement of bone formation in murine tibiae and femora with knee loading. J Bone Miner Metab. 2007;25:383–91. doi: 10.1007/s00774-007-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone. 2004;34:261–70. doi: 10.1016/j.bone.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012;27:1784–93. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Willie BM, Beach JM, Wright TM, van der Meulen MC, Bostrom MP. Trabecular bone adaptation to loading in a rabbit model is not magnitude-dependent. J Orthop Res. 2013;31:930–4. doi: 10.1002/jor.22316. [DOI] [PubMed] [Google Scholar]
- 26.Qin YX, Lam H, Ferreri S, Rubin C. Dynamic skeletal muscle stimulation and its potential in bone adaptation. J Musculoskelet Neuronal Interact. 2010;10:12–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Winet H. A bone fluid flow hypothesis for muscle pump-driven capillary filtration: II. Proposed role for exercise in erodible scaffold implant incorporation. Eur Cell Mater. 2003;6:1–10. doi: 10.22203/ecm.v006a01. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 28.Otter MW, Qin YX, Rubin CT, McLeod KJ. Does bone perfusion/reperfusion initiate bone remodeling and the stress fracture syndrome? Med Hypotheses. 1999;53:363–8. doi: 10.1054/mehy.1998.0782. [DOI] [PubMed] [Google Scholar]
- 29.Laughlin MH. The muscle pump: what question do we want to answer? J Appl Physiol. 2005;99:774. doi: 10.1152/japplphysiol.00578.2005. [DOI] [PubMed] [Google Scholar]
- 30.Hu M, Yeh R, Lien M, Teeratananon M, Agarwal K, Qin YX. Dynamic Fluid Flow Mechanical Stimulation Modulates Bone Marrow Mesenchymal Stem Cells. Bone Research. 2013:98–104. doi: 10.4248/BR201301007. [DOI] [PMC free article] [PubMed] [Google Scholar]