Abstract
Stressful early life experiences are implicated in lifelong health. However, little is known about the consequences of emotional or physical stress on neurobiology. Therefore, the following set of experiments was designed to assess changes in transcription and translation of key proteins within the nucleus accumbens (NAc). Male adolescent (postnatal day [PD] 35) or adult (eight-week old) mice were exposed to emotional (ES) or physical stress (PS) using a vicarious social defeat paradigm. Twenty-four hours after the last stress session, we measured levels of specific mRNAs and proteins within the NAc. Spine density was also assessed in separate groups of mice. Exposure to ES or PS disrupted ERK2, reduced transcription of ΔFosB, and had no effect on CREB mRNA. Western blots revealed that exposure to ES or PS decreased ERK2 phosphorylation in adolescents, whereas the same stress regimen increased ERK2 phosphorylation in adults. Exposure to ES or PS had no effect on ΔFosB or CREB phosphorylation. ES and PS increased spine density in the NAc of adolescent-exposed mice, but only exposure to PS increased spine density in adults. Together, these findings demonstrate that exposure to ES or PS is a potent stressor in adolescent and adult mice, and can disturb the integrity of the NAc by altering transcription and translation of important signaling molecules in an age-dependent manner. Furthermore, exposure to ES and PS induces substantial synaptic plasticity of the NAc.
Keywords: ERK, ΔFosB, dendritic spines, nucleus accumbens, depression, social defeat, adolescence
Introduction
Stressful early life experiences can have devastating consequences associated with mental illness and behavioral malfunctioning that extend well into adulthood [1]. Individuals with childhood history of abuse, whether physical, emotional, or sexual, have increased risk of life-long negative outcomes [1–3]. These individuals die by suicide more frequently, and are more likely to succumb to depression and anxiety disorders [4–7]. However, little is known about the different effects of specific types of abuse. Clinical and epidemiological studies in people with history of abuse have largely been unable to tease apart the potentially disparate effects of emotional or physical stress since these are rarely experienced in isolation [8–10]. Nevertheless, studies that attempt to measure these differences find surprising outcomes. Individuals with childhood history of emotional abuse are at increased risk for developing post-traumatic stress disorder, suicide, substance abuse and obesity, and it appears that emotional maltreatment may predispose a person to developing depression and anxiety symptoms more so than when compared to those with physical maltreatment or sexual abuse alone [5,10–12].
Preclinical models attempting to measure the differences between emotional and physical stress also indicate substantial functional and biochemical changes after exposure to emotional stress. Mice exposed to emotional or physical stress as adults have long-lasting deficits in behavioral tasks designed to assess changes in mood-related behavior [13]. Furthermore, exposure to emotional or physical stress powerfully disrupts cell biology of ventral tegmental area (VTA) neurons in these mice [13]. The VTA is part of the mesolimbic dopamine system, a brain region that is heavily involved in natural and drug reward [14–16], and is now increasingly being looked at for its role in managing motivation- and mood-related functioning [17–19]. The nucleus accumbens (NAc) is densely innervated by the VTA, and it also plays a pivotal role in reward and mood processes [17–19]. Therefore, it was of interest to determine whether emotional or physical stress could alter neurobiology and synaptic plasticity within the NAc.
Converging lines of evidence indicate that neurotrophin signaling has profound influence on mood. In particular, extracellular signal-related kinase 2 (ERK2), a downstream target of the neurotrophin BDNF, has emerged as an important mediator ccof mood. Chronic stress increases, whereas chronic exposure to the antidepressant fluoxetine decreases ERK2 expression within the VTA [20–22]. Additionally, viral-mediated overexpression of ERK2 results in susceptibility to stress, while knockdown with a dominant negative of ERK2 results in a stress-resistant phenotype in rodents that have had their VTA transfected with the respective viruses [20,21]. Not surprisingly, then, dysregulation of two of ERK’s downstream targets, ΔFosb and cAMP response element binding protein (CREB), have also been shown to be intimately linked with deficits in mood-related functioning [23–28]. In the NAc, activation of CREB is associated with increased anxiety- and depression-like behavior in adult rodents [24,29,30]. Conversely, induction of ΔFosB in the NAc in adulthood appears to be related to resilience to stress [27]. These signaling molecules also appear to be involved in synaptogenesis [14,31,32], a type of neural plasticity implicated in susceptibility and resilience to stress [32]. Because adolescence is thought to be a period of increased synaptic plasticity, determining the consequences of exposure to emotional and physical stress on ERK2, ΔFosB, and CREB in relation to synaptic plasticity was tantalizing.
While studies assessing the deleterious effects of stress have generally been conducted in adulthood, much less is known about the effects of stressors during adolescence, a critical developmental period of increased sensitivity to stress [33–35]. This is surprising, since adolescence is a period when psychiatric, drug abuse, and conduct disorders often emerge [36–38]. Thus, the focus of this study was to assess the effects of emotional (ES) or physical (PS) stress in adolescent and adult male mice on NAc biochemistry and synaptic plasticity.
Methods
Animals
Mice were male, fed ad libitum, allowed a 1-week habituation period before experimental manipulation, and housed at 23–25°C on a 12 hr light/dark cycle (lights on at 7 AM). Adolescent (postnatal day [PD] 35) and adult (eight-week old) male c57BL/6J mice (Jackson Labs, Bar Harbor, Maine), and CD1 retired breeders (Charles River), were used in this study. Mice were housed in clear polypropylene boxes containing wood shavings (four per cage prior to stress, singly housed following stress), and CD1 (housed one per cage immediately upon arrival). Experiments were conducted in compliance with the guidelines for the Care and Use of Laboratory Animals [39], and approved by the Florida State University Animal Care and Use Committee.
Stress exposure and experimental design
Emotional and physical stress procedures were performed as previously described [13]. Adolescent mice were randomly assigned to a daily session (10 min per session) of control (CON), emotional (ES), or physical (PS) stress for 10 consecutive days (PD35-44 for adolescents). Briefly, the home cage (23.5cm × 45.5cm × 15cm) of a CD-1 retired breeder was separated into two compartments by a perforated clear Plexiglas divider, allowing olfactory, acoustic and visual signals to be shared between the compartments. The mouse in the ES condition was placed into the empty compartment adjacent to the CD-1 aggressor, while the mouse in the PS condition was placed into the compartment containing the aggressor, as previously described [40–42]. During this time, the PS mouse was subjugated by the CD-1 mouse and adopted a defensive posture. To minimize physical injury and subject attrition, the daily social defeat episode would be terminated immediately in the event that the CD1 displayed extreme physical aggression (i.e., continuous biting after submissive posturing by the c57BL/6J mouse) [43]. After ten minutes, the ES exposed mouse was transferred to a novel cage, in the compartment adjacent to a novel CD-1, to minimize exposure to any latent stimuli potentially produced by the PS-exposed mouse. The PS-exposed mouse was left overnight in the compartment adjacent to the CD-1 that socially defeated it. This process was repeated for ten consecutive days, such that each day the ES-exposed mouse ‘witnessed’ the defeat of a novel mouse by a novel CD-1. The term ‘witness’ in this model refers to all sensory stimuli associated with the ES experience and not visual stimuli alone [13]. CON-exposed mice were housed by pair, one on each side of a perforated Plexiglas partition, and handled daily [41]. Behavioral testing began 24 h after the last stress session. All animals (adolescent and adults) were single housed at the initiation of stress treatment and remained single housed for the duration of the study.
Social Interaction Test
The social interaction test is a test of social avoidance [41–43]. Briefly, this is a two-session test. In the first session, a mouse is allowed to explore an open field arena (40 cm × 40 cm) for 2.5 minutes. Along one side of the arena is a wire mesh cage that remains empty during the first trial (no target). This mouse is removed and a novel CD-1 male mouse is placed into the wire mesh cage. The test mouse is replaced and the amount of time he spends in the “interaction zone” (an 8 cm wide corridor surrounding the cage), as well as the time spent in the “corners” farthest from the mesh cage, are measured during the 2.5-minute trial (target present). Socially defeated mice explore the interaction zone less when another mouse is present and spend more time in the corners. Interestingly, chronic antidepressant treatment alleviates this behavioral phenotype, but acute treatment does not [13,44,45]. This makes the social interaction test a highly valid model of antidepressant efficacy and is thus a good model to test for depression-like affect.
Real Time PCR
Mice were sacrificed either 24-h after the last exposure to CON, ES, or PS and 1.25 mm punches were taken from NAc (including both core and shell) and stored at − 80°C until use. RNA was isolated using RNEasy Micro kits (Qaigen) and cDNA was created from these samples using iScript cDNA synthesis kit (Bio-Rad; Hercules, California). qPCRs were performed in triplicate using 96 well PCR plates and RealMasterMix (Eppendorf) with an Eppendorf MasterCycler Realplex2 according to manufacturer’s instructions. Threshold cycle [C(t)] values were measured using the supplied software and analyzed with the ΔΔC(t) method as described previously [27,46]. Primer sequences for Mapk1 (ERK2), Deltafosb (ΔFosB), Creb1 (CREB), and Gapdh were generated from the Harvard Primer Bank and are listed in Table 1.
Table 1.
qPCR Primers for Gapdh, Mapk1, Creb1, FosB are listed in Table 1.
| Gene | Reverse Sequence | Forward Sequence |
|---|---|---|
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Creb1 | AGTGACTGAGGAGCTTGTACCA | TGTGGCTGGGCTTGAAC |
| ΔFosb | AGGCAGAGCTGGAGTCGGAGAT | GCCGAGGACTTGAACTTCACTCG |
Western blotting
Tissue punches of NAc (1.25 mm) from mice were sonicated in a standard lysis buffer and then centrifuged at 14,000 rpm for 15 min. Each punch contained tissue from both core and shell. Samples (20 μg; estimated through Bradford assay) were treated with β-mercaptoethanol and subsequently electrophoresed on precast 4%–20% gradient gels (Biorad, Hercules, California), as previously described [47]. Proteins were transferred to a polyvinylidene fluoride membrane, washed in 1X Tris-buffered saline with 0.1% Tween-20 (TBST), and blocked in milk dissolved in TBST (5% weight/volume) for one h at 25°C. Blots were probed (overnight at 4°C) with antibodies against ΔFosB (1:1000), phosphorylated ERK1/2 (1:2000) and CREB (1:2000), stripped with Restore (Pierce Biotechnology, Rockford, Illinois) and re-probed with antibodies against total ERK1/2 (1:2000), CREB (1:2000), and GAPDH (1:2000). All antibodies were from Cell Signaling (Beverly, Massachusetts) and were used according to manufacturer’s instructions in 5% milk dissolved in TBST. After further washes, membranes were incubated with peroxidase-labeled goat anti-rabbit IgG or Goat anti-mouse IgG (1:5,000; Vector Labs, Burlingame, California). Bands were visualized with SuperSignal West Dura substrate (Pierce Biotechnology, Rockford, Illinois), quantified using ImageJ (NIH) and normalized to total protein (optical density phosphorylated protein ÷ optical density total protein) or to GAPDH (optical density target protein ÷ optical density GAPDH). Data are presented as percentage of control expression (Test value ÷ CON value × 100). Because ERK1 and ERK2 bands are clearly discernable, only the ERK2 bands were quantified.
Golgi Staining and Spine Analysis
Mice were given an overdose of Ketmaine/Xylazine and perfused transcardially with ice-cold saline. Whole brains were dissected out and stained with FD Rapid GolgiStain kit, as per manufacturer’s instructions (FD Neurotech). After staining, brains were sliced into 100 um sections and mounted on gelatin coated slides, dehydrated with ascending concentrations of ethanol, defatted in xylene, and coverslipped using permount. Dendritic spines from the NAc core and shell were imaged using StereoInvestigator (MBF Bioscience, Williston, VT) and spine analysis was performed using ImageJ (National Institute of Health) by two observers blind to treatment conditions. More specifically, the total number of spines was counted on a given length of dendrite and the spine density was derived (total number of spines ÷ length of dendrite in um) as described previously [48{Perez-Cruz, 2011 #29217]}. A correlation between values from the two raters demonstrated high concordance.
Statistical analyses
Data were analyzed using mixed-design (between and within variables) analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post hoc tests. When appropriate, Student’s t-tests were used to determine statistical significance of preplanned comparisons. Data are expressed as the mean ± SEM. Statistical significance was defined as pitalic> 0.05.
Results
Social interaction following exposure to CON, ES, or PS in adolescent and adult C57BL/6J mice
We first assessed the consequences of 10 days of CON, ES, or PS exposure on social interaction 24 h after the last stress session in either adolescent or adult mice. One-way ANOVA revealed that social interaction time varied as a function of stress condition (F(2,27)= 11.7, pbold> 0.001) in the adolescent mice. Exposure to PS reduced social interaction compared to the CON-exposed mice (Fig. 1A, left panel). Interestingly, ES-exposed mice also showed social avoidance when compared to the CON-exposed mice, indicating that social avoidance can be induced vicariously in adolescent mice (p< 0.05). To determine whether stress exposure during adulthood could induce similar changes, a separate group of mice was exposed to CON, ES, or PS for 10 consecutive days as adults and then social interaction was assessed 24 h later (Fig. 1A, right panel). As expected, we found that exposure to ES and PS induced social avoidance in adult mice (F(2,27)= 29.1, p< 0.001). Together, these findings demonstrate that ES and PS exposure induce social avoidance in both adolescent and adult mice.
Fig. 1.
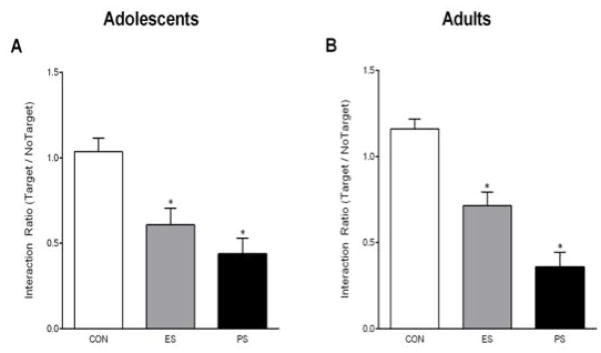
Emotional (ES) and physical (PS) stress alters social interaction 24-hr after the last stress exposure. (A) ES and PS exposure during adolescence reduced the time spent interacting with the CD-1 mouse when compared to control (CON) mice (n=30; p< 0.05). (B) ES and PS exposure during adulthood reduced the time spent interacting with the CD-1 mouse when compared to CON-exposed mice (n=30; p< 0.05). *Significantly different than CON-exposed mice.
Effects of CON, ES, or PS exposure on ERK2, ΔFosB, and CREB within the NAc of adolescent and adult C57BL/6J mice
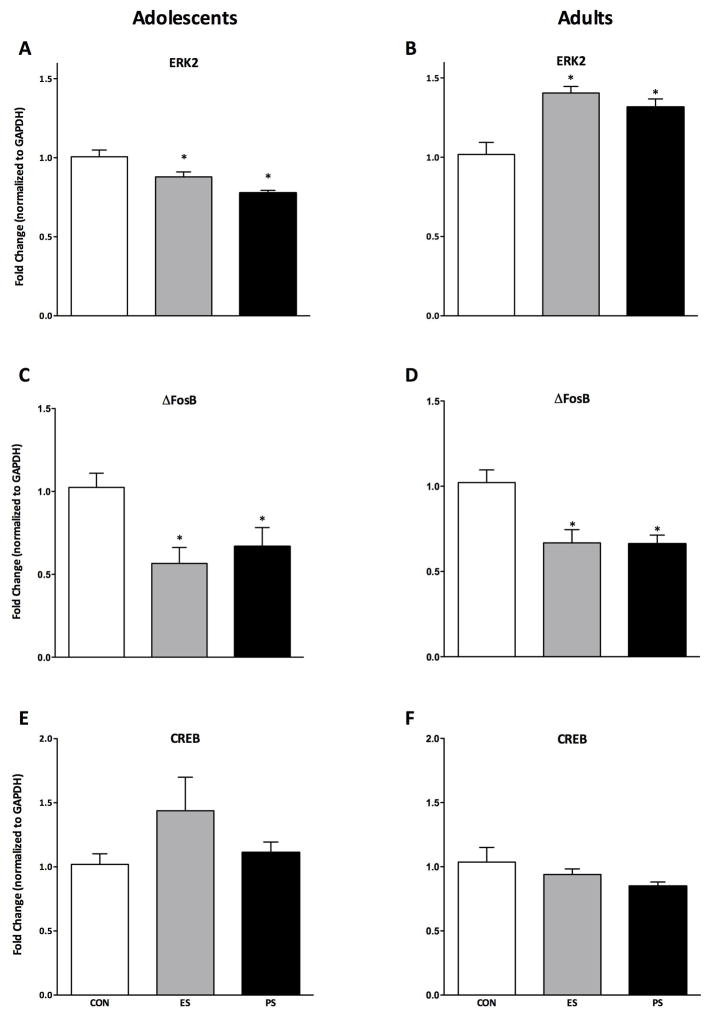
ERK2, ΔFosB, and CREB mRNA levels within the NAc were measured 24 h after either adolescent or adult exposure to CON, ES, or PS using qPCR (Fig. 2A–F). ERK2 levels varied as a function of stress exposure in both adolescent- (F(2,21)= 12.1, p< 0.001; Fig. 2A) and adult-exposed mice (F(2,21)= 12.9, p< 0.001; Fig. 2B) when compared to CON-exposed mice. ERK2 mRNA was surprisingly decreased in mice exposed to ES and PS as adolescents, but increased in mice exposed to ES and PS as adults (p< 0.05, respectively). ΔFosB mRNA levels also varied as a function of stress exposure in both adolescent (F(2,21)= 5.9, p< 0.01; Fig. 2C) and adult (F(2,21)= 8.9, p< 0.01; Fig. 2D) exposed mice. Interestingly, ES and PS exposure decreased ΔFosB mRNA in both adolescent and adult-exposed mice (p< 0.05). Lastly, we also measured the effect of ES or PS on CREB expression. CREB mRNA did not vary be stress exposure in either adolescent (Fig. 2E) or adult (Fig. 2F) mice.
Fig. 2.
Emotional (ES) and physical (PS) stress alters mRNA levels 24-hr after the last stress exposure. (A) ES and PS exposure during adolescence increased levels of ERK2 mRNA when compared to control (CON) mice (n=24; p< 0.05). (B) ES and PS exposure during adulthood reduced levels of ERK2 mRNA when compared to CON-exposed mice (n=24; p< 0.05). (C) ES and PS exposure during adolescence reduced levels of ΔFosB mRNA when compared to CON-exposed mice (n=24; p< 0.05). (D) ES and PS exposure during adulthood reduced levels of ΔFosB mRNA when compared to CON-exposed mice (n=24; p< 0.05). (E) ES and PS exposure during adolescence (n=24; p> 0.05) or adulthood (F) had no effect on CREB mRNA when compared to CON-exposed mice (n=24; p> 0.05). Data are presented as fold change from CON-exposed mice. *Significantly different than CON-exposed mice.
Effects of CON, ES, or PS on protein phosphorylation and expression on ERK2, ΔFosB, and CREB within the NAc of adolescent and adult C57BL/6J mice
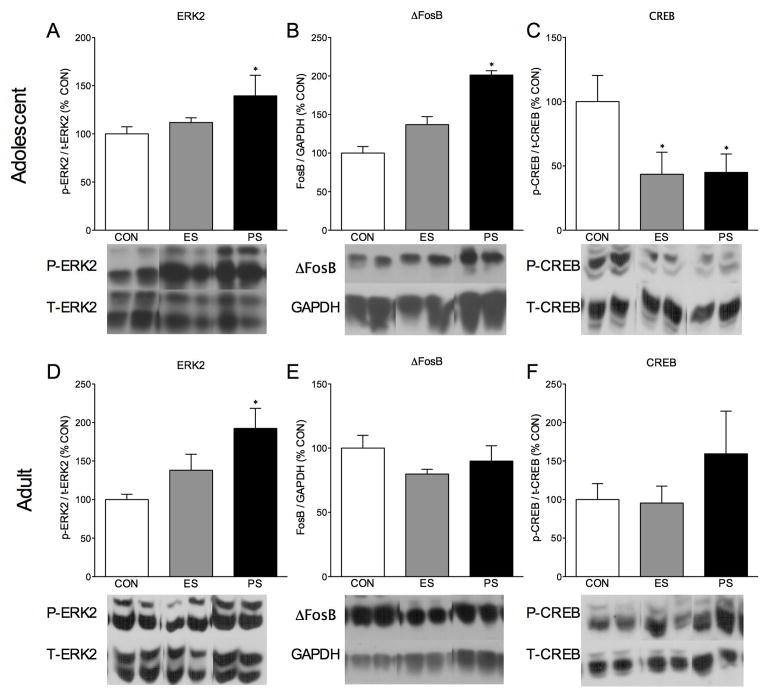
Since changes in mRNA do not always translate into changes in protein levels, we also assessed the effects of ES or PS on total and phosphorylated levels of protein. Phosphorylated ERK2 protein levels varied as a function of CON, ES, or PS exposure in adolescent mice (F(2,20)=4.50, p< 0.05; Fig. 3A). More specifically, we found an increase in ERK2 phosphorylation in the NAc of adolescents when compared to the CON-exposed controls (p< 0.05) after PS exposure. Similarly, levels of NAc ERK2 phosphorylation also varied as a function of stress exposure in adults (F(2,21)= 4.18, p< 0.03; Fig. 3D), after PS exposure when compared to the CON-exposed adult mice 24 h after the last stress session (p< 0.05). Total levels of ΔFosB varied as a function of stress exposure during adolescence (F(2,20)=4.65, p< 0.05; Fig. 3B), but not adult mice (Fig. 3E, p>0.05.) Interestingly, in mice exposed to ES or PS as adolescents, phosphorylation of CREB varied as a function of stress exposure (F(2,20)=6.10, p< 0.01; Fig. 3C), with no effect in the adult mice (Fig. 3F, p>0.05) when compared to CON-exposed mice. More specifically, exposure to ES or PS during adolescence robustly decreased CREB phosphorylation when compared to CON-exposed mice (p<0.05). No significant differences were seen when phosphorylated ERK2, total ERK2, or total CREB when compared to CON mice in adolescent mice (p> 0.05). Phosphorylated CREB was significantly influenced when normalized to GAPDH instead of total CREB (F(2,20)=6.1, p< 0.01; data not shown) in adolescent mice. In adults, only phosphorylated ERK2 was significantly influenced by ES or PS exposure when normalized to GAPDH instead of total ERK (F(2,21)=10.88, p< 0.01; data not shown). These differences are expected, since they do not account for changes in total protein levels.
Fig. 3.
Emotional (ES) and physical (PS) stress alters protein levels 24-hr after the last stress exposure. (A) ES and PS exposure during adolescence increased ERK2 phosphorylation and increased ΔFosB when compared to control (CON) mice (n=23; p< 0.05). (C) ES and PS exposure decreased CREB phosphorylation in adolescent mice when compared to CON-exposed mice (n=23; p< 0.05). In adults, PS, but not ES exposure increased levels of ERK2 phosphorylation when compared to CON-exposed mice (n=24; p< 0.05), (E) but had no effect on ΔFosB (n=24; p> 0.05) or (F) CREB phosphorylation (n=24; p> 0.05) when compared to CON-exposed mice. Data are presented as % of CON levels of immunoreactivity. *Significantly different than CON-exposed mice.
Effects of CON, ES, or PS exposure on spine density within the NAc of adolescent and adult C57BL/6J mice
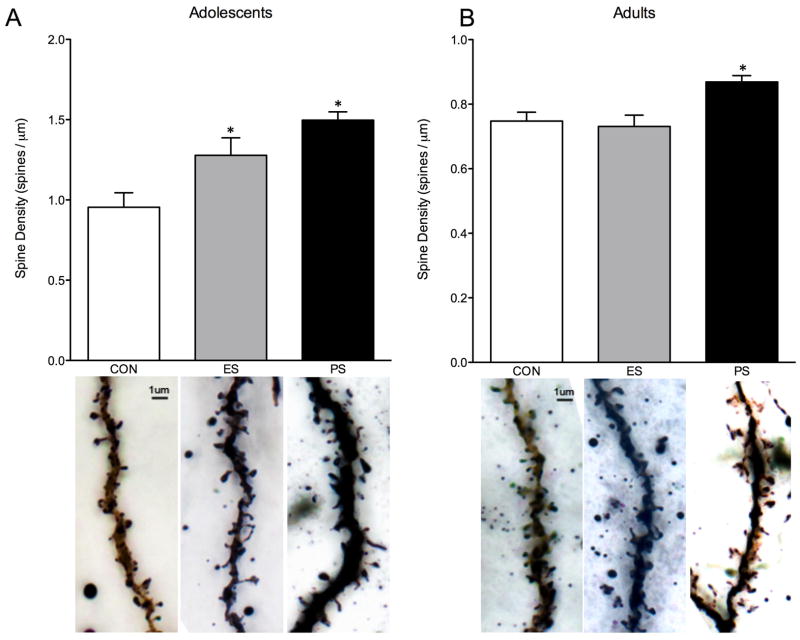
Because changes in intracellular signaling and gene expression often suggest changes in synaptic plasticity, we also assessed the effects of stress exposure on spine density within the NAc. Figure 4 shows the effect of CON, ES, or PS during adolescence (Fig. 4A) or adulthood (Fig. 4B) on spine density. In adolescent mice, spine density was found to significantly vary as a function of stress exposure (F(2,26)= 9.7, p< 0.001; Fig. 4A). Specifically, mice exposed to ES or PS as adolescents had increased spine density within the NAc, indicating increased synaptic plasticity. In adults, there was also an effect of stress exposure (F(2,14)= 6.3, p< 0.05; Fig. 4B); however, only PS-exposure increased spine density in the NAc when compared to the CON-exposed mice.
Fig. 4.
Emotional (ES) and physical (PS) stress alters spine density 24-hr after the last stress exposure. (A) ES and PS exposure during adolescence increased spine density when compared to control (CON) mice (n=29; p< 0.05). (B) PS, but not ES exposure during adulthood increased spine density when compared to CON-exposed mice (n=17; p< 0.05). *Significantly different than CON-exposed mice.
Discussion
The present study assessed whether exposure to emotional (ES) or physical (PS) stress could disrupt the integrity of the NAc of adolescent and adult male mice 24 h after stress exposure. Here, we provide evidence that exposure to ES or PS disrupts social interaction, and that it induces unique, age-dependent adaptations on gene transcription, protein phosphorylation, and synaptic plasticity.
Exposure to ES and PS both induced a depression-like state as indexed in the social interaction test. In this task, a mouse is allowed to interact with a conspecific in an open field arena. This behavioral task is one of the most important tests currently used to assess behavioral state in socially defeated mice [40,41]. We find that mice exposed to ES or PS during adolescence or adulthood spent significantly less time interacting with a social target than the CON-exposed mice. This is interesting because it suggests that exposure to ES can induce social avoidance even in young mice. Furthermore, simply ‘witnessing’ the physical stressor appears to be enough to induce social avoidance in ES-exposed mice. Exposure to social defeat, as in the PS-exposed mice, is known to induce a robust and reliable social avoidance in adult mice [13,21,40–44]. Here, we show that PS exposure can produce this effect in adolescent mice as well, and further extend these findings to also demonstrate that it can be induced vicariously through ES exposure [13]. In contrast to previous reports showing a distribution of behavioral response to social defeat (i.e., PS) from ‘resilient’ to ‘susceptible’, we found relatively few ‘resilient’ mice. Because these ‘resilient’ mice were not statistical outliers from the mean, they were included with ‘susceptible’ mice into the PS group. Given the relatively small number of resilient mice, it is unlikely that susceptible versus resilient effects could have been found. Nevertheless, we believe that future studies using more mice may be able to uncover differences gene expression and sine density by susceptibility.
While there is substantial evidence demonstrating that the NAc plays a role in mediating depression-like behavior in adults [23,27,30,41,46,49], significantly less is known about its role in adolescence, a period of increased synaptic plasticity [50,51]. The NAc receives dopaminergic input from the VTA and, together, these brain areas form part of the mesolimbic dopamine system [52,53]. This circuit is widely believed to be responsible for mediating the rewarding effects of drugs of abuse, natural rewards, and more recently, in mediating responses to emotion-eliciting stimuli [17,54–56]. Here, we show that the NAc undergoes substantial changes in response to ES and PS exposure both in adolescence and adulthood. First, exposure to ES and PS differentially regulated ERK2 mRNA in adolescent and adult mice. ERK2 mRNA was significantly reduced following ES and PS in adolescent mice, but elevated in the NAc of adults. Surprisingly, we found that the decreases in ERK2 mRNA were accompanied by increases in ERK2 phosphorylation in the NAc of adolescent mice. And while both ES and PS influenced mRNA levels of ERK2, only PS influenced ERK2 phosphorylation. ERK2 is a protein kinase that must be phosphorylated to become active, and therefore, it is expected that changes in ERK2 expression might accompany changes in ERK2 phosphorylation. However, this is not always the case [57,58]. The relationship between activity of signaling proteins and the expression of their mRNA can be complex, and it is likely that there are several factors controlling expression of ERK2 beyond its activation. The stress-induced decreases in ERK2 mRNA and increased phosphorylation were both robust and unique to adolescence, making it particularly interesting. The mechanism(s) underlying this effect is unknown. Given the role ERK2 play in drug abuse processes [59–61] and that drugs of abuse and stress can cross-sensitize [62,63], we expected that ES or PS exposure would have resulted in increased ERK2 expression and activity in the adolescent mice. This finding, in particular, is surprising because we have reported increases in ERK2 phosphorylation in the VTA of adult rodents exposed to chronic unpredictable stress [21]. The paradoxical finding between adolescent and adult mice is intriguing. It is likely that these effects may be due, at least in part, to uniquely age-dependent responses to stress often seen through periods prior to adulthood [33,34,64,65].
Exposure to ES and PS reduced expression of ΔfosB mRNA in both adolescent and adult mice. This finding is surprising, since one might expect to see an increase in ΔfosB, especially given previous reports that exposure to social defeat and other stressors increase ΔfosB in the NAc [27,66–68]. Recently, it was demonstrated that ΔfosB may play a role in resilience to social defeat stress, rather than susceptibility: social isolation reduced ΔfosB levels in the NAc, thus rendering mice susceptible to stress, and lower levels of this protein were found in postmortem NAc tissue from depressed patients, whereas mice with a larger induction of ΔfosB, following exposure to social defeat, appeared more resilient than those with a smaller induction [27]. It is possible that because we did not look until twenty-four hours after the last stress session, levels of ΔfosB transcription have decreased to compensate for higher levels of stress-induced Fos transcription in the NAc. However, within this framework, one might still expect to see elevated levels of ΔfosB protein in the NAc, given its high level of stability [69]. Interestingly, we do see elevated levels of ΔfosB protein in the NAc. Which lends support to this hypothesis. Because of the relationship between ΔfosB and resilience, it is somewhat surprising to see increased levels of this transcription factor given the robust social avoidance demonstrated by our mice. However, given increased ERK2 phosphorylation, it is not surprising that ΔfosB, a downstream target of ERK2 {Hazzalin, 1997 #8}{Pavon, 2006 #9}, is increased. In addition, this increase could be because we did not distinguish between core and shell during dissection, as differential expression between these two NAc subregions is often found [27,67,70,71]. Future studies are clearly necessary to assess the specific features of this notion within the context of differential gene expression across the lifespan.
We also assessed potential changes in the expression of NAc CREB to gain a deeper understanding of the molecular changes taking place in ES- and PS-exposed mice. Here, we found decreased CREB phosphorylation in mice exposed to ES and PS as adolescents. It is not surprising that we find changes in CREB expression, since CREB is usually induced in the NAc in response to stress [23,24,29,30]. However, in cases of prolonged stress, such as social isolation, CREB is inhibited and induces an anxiogenic- and anhedonia-like state [30]. Although speculative, since these mice were exposed to a ten-day social defeat paradigm, it is possible that the chronic stress of social instability and constant threat could be acting on CREB in a similar way to prolonged mild stress. Another possibility is that our experimental manipulations result in inducible cAMP early repressor (ICER), a potent repressor of CRE-mediated transcription, thus serving as an important negative-feedback mechanism for shutting off CREB-induced transcription [72,73]. Because our biochemical analysis was performed 24 h after behavioral testing, it is plausible that CREB induction was already reversed at this time point. Within this context, CREB protein levels would likely be downregulated or brought back to baseline. However, given previous reports that social defeat increases CREB activity in the shell of the NAc [45], decreases in CREB signaling after ES or PS was unanticipated. The relationship between induction of CREB and functional outputs within the NAc is complex [23,24,74,75] and future studies should examine these possibilities.
Because changes in ERK2, ΔfosB, and CREB each can result in changes in synaptic plasticity [76–79], and because spine density changes are known to accompany adolescence {Hazzalin, 1997 #8}{Pavon, 2006 #9}, we were also interested in determining whether exposure to ES or PS would impact synaptic plasticity by assessing spine density within the NAc. Here, we show that exposure to ES or PS during adolescence does increase spine density in the NAc twenty-four hours after the last stressor. As a positive control, we also assessed spine density in adult-exposed mice. Not surprisingly, adult PS-, but not ES-, exposed mice had elevated spine density in the NAc [46,71,80]. Increased spine density after adolescent PS exposure was expected given previous reports showing that stress during postnatal development increase spine density within the NAc [81]. Interestingly, our results also show that exposure to ES during adolescence induced changes similar to that observed in the PS-exposed mice, indicating that ES is a potent stressor [13]. This suggests that the adolescent NAc undergoes substantial restructuring, even after more subtle stressors. Perhaps this is not unexpected, since the developing brain goes through substantial overproduction and pruning of synapses and relatively high levels of dendritic spine turnover [34,51]. Indeed, it has been hypothesized that different levels of synaptic turnover in discrete brain areas could reflect critical developmental periods for these brain regions [34,50,51]. Given that adolescence is a period of increased risk of initiating drug use, increased sensitivity to reward, and increased risk taking [37,82,83], it is plausible that the NAc could be undergoing a particularly sensitive period of turmoil during adolescence. This could explain why we see higher levels of spine plasticity in adolescents and could partially be responsible for adolescents’ increased sensitivity to stress. However, it is currently unknown whether exposure to stress increases different subtypes of spines. Due to inherent limitations surrounding the methodologies used, determination of spine subtype was unreliable. Future experiments will be needed to fully elucidate the effects of stress during adolescence on synaptogenesis of discrete spine types. Furthermore, this study did not differentiate between effects of stress on core versus shell. It is plausible, given reports of contrasting functioning between these NAc anatomical distinctions, that there may be different effects in these two NAc subregions. Nevertheless, these data suggest that considerable synaptic plasticity is taking place in the NAc, and future studies will be aimed at determining potential unique effects of ES and PS on core versus shell of the adolescent NAc.
In summary, we show that exposure to ES or PS induces different neurobiological effects in the NAc depending on the developmental stage of the mice. Adolescent and adult male mice exposed to ES or PS demonstrated aberrant behavioral reactivity to social stimuli, altered NAc gene expression, protein phosphorylation, and dendritic spine plasticity. Importantly, we show that mice that witness, but do not physically experience, stress have nearly identical changes as those subjected to PS. Taken together, exposure to ES and PS are potent stressors in adolescent and adult mice capable of disrupting the integrity of the NAc. Ongoing studies are assessing whether these stress-induced biochemical changes are long lasting, and how they may affect functional behavioral outputs.
Acknowledgments
This work was supported by grant R01DA026854 from the National Institute on Drug Abuse and Planning Grant from Florida State University. BL Warren was supported by a Neuroscience Fellowship from Florida State University and a training grant T32MH093311 from the National Institute of Mental Health. LF Alcantara was supported by a McKnight Fellowship from the Florida Education Fund.
References
- 1.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JE, Gotlib IH. Lifetime episodes of dysphoria: Gender, early childhood loss and personality. Br J Clin Psychol. 1997;36 (Pt 2):195–208. doi: 10.1111/j.2044-8260.1997.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilmer WS, McKinney WT. Early experience and depressive disorders: Human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 4.Anda RF, Brown DW, Felitti VJ, Dube SR, Giles WH. Adverse childhood experiences and prescription drug use in a cohort study of adult hmo patients. BMC Public Health. 2008;8:198. doi: 10.1186/1471-2458-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: Relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 6.Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, Giles WH. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28:430–438. doi: 10.1016/j.amepre.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 8.Rees CA. Understanding emotional abuse. Archives of disease in childhood. 2010;95:59–67. doi: 10.1136/adc.2008.143156. [DOI] [PubMed] [Google Scholar]
- 9.Dubowitz H, Newton RR, Litrownik AJ, Lewis T, Briggs EC, Thompson R, English D, Lee LC, Feerick MM. Examination of a conceptual model of child neglect. Child Maltreat. 2005;10:173–189. doi: 10.1177/1077559505275014. [DOI] [PubMed] [Google Scholar]
- 10.Hornor G. Emotional maltreatment. Journal of pediatric health care: official publication of National Association of Pediatric Nurse Associates & Practitioners. 2012;26:436–442. doi: 10.1016/j.pedhc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Wright MO, Crawford E, Del Castillo D. Childhood emotional maltreatment and later psychological distress among college students: The mediating role of maladaptive schemas. Child Abuse Negl. 2009;33:59–68. doi: 10.1016/j.chiabu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolaños-Guzmán CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF. Negative reinforcement in drug addiction: The darkness within. Current opinion in neurobiology. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Russo SJ, Bolaños CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. Irs2-akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- 17.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 20.Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward-and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem. 2005;93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- 23.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. Creb activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of creb. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltafosb in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA, Nestler EJ. Serum response factor promotes resilience to chronic social stress through the induction of deltafosb. J Neurosci. 2010;30:14585–14592. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iñiguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolaños CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. Deltafosb in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iñiguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños-Guzmán CA. The influence of deltafosb in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated camp response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ. Creb regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poo MM. Neurotrophins as synaptic modulators. Nature reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 32.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 36.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 37.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 38.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Council NR. Guideliness for the care and use of mammals in neuroscience and behavioral research. Washingnton: National Academy Press; 2003. [PubMed] [Google Scholar]
- 40.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolaños CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of bdnf in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. Akt signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iñiguez SD, Charntikov S, Baella SA, Herbert MS, Bolaños-Guzmán CA, Crawford CA. Post-training cocaine exposure facilitates spatial memory consolidation in c57bl/6 mice. Hippocampus. 2011;21 doi: 10.1002/hipo.20941. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibb R, Kolb B. A method for vibratome sectioning of golgi-cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 49.Yadid G, Overstreet DH, Zangen A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001;896:43–47. doi: 10.1016/s0006-8993(00)03248-0. [DOI] [PubMed] [Google Scholar]
- 50.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nature reviews Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 53.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 54.Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: A neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 57.Mehra A, Lee KH, Hatzimanikatis V. Insights into the relation between mrna and protein expression patterns: I. Theoretical considerations. Biotechnology and bioengineering. 2003;84:822–833. doi: 10.1002/bit.10860. [DOI] [PubMed] [Google Scholar]
- 58.Lee PS, Shaw LB, Choe LH, Mehra A, Hatzimanikatis V, Lee KH. Insights into the relation between mrna and protein expression patterns: Ii. Experimental observations in escherichia coli. Biotechnology and bioengineering. 2003;84:834–841. doi: 10.1002/bit.10841. [DOI] [PubMed] [Google Scholar]
- 59.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of erk in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of erk pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños-Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- 65.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 66.Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP., Jr Intermittent social defeat stress enhances mesocorticolimbic deltafosb/bdnf co-expression and persistently activates corticotegmental neurons: Implication for vulnerability to psychostimulants. Neuroscience. 2012;212:38–48. doi: 10.1016/j.neuroscience.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltafosb in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP., Jr Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: Time course of mu-opioid receptor mrna and fosb/deltafosb immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews Neuroscience. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, Dinieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. Deltafosb induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee S, Koob G, Turecki G, Neve R, Thomas M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feedforward loop involving deltafosb and calcium/calmodulin-dependent protein kinase ii in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling camp signaling to transcription in the liver: Pivotal role of creb and crem. Experimental cell research. 2002;275:143–154. doi: 10.1006/excr.2002.5491. [DOI] [PubMed] [Google Scholar]
- 73.Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and camp-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25:4073–4081. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible camp early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrot M, Wallace DL, Bolaños CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, Nestler EJ. Regulation of anxiety and initiation of sexual behavior by creb in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patterson MA, Szatmari EM, Yasuda R. Ampa receptors are exocytosed in stimulated spines and adjacent dendrites in a ras-erk-dependent manner during long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. Fosb differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with deltafosb as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki S, Zhou H, Neumaier JF, Pham TA. Opposing functions of creb and mkk1 synergistically regulate the geometry of dendritic spines in visual cortex. J Comp Neurol. 2007;503:605–617. doi: 10.1002/cne.21424. [DOI] [PubMed] [Google Scholar]
- 80.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic regulation of rac1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 82.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 83.Andersen SL, Navalta CP. Altering the course of neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]