Abstract
HTLV-1 is a human retrovirus that is associated with the neuroinflammatory disorder HTLV-1 associated myelopathy/ tropical spastic paraparesis (HAM/TSP). In these patients, HTLV-1 is primarily found in the CD4+CD25+ T cell subset (Regulatory T cells:Tregs), which is responsible for peripheral immune tolerance and is known to be dysfunctional in HAM/TSP. Recent evidence suggests that FoxP3 expression and function is determined epigenetically through DNA demethylation in the Treg-specific demethylated region (TSDR). We analyzed the methylation of the TSDR in PBMCs, CD4+ T cells, and CD4+CD25+ T cells from normal healthy donors (NDs) and HAM/TSP patients. We demonstrated that there is decreased demethylation in analyzed PBMCs and CD4+CD25+ T cells from HAM/TSP patients as compared to NDs. Furthermore, decreased TSDR demethylation was associated with decreased functional suppression by Tregs. Additionally, increased HTLV-1 Tax expression in HAM/TSP PBMC culture correlated with a concomitant decline in FoxP3 TSDR demethylation. Overall, we suggest that HTLV-1 infection decreases Treg functional suppressive capacity in HAM/TSP through modification of FoxP3 TSDR demethylation and that dysregulated Treg function may contribute to HAM/TSP disease pathogenesis.
Keywords: Regulatory T cells, HTLV-1, Treg specific demethylation region (TSDR), epigenetic regulation, suppressive capacity, demethylation
Introduction
Human T cell Lymphotropic Virus-1 (HTLV-1) is a human retrovirus initially isolated in 1980 from a patient with a cutaneous T cell lymphoma (1). It is estimated that 15-20 million people worldwide are infected, though the vast majority will remain asymptomatic carriers(2, 3). Approximately 0.25-4% will develop HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) with another 1-5% developing Adult T cell leukemia/lymphoma (ATL/L)(2). HAM/TSP is a neuroinflammatory disorder characterized by perivascular inflammatory infiltration in the brain and spinal cord (4). Activated cytotoxic T cells (CD8+ T cells) and the production of inflammatory cytokines, including IFN-γ and TNF-α, have been associated with damage to the central nervous system (CNS) (5), eventually leading to urinary and bowel incontinence, spastic limb paraparesis, parasthesias and ataxia manifesting after a long asymptomatic phase of infection (6). In all HTLV-1 infected individuals the primary viral reservoir is CD4+CD25+ T cells (7), a subset of which is comprised of regulatory T cells (Tregs). Given that Treg dysfunction has been reported in HAM/TSP patients (8), proper characterization of Tregs and their functional abnormalities may provide an assessment of the immune dysregulation observed in HAM/TSP patients and serve as a valuable biomarker in clinical trials.
Tregs are essential in maintaining peripheral immune tolerance through their ability to actively suppress auto-reactive T cells and other inflammatory immune responses (9). They have been implicated in cancer, infectious disease, autoimmunity, transplant medicine and allergy, but their characterization and isolation has proven to be complex (10-12). Although the cell surface markers CD4 and CD25 are routinely used for their isolation, in humans the CD4+CD25+subset also includes activated T cells. Notably, only the top 1-2% of these cells (CD4+CD25hi) is considered to be functionally suppressive (13), and this frequency varies physiologically, especially with increased age (14). Several additional markers used to characterize Tregs in healthy donors, including GITR, CD127, and CTLA4, are unreliable here due to their alteration by HTLV-1 related activation (15, 16).
FoxP3 was first identified as a specific Treg marker in the context of the genetic disorder Immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) in which the gene is mutated (17). IPEX patients develop life-threatening autoimmune complications due to a defect in Tregs (18). Furthermore, when CD4+CD25- T cells (Teffs) were induced to express FoxP3, they acquired a Treg phenotype indicating that FoxP3 is a Treg lineage specific factor (19). However, in the human immune system, the FoxP3 expressing subset includes many cell types (i.e natural, induced, naive, memory, etc.) with different functions and FoxP3 expression levels (20). Additionally, there is up-regulation of FoxP3 transiently upon activation of T cells (21). Therefore, in inflammatory disorders such as HAM/TSP, Foxp3 expression may not be a reliable marker of Tregs.
Recent research has highlighted the importance of DNA methylation in the epigenetic control of Foxp3 gene expression (22, 23). Studies have shown that selective demethylation of a conserved CpG island within the Foxp3 locus termed the Treg-specific demethylated region (TSDR) leads to stable Foxp3 expression and defines thymic-derived natural Tregs (24). On the other hand, activated T cells and peripherally-induced Tregs (iTregs) exhibit a variably methylated FoxP3 TSDR (25). Importantly, stable expression of FoxP3 through demethylation of the TSDR is necessary for Treg suppression (26).
We examined levels of TSDR demethylation in PBMCs, CD4+ T cells and CD4+CD25+ T cells obtained from normal healthy donors (NDs) and HAM/TSP patients. The results demonstrated decreased demethylation in the FoxP3 TSDR of HAM/TSP patients as compared to NDs that correlates with the decreased suppressive capacity of CD4+CD25+ T cells in these patients. The lower levels of demethylation seen in the CD4+CD25+ subset of our HAM/TSP patients may also serve as an indicator of disease progression and suggests that a virus or viral gene may contribute to dysregulation of Tregs.
Materials and Methods
Patient samples
A total of 10 ND PBMCs and 9 HAM/TSP PBMCs samples were used for this study. An additional 3 ND PBMC samples and 6 HAM/TSP samples were used for 24h PBMC culture. ND ranged from 28-64 years old while HAM/TSP patients ranged from 31-70 years old. PBMCs were isolated by Ficoll-Hypaque (Lonza, Walkersville, MD) centrifugation, and were cryopreserved in liquid nitrogen until use. All NDs were noted to be healthy and HTLV-1 negative. The study was reviewed and approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. Informed consent was written and obtained from each subject in accordance with the Declaration of Helsinki.
Cell lines
HTLV-1-infected human cell lines HUT102 and MT2 and the uninfected cell lines Jurkat and MOLT3 were used. All the cells were cultured in RPMI 1640 supplemented with 10% FBS, 100U/mL penicillin, 100μg/mL streptomycin sulfate, and 2mM L-glutamine in 5%CO2 incubator at 37°C.
DNA isolation
Total DNA was isolated from cell lines or cells obtained from NDs and HAM/TSP patients using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD). All samples were incubated with RNAse A at 37°C for 30min to ensure removal of all RNA.
Bisulfite conversion
Due to the harshness of the reaction, at least 1μg of DNA was utilized when possible. All samples were initially cut with Fast Digest EcoRI (Fermentas, Glen Burnie, MD). Bisulfite conversions were completed using the Cells-to-CpG kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
Quantitative PCR
Bisulfite converted DNA was amplified in two separate reactions on a ViiA7 thermocycler (Applied Biosystems, Foster City, CA). One reaction amplied demethylated FoxP3 and the other amplified methylated FoxP3 using primers and probes directed against FoxP3 intron1(27). The reactions occurred in a total volume of 20 μl with 5 μl of bisulfite converted DNA. The thermal cycler conditions were as follows: 50°C for 2 min, 95°C for 10min, 40 cycles at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing and extension), and 25°C for 2 min. The level of demethylation was calculated as published previously (27) and values from female patients were doubled to account for 2 X chromosomes.
Flow cytometry
For analysis of CD4+ T cell population, cells were stained with CD3-APC-Cy7, CD4-PE-Cy7, and CD25-PE (BD Bioscience, San Jose, CA) and then fixed with Fixation Buffer (eBioscience, San Diego, CA) following their supplied protocol. After washing the cells with permiabilization buffer (eBioscience), FoxP3 antibody (236A/E7; eBioscience) was added to cells for the intracellular staining. Monoclonal isotype controls were used for each Ab and set as the negative control. Flow cytometric analysis was performed using a LSRII (BD Biosciences). All data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
CD4+ CD25+ T cell enrichment
PBMCs were thawed and then enriched for CD4+ T cells using CD4+CD25+ Treg isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ T cells were then labeled with CD25-biotin beads and separated on a column for isolation of CD4+CD25+cells and CD4+CD25- cells. Purity was assessed by flow cytometry using an CD25-PE antibody (BD Biosciences clone MA251). The results in this study on levels of FoxP3 TSDR demethylation was adjusted (normalized) to the purity of the CD4+CD25+ T cell bead isolation from each patient (that ranged from 50.7-90.6% mean = 65.5 in HAM/TSP and 22.7-80.1% mean = 53.03 in HD, p=NS).
Suppression assays
Suppressive function of Tregs in ND and HAM/TSP patients was assessed by inhibitory effects of allogeneic cell proliferation. CD4+CD25+ T cells were magnetically isolated from 3 ND and 2 HAM/TSP patients. 2.5 M CFSE (Invitrogen Life Technologies, Carlsbad, CA) was integrated into CD4+CD25- T cells (Teffs) isolated from the same ND for each experiment. Teffs were added at 2x104 cells in 96 U-bottom microplates containing varying amounts of irradiated Tregs for final ratio of Treg:Teff of 0.25:1 to 1:1, 500ng/mL anti-CD3 HIT3a (BD Biosciences) and 5x104 cells of autologous irradiated ND PBMCs (used as feeder cells) in 5% Human AB serum in RPMI 1640 with 100U/mL penicillin, 100 μg/mL streptomycin sulfate, and 2mM L-glutamine. PBMC and Tregs were gamma irradiated to 3000rad using a Cs irradiation source. After culture for 3 days, cells were stained with CD3-APC, CD4-Alexa700, and CD-25PE (BD Biosciences) and then analyzed on LSRII for proliferation. Cells stained with monoclonal isotype Abs were used as negative controls.
Tax and HBZ mRNA quantification
Total RNA was extracted from PBMCs using RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. 85 ng of total RNA was converted into cDNA and amplified in a one step reaction using TaqMan® RNA-to-CtTM 1-Step Kit (Applied Biosystems) according to the manufacturer's instructions. Tax primer and probe sequences (28) or HBZ primer probe (29) were added to mRNA samples and amplified on a Viia7 (Applied Biosystems) thermocycler as follows: 48°C for 15 min, 95°C for 10min, and 45 cycles at 95°C for 15 s and 60°C for 1 min. HPRT primers and probe were added to mRNA for an assessment of RNA quantity and quality on samples in each run. MT-2 was used as a calibrator sample and the level of tax and HBZ mRNA expression was then calculated using the comparative CT method on ViiA 7 software.
Tax expression
6 HAM/TSP and 3 ND PBMCs were incubated at 37°C in RPMI 10%FBS for 24h to allow for peak expression of HTLV-1 Tax (30). Cells were then stained with CD3-Pacific Blue, CD4-PECy7, CD25-PE, CD8-PerCp5.5 (BD Biosciences) for cell surface staining. FoxP3-APC (eBioscience), and Lt-4-Alexa Flour® 488 (kindly provided by Dr. Tanaka) were added for intracellular staining according to the manufacturer's protocol. Cells were also stained with monoclonal isotype control Abs as negative controls and analyzed on LSRII for staining intensity. PBMCs were collected before and after culture to extract total DNA and then analyze FoxP3 TSDR demethylation.
Proviral load
Proviral load was determined from DNA using the same tax primers and probes mentioned previously (28) and amplified as a standard curve against TARL2 DNA standards. Relative proviral load was determined against actin quantity in the samples and run on a ViiA7 thermocycler as noted for quantitative PCR.
Statistical analysis
TSDR demethylation, frequency of CD4+CD25+T cells and FoxP3 expression in NDs and HAM/TSP patients were analyzed by the Student's unpaired t-test. Suppression assays were grouped and analyzed by Two-way Anova. Intersample and intrasample comparisons of Treg:Teff ratios were analyzed by the Student's unpaired t-test. A linear regression was performed to determine correlation between TSDR demethylation and %suppression and between the change in TSDR demehtylation and Tax expression in CD4+CD25+ T cells after culture. All statistical analyses were performed using Prism (GraphPad software). p-values <0.05% were considered significant.
Results
FoxP3 TSDR demethylation in HAM/TSP patients
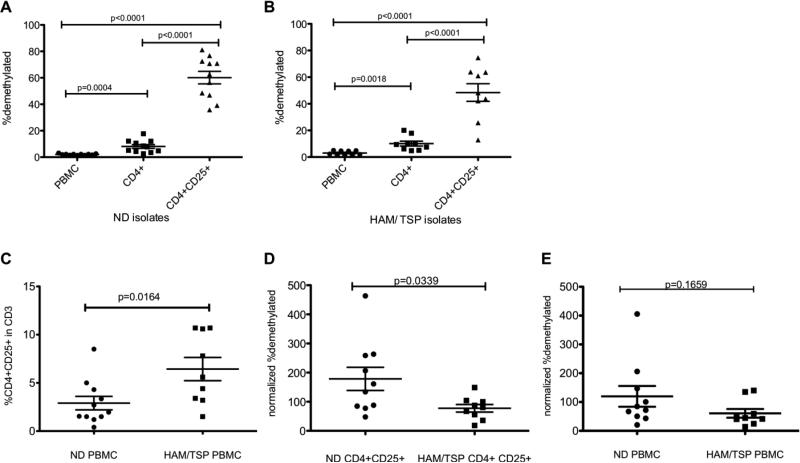
To examine TSDR demethylation in HAM/TSP primary T cells, DNA from whole PBMCs, CD4+ T cells, and CD4+CD25+T cells was isolated and compared to NDs for FoxP3 TSDR methylation status. TSDR demethylation was calculated as the percentage of DNA in FoxP3 intron 1 that amplified with primers directed against demethylated CpG islands in FoxP3 Intron 1 versus DNA that amplified with primers against methylated CpG islands in FoxP3 Intron 1 (Materials and Methods; (24, 31). In NDs, 2.066% (s.d.+/- 0.154%) of FoxP3 TSDR demethylation was detected in whole PBMCs (Fig. 1A). A significant increase in demethylation was detected in the total CD4+ T cell (8.097%) population and even higher in the isolated CD4+ CD25+ T cell subset (60.15%) compared to whole PBMCS (p=0.0004 and p< 0.0001, respectively; Fig. 1B). Similarly to ND, whole HAM/TSP PBMCs showed 3.022% (s.d.+/- 0.552) of FoxP3 TSDR demethylation with a statistically significant increase in demethylation in CD4+ T cell (10.11%) and CD4+ CD25+ T cell subsets (48.43%) compared to whole PBMCs (p=0.0018 and p<0.0001, respectively; Fig. 1B). Thus, the enrichment of CD4+CD25+ T cells from whole PBMC significantly increases the percentage of FoxP3 TSDR demethylation and is consistent with previous studies (32).
Fig. 1.
(A) % FoxP3 TSDR demethylation in ND PBMC (n=10), isolated CD4+ T cells, and isolated CD4+CD25+ T cells. (B) % FoxP3 TSDR demethylation in HAM/TSP PBMC (n=9), isolated CD4+ T cells, and isolated CD4+CD25+ T cells. The long horizontal bars represent the mean for each group while the shorter bars represent the standard deviation. (C) A comparison of the %CD4+CD25+ in CD3+ T cells of ND and HAM/TSP PBMC. (D) Normalized % FoxP3 TSDR demethylation in isolated CD4+CD25+ T cells of ND and HAM/TSP patients. % FoxP3 TSDR demethylation was normalized to the % CD4+CD25+. (E) Normalized % FoxP3 TSDR demethylation in PBMC of ND and HAM/TSP patients. % FoxP3 TSDR demethylation was normalized to the % CD4+CD25+.
Since the frequency of CD4+CD25+ T cells is known to be elevated in HAM/TSP patients compared to NDs (33), it was important to incorporate this when measuring FoxP3 TSDR methylation. As shown in Fig. 1C, indeed the frequency of CD4+CD25+ T cells was significantly higher in HAM/TSP patients than NDs (p=0.0164). There was no significant difference in the percentage of CD4+ T cells between the two groups (unpublished observations). Therefore, after normalization to the frequency of CD4+CD25+ T cells, HAM/TSP patients were found to have a statistically significant decreased demethylated percentage in isolated CD4+ CD25+ T cells as compared to NDs (p=0.0339; Fig. 1D). A similar trend was also observed in whole PBMCs although this relationship did not reach statistical significance (p=0.1659; Fig.1E) probably due to the low frequency of CD4+CD25+ cells in PBMC (Fig. 1C).
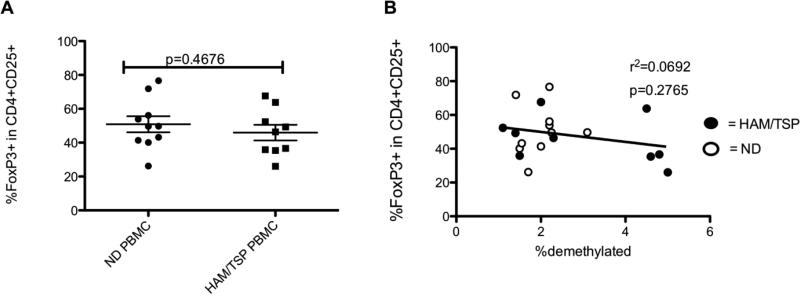
No correlation between the percent FoxP3+ cells and FoxP3 demethylation
Many investigators have relied upon FoxP3 by flow cytometry for characterization of Tregs (20). Although in NDs it appears to be a reliable marker, FoxP3 can also be increased upon T cell activation (21). FoxP3 mRNA and protein has been demonstrated to be decreased in HAM/TSP patients, although this was demonstrated in isolated T cell subsets (34). Interestingly, when the percentage of FoxP3+ cells in HAM/TSP PBMCs were compared by flow cytometry, CD4+CD25+ cells were shown to have equivalent FoxP3 percentage compared to NDs (p=0.4676; Fig. 2A). In particular, the percent FoxP3+ cells in PBMCs and the level of FoxP3 TSDR demethylation did not correlate in our experiments (p= 0.2765, r2= 0.0692; Fig. 2B). These results are likely due to transient expression of FoxP3 in activated T cells, which are increased in HAM/TSP patients compared to NDs (35). Therefore, the percent of FoxP3+ cells by flow analysis does not correlate with FoxP3 TSDR demethylation in human PBMCs.
Fig. 2.
(A) % FoxP3+ cells in CD4+CD25+ T cells of ND and HAM/TSP patients. The long horizontal bars represent the mean for each group while the shorter bars represent the standard deviation. (B) No correlation of the %FoxP3 TSDR demethylation in PBMCs with the % FoxP3+ cells in CD4+CD25+ T cells of ND (opened circles) and HAM/TSP patients (closed circles).
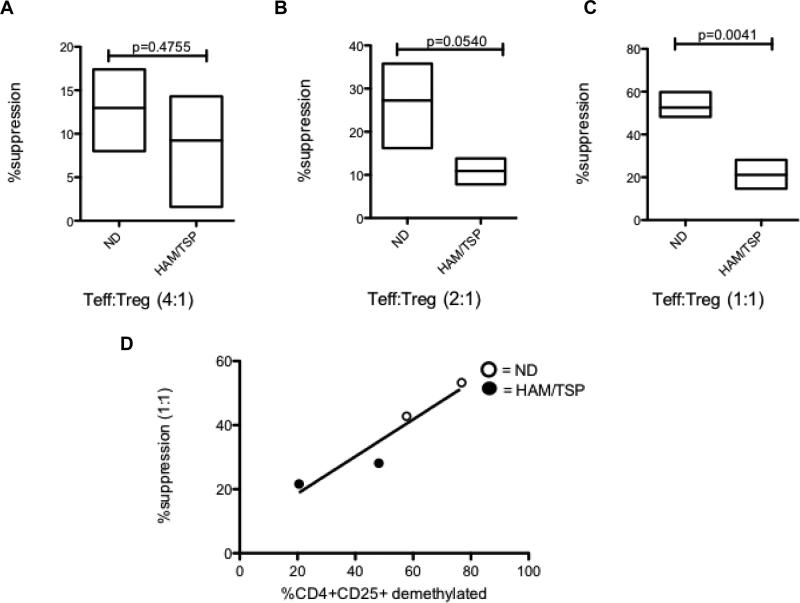
Correlation of FoxP3 TSDR demethylation with Treg suppressive function
The level of FoxP3 TSDR demethylation in isolated CD4+CD25+ T cells varied widely between the HAM/TSP patients included in our study. To determine if there was a correlation between methylation status of the FoxP3 TSDR and functional suppression of isolated CD4+CD25+ T cells, allogeneic Treg suppression assays were performed. NDs with previously confirmed demethylated TSDRs were compared with HAM/TSP patients with confirmed methylated TSDRs. As a control, ND CD4+CD25+ T cells were able to suppress allogeneic activated ND CD4+CD25- T cells (Teffs) and this suppressive capacity increased depending on Treg:Teff ratio (Fig. 3A-C). In contrast, HAM/TSP patients CD4+ CD25+ T cells suppressed to a significantly lesser extent, especially at high Treg:Teff (1:1) (p= 0.0041;Fig. 3C). A two-way ANOVA analysis demonstrated a significant difference in suppression when assessing both patient type (HAM/TSP vs. ND) and Treg:Teff ratio. In addition, there was a trend (with 2 normal donors and 2 HAM/TSP patients) for the level of demethylation in the FoxP3 TSDR to positively associate with the level of suppression. These results demonstrated that the decrease of Treg suppressive function in HAM/TSP patients may be associated with the demethylation status of FoxP3 TSDR.
Fig. 3.
Lymphoproliferation suppression assays were set up and ND (n=3) or HAM/TSP (n=2) CD4+CD25+ T cells were added at different ratios of Treg:Teff = 0.25:1 (A), 0.5:1 (B) and 1:1 (C). The % suppressions of Treg were normalized to Teff proliferation without Treg. (D) Correlation between CD4+CD25+ TSDR % demethylation and suppressive capacity as determined in lymphoproliferation suppression assays at Treg:Teff ratio of 1:1.
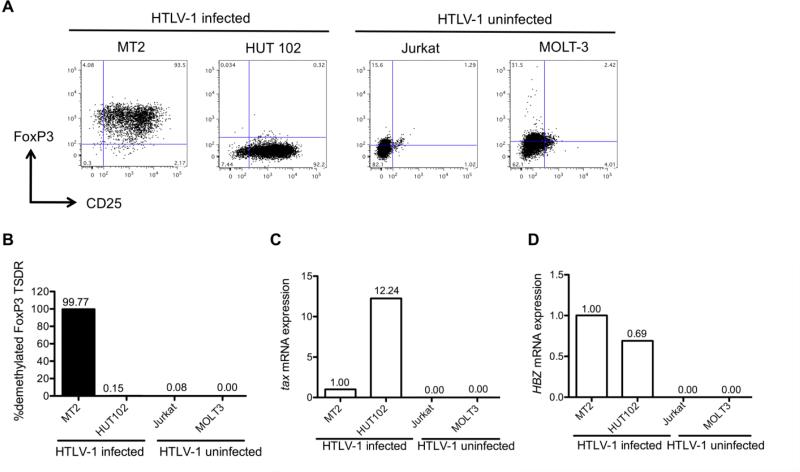
FoxP3 demethylation in HTLV-1 CD4+T cell lines
Initially, HTLV-1-infected and uninfected cell lines were characterized for expression of virus specific gene products, Treg markers (CD25 and FoxP3) and the demethylation levels of the FoxP3 TSDR.
Both HTLV-1-infected cell lines MT2 and HUT102 express CD25 (95.7%, 96.5% respectively) (Fig. 4A) while the HTLV-1-uninfected cell lines Jurkat and MOLT-3 were CD25 negative (Fig. 4A). Only MT2 expressed a high percentage of FoxP3+ cells in the CD25+ T cell population (97.6%) (Fig. 4A). In contrast, HUT102 had very few CD25+ FoxP3+cells (1.3%) (Fig. 4A). The two uninfected CD25- T cell lines had low levels of FoxP3 positivity (Fig. 4A) consistent with previous reports (36).
Fig.4.
HTLV-1-infected and HTLV-1-uninfected cell line profiles. (A) Flow cytometry profiles of HTLV-1-infected and HTLV-1-uninfected cell lines. The x-axis denotes CD25 expression and the y-axis denotes FoxP3 expression. (B) FoxP3 TSDR demethylation analysis of HTLV-1-infected and HTLV-1-uninfected cell lines. (C) Tax mRNA expression in the cell lines by RTPCR. The data were calculated as relative amount to MT2 (1.00). (D) HBZ mRNA expression in the cell lines by RT-PCR. The data were calculated as relative amount to MT2 (1.00).
Given the variation of CD25 and the percent FoxP3 positivity in these cell lines, the level of FoxP3 TSDR demethylation was determined in each. The MT2 cell line, which had the highest percentage of FoxP3 by flow cytometry, had a FoxP3 TSDR that was completely demethylated, displaying similar levels of demethylation to that reported in natural thymus-derived Tregs from healthy donors (95-99.98%) (Fig. 4B) (37). By contrast, HUT102 was completely methylated (Fig. 4B). Similarly, the two uninfected T cell lines that had low FoxP3 positivity by flow cytometry also displayed complete methylation.
Since HTLV-1 gene products including Tax and HTLV-1 basic leucine zipper factor (HBZ) have previously been suggested to interact with FoxP3 (38, 39), we next determined if FoxP3 methylation status was associated with expression of these viral proteins. Since MT-2 expressed both tax and HBZ mRNA, all the data was normalized to tax and HBZ mRNA expressions in MT-2 as 1.00. When compared to MT-2, HUT102 expressed more tax mRNA (12.24 times) but had lower HBZ mRNA levels (Fig. 4C and 4D), as previously reported (40). As expected, tax and HBZ mRNA were not detected in Jurkat and MOLT3, the two HTLV-1-uninfected cell lines. Thus, tax mRNA expression levels were elevated in the HTLV-1-infected cell line that had complete FoxP3 TSDR methylation (HUT 102, Fig. 4B). By contrast, the MT2 cell line which had lower levels of tax expression had a completely demethylated FoxP3 TSDR. Since both HTLV-1 infected T cell lines expressed HTLV-1 HBZ, there appeared to be no relationship with FoxP3 TSDR methylation.
Negative correlation of FoxP3 TSDR demethylation with HTLV-1 Tax expression
HTLV-1 Tax is a pleiotropic viral protein that transactivates a number of cellular pathways, directly regulates interleukin production, promotes IL-2 independent growth in infected T cells, and impacts gene expression through chromatin remodeling (41). HAM/TSP patients are known to express more of this viral gene than asymptomatic carriers (AC) (42). Moreover, Tax has previously been shown to decrease FoxP3 levels after transfection into ND CD4+CD25+ T cells (8). We therefore asked if there was also a correlation with increased HTLV-1 Tax expression in HAM/TSP CD4+CD25+ T cells and decreased FoxP3 TSDR demethylation.
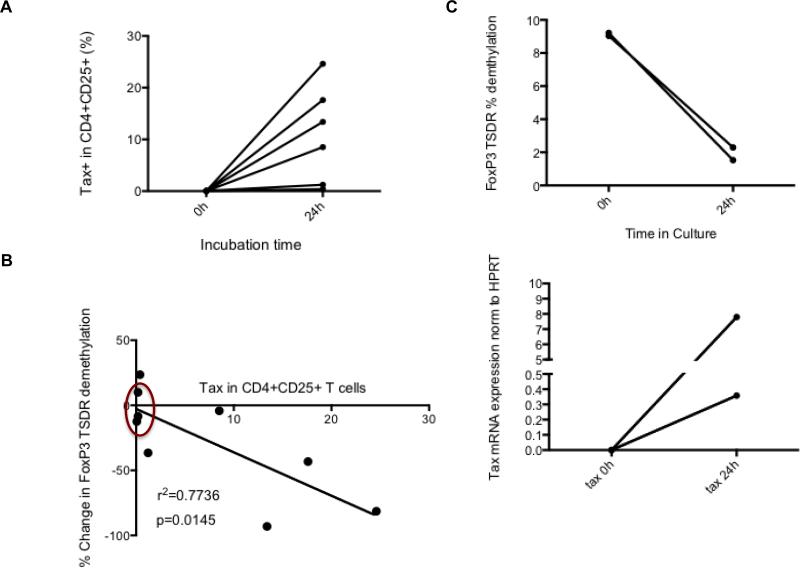
Freshly thawed PBMCs do not express HTLV-1 Tax at baseline by flow cytometry (Fig. 5A), however, after short-term culture (24 hours), variable levels of Tax expression were observed, particularly in the CD4+CD25+ T cell subset (Fig. 5A). Proof of concept was performed by observing FoxP3 demethylation before and after culture in isolated CD4+T cells from 2 HAM/TSP patients. Both showed a significant decline in FoxP3 TSDR demethylation after culture. Increasing levels of HTLV-1 Tax in CD4+CD25+ HAM/TSP T cells had a strong linear correlation (Coefficient of determination r2= 0.7736, p=0.0145; Fig. 5B) with a decline in the FoxP3 TSDR demethylation from HAM/TSP PBMC. This decreased FoxP3 TSDR demethylation in HAM/TSP also correlated with the levels of HTLV-1 tax mRNA expression in PBMC after 24 hour culture (unpublished observations). As controls, there were no changes in FoxP3 TSDR demethylation in short-term cultures of ND PBMCs that were HTLV-1 Tax negative (Fig. 5B, circled points). To assess for the possibility that the decline in FoxP3 TSDR demethylation may be attributed to Treg death via CD8+ cytotoxic T lymphocyte (CTL) killing, two of our patient samples were depleted of CD8+ T cells by bead isolation. As shown in Figure 5C, in the absence of CD8+ T cells, both patients still demonstrated a large decline in FoxP3 TSDR demethylation post-culture that mirrored a concomitant increase in tax mRNA expression (Fig. 5C).
Fig. 5.
Increased Tax expression correlates with reduced FoxP3 TSDR demethylation. (A) Tax protein expression in CD4+CD25+ T cells of HAM/TSP patients before and after culture. PBMCs of HAM/TSP patients (n=6) were cultured for 24 hours. (B) Correlation of the Tax protein expression in CD4+CD25+ T cells of HAM/TSP patients with the change in FoxP3 TSDR demethylation before and after culture for 24 hours. ND samples are circled in red. Coefficient of determination r2= 0. 7736 (C) %FoxP3 TSDR demethylation and tax mRNA expression in isolated HAM/TSP CD4+ T cells ex vivo.
Discussion
Immune dysregulation is a prominent feature in HTLV-1-associated disorders, although how the virus contributes to this outcome remains incompletely understood. Of particular interest, HAM/TSP patients display an activated inflammatory immune response associated with a decreased suppressive capacity in Treg cells (43). In contrast, it has been demonstrated that the immunocompromised state seen in ATLL patients may be a result of clonal expansion of a Treg precursor, suppressing anti-tumor immunity (44). Flow cytometry, has been used to characterize these Tregs, based on a number of cell surface markers and intracellular expression of FoxP3. However many of these same markers, including FoxP3, are also seen on activated T cells (21). Furthermore, the use of FoxP3 as a Treg lineage specific marker has been difficult due to the heterogeneity in phenotype and function within the human FoxP3 subset (45).
FoxP3 TSDR methylation status has been suggested to be a better indicator of sustained Treg suppressive function, particularly in inflammatory environments (46). Recently, it has been demonstrated that FoxP3 TSDR demethylation can be characterized from whole blood PBMCs if used in conjunction with normalization to a specific T cell population (47). We took a similar approach by enumerating demethylation of the FoxP3 TSDR in i) PBMCs; ii) CD4+ T cells; iii) CD4+ CD25+ T cells of HAM/TSP patients normalized to the activated CD4+ T cell population as determined by flow cytometry. Since HAM/TSP patients have a higher percentage of CD4+CD25+ T cells (Fig. 1C) reflective of the activated T cell population known to be elevated in this disease (33, 35), it is informative to normalize the levels of FoxP3 TSDR demethylation to the percentage of CD4+ CD25+ T cells. After normalization, there was, a significant decrease in the percentage of FoxP3 TSDR demethylation observed in HAM/TSP patient CD4+CD25+ T cells compared to ND. In addition, attempts to more stringently select Treg cells and eliminate activated T cells by using CD4+CD25+CD127- bead isolation did not increase the FoxP3 TSDR demethylation of isolated cells compared with the Foxp3 TSDR demethylation calculated from cells isolated with CD4+CD25+ beads (unpublished observation). This suggested that levels of TSDR demethylation in purified CD+CD25+ T cells and normalization to CD25 expression is a reasonable method to purify Tregs.
The ultimate indicator of Treg function is the suppressive capacity of Tregs as determined by an in vitro functional assay such as lymphoproliferation (48). Indeed, in HAM/TSP CD4+CD25+ T cells with lower levels of FoxP3 TSDR demethylation we observed substantially reduced Treg suppression compared to ND CD4+CD25+ T cells with higher levels of FoxP3 TSDR demethylation (Fig. 4) and a trend of an association with the level of FoxP3 TSDR demethylation with Treg suppression (Fig 3). While additional patients and controls will be required to confirm this observation, these results suggest that FoxP3 TSDR demethylation may be a good surrogate for Treg functional suppressive capacity. In addition, it would be of interest to compare FoxP3 levels, as assessed by FoxP3 protein expression or MFI analysis by flow, with Treg suppression. This dysregulation of Tregs may foster a proinflammatory environment that contributes to the pathogenesis of HAM/TSP (38).
The results in this study are consistent with previous reports demonstrating decreased Treg function in HAM/TSP (8) though it had been unclear if this is due to a decreased Treg population, Treg function or both. Since it had been shown that the expression of an HTLV-1 tax was associated with a decrease in FoxP3 expression (8) and that HTLV-1 Tax increases after short-term in vitro culture of HAM/TSP PBMC (30), we asked if there was also a correlation between the HTLV-1 proviral load and the observed decrease in FoxP3 TSDR demethylation. The observation of decreased FoxP3 demethylation in vitro concurrently with increasing Tax expression led us to believe that these changes in demethylation are a direct consequence of HTLV-1 protein expression since Tax expression did not affect the population of CD4+CD25+ T cells after culture (unpublished observation). Furthermore, the reduction of FoxP3 demethylation in isolated CD4+ T cells (Fig. 5C) in the absence of CTLs suggests a direct effect of Tax on FoxP3 expression rather than CTL mediated lysis of the Treg population. We conclude that the observed dysregulation of Tregs in HAM/TSP is associated with a decrease in Treg function rather than Treg numbers and suggest that this inhibition of Treg function is mediated by the trans-activating properties of the HTLV-1 tax gene.
HAM/TSP patients are known to express higher levels of tax mRNA compared to asymptomatic carriers (42). Additionally, we have shown that the HUT102 cell line that had higher levels of tax expression compared to MT2 was associated with a completely methylated TSDR (Fig. 4). Collectively, these observations on the ex vivo association of reduced Fox P3 TSDR demethylation and high HTLV-1 tax expression coupled with the in vitro correlates of Tax expression with decreased demethylation supports the potential role for HTLV-1 tax in the dysregulation of the FoxP3 TSDR methylation in HAM/TSP.
Since we have shown that increased virus protein expression was associated with decreased FoxP3 TSDR demethylation, we asked if this also correlated with clinical outcome measures in HAM/TSP. Of the three patients with the largest decrease in FoxP3 TSDR demethylation (patients 1-3 in Table 1) two had high clinical disability scores (IPEC >13). Patients 1 and 2 also had rapid clinical deterioration and shorter duration of disease. Patient 3 had multiple brain lesions on MRI that have progressed since disease onset. Although proviral load correlated with HTLV-1 Tax expression after short-term culture (unpublished observation), it did not correlate with FoxP3 TSDR demethylation. This may be due to measurement of proviral load in whole PBMCs rather than in the main HTLV-1 peripheral reservoir, CD4+CD25+ T cells. However, if decreased FoxP3 TSDR demethylation is confirmed to be associated with poorer clinical outcome measures in larger cohorts of HAM/TSP patients, this would not only support dysregulation of Tregs as an immunopathological mechanisms in HTLV-1 neurologic disease (8, 38) but would also introduce potential therapeutic strategies that target epigenetic modification of Treg cells. Many studies have utilized demethylating agents such as 5-aza-2′-deoxycytydine (DAC) and procainamide to demethylate the TSDR in Teffs, thereby transforming them into functionally suppressive Tregs (49, 50). Similar agents such as 5-azacytidine and valproate have been explored in HTLV-1 infection but only for their potential anti-retroviral and anti-leukemic properties (51, 52). To date, no one has explored their use in restoring Treg function in HAM/TSP.
Table 1.
HAM/TSP patient clinical assessment
| HAM/TSP Patient |
Onset of symptoms |
Peripheral inflammatory involvement |
IPEC Disability Scale |
EDSS | AI | Progression | Tax expression in CD4+CD25+ after culture |
PVL |
|---|---|---|---|---|---|---|---|---|
| 1 | 6Y prior to sample collection | None | 15 | 4 | 4 | initial rapid decline, then slow | 17.6 | 3.70 |
| 2 | 4Y prior to sample collection | Multiple: bilateral uveitis, dermatitis. | 13 | UN | 12 | rapid progression, ongoing | 24.6 | 40.0 |
| 3 | 2Y prior to sample collection | None | 1, multiple MRI lesions | 1 | 0 | Relatively stable, cognitive decline | 13.4 | 25.2 |
| 4 | 2Y prior to sample collection | None | 12 | 6 | 4 | initial rapid decline , then slow progression | 1.23 | 14.0 |
| 5 | insidious course, unclear date of onset | None | UN | 4 | 2 | insidious, slow progression | 8.52 | 10.0 |
| 6 | 13Y prior to sample collection | Uveitis, eczema | 7 | 3.5 | UN | insidious, slow progression | 0.39 | 6.32 |
UN= unknown EDSS=Expanded disability status score IPEC= Instituta de Psquisa Clinica Evandro Chagas AI=Ambulatory Index PVL= proviral load
In summary, the results in this study support the observation that HAM/TSP patients have decreased demethylation in the FoxP3 TSDR that associated with reduced Treg function. These changes in the TSDR appear to be related to HTLV-1 Tax expression in vitro and might inform clinical status in HAM/TSP patients. FoxP3 TSDR methylation status may also serve as a good surrogate biomarker for Treg function in conjunction with other disease parameters in HTLV-1 infected patients. Moreover, these observations provide for a new avenue of therapeutic interventional strategies that may be beneficial to patients with HAM/TSP.
Acknowledgments
Special thanks to Mr. Matt McCormick for his contributions to the demethylation protocols developed for this study.
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 4.Izumo S. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP). Neuropathology : official journal of the Japanese Society of Neuropathology. 2010 doi: 10.1111/j.1440-1789.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 5.Barmak K, Harhaj EW, Wigdahl B. Mediators of central nervous system damage during the progression of human T-cell leukemia type I-associated myelopathy/tropical spastic paraparesis. Journal of neurovirology. 2003;9:522–529. doi: 10.1080/13550280390218689. [DOI] [PubMed] [Google Scholar]
- 6.Evangelou IE, Oh U, Massoud R, Jacobson S. HTLV-I-Associated Myelopathy/Tropical Spastic Paraparesis: Semiautomatic Quantification of Spinal Cord Atrophy from 3-Dimensional MR Images. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2012 doi: 10.1111/j.1552-6569.2011.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamano Y, Cohen CJ, Takenouchi N, Yao K, Tomaru U, Li HC, Reiter Y, Jacobson S. Increased expression of human T lymphocyte virus type I (HTLV-I) Tax11-19 peptide-human histocompatibility leukocyte antigen A*201 complexes on CD4+ CD25+ T Cells detected by peptide-specific, major histocompatibility complex-restricted antibodies in patients with HTLV-I-associated neurologic disease. The Journal of experimental medicine. 2004;199:1367–1377. doi: 10.1084/jem.20032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamano Y, Takenouchi N, Li HC, Tomaru U, Yao K, Grant CW, Maric DA, Jacobson S. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I-associated neuroimmunological disease. The Journal of clinical investigation. 2005;115:1361–1368. doi: 10.1172/JCI23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature immunology. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 10.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Zhou B, Li M, Deng Q, Wu X, Le X, Wu C, Larmonier N, Zhang W, Zhang H, Wang H, Katsanis E. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol. 2007;123:50–59. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Weiss VL, Lee TH, Jaffee EM, Armstrong TD. Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology. 2012;1:1191–1193. doi: 10.4161/onci.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 14.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clinical and experimental immunology. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez E, Cartier L, Rodriguez L, Alberti C, Valenzuela MA. In vivo fluctuation of Tax, Foxp3, CTLA-4, and GITR mRNA expression in CD4(+)CD25(+) T cells of patients with human T-lymphotropic virus type 1-associated myelopathy. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 2010;43:1109–1115. doi: 10.1590/s0100-879x2010007500107. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsson J, Barbosa HM, Jordan KA, Chapman JM, Brunialti MK, Neto WK, Nukui Y, Sabino EC, Chieia MA, Oliveira AS, Nixon DF, Kallas EG. The frequency of CD127low expressing CD4+CD25high T regulatory cells is inversely correlated with human T lymphotrophic virus type-1 (HTLV-1) proviral load in HTLV-1-infection and HTLV-1-associated myelopathy/tropical spastic paraparesis. BMC immunology. 2008;9:41. doi: 10.1186/1471-2172-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 18.van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clinical & developmental immunology. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loser K, Hansen W, Apelt J, Balkow S, Buer J, Beissert S. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene therapy. 2005;12:1294–1304. doi: 10.1038/sj.gt.3302567. [DOI] [PubMed] [Google Scholar]
- 20.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. European journal of immunology. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 22.Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, Ziegler SF, Huehn J, Pardoll DM, Vignali DA. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36:717–730. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toker A, Huehn J. To be or not to be a Treg cell: lineage decisions controlled by epigenetic mechanisms. Science signaling. 2011;4:pe4. doi: 10.1126/scisignal.2001783. [DOI] [PubMed] [Google Scholar]
- 25.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Turbachova I, Hamann A, Olek S, Huehn J. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. European journal of immunology. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 26.Polansky JK, Schreiber L, Thelemann C, Ludwig L, Kruger M, Baumgrass R, Cording S, Floess S, Hamann A, Huehn J. Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl) 2010;88:1029–1040. doi: 10.1007/s00109-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockis J, Fink W, Francois V, Connerotte T, de Smet C, Knoops L, van der Bruggen P, Boon T, Coulie PG, Lucas S. Comparison of stable human Treg and Th clones by transcriptional profiling. European journal of immunology. 2009;39:869–882. doi: 10.1002/eji.200838807. [DOI] [PubMed] [Google Scholar]
- 28.Oh U, Yamano Y, Mora CA, Ohayon J, Bagnato F, Butman JA, Dambrosia J, Leist TP, McFarland H, Jacobson S. Interferon-beta1a therapy in human T-lymphotropic virus type I-associated neurologic disease. Annals of neurology. 2005;57:526–534. doi: 10.1002/ana.20429. [DOI] [PubMed] [Google Scholar]
- 29.Enose-Akahata Y, Abrams A, Massoud R, Bialuk I, Johnson KR, Green PL, Maloney EM, Jacobson S. Humoral immune response to HTLV-1 basic leucine zipper factor (HBZ) in HTLV-1-infected individuals. Retrovirology. 2013;10:19. doi: 10.1186/1742-4690-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rende F, Cavallari I, Corradin A, Silic-Benussi M, Toulza F, Toffolo GM, Tanaka Y, Jacobson S, Taylor GP, D'Agostino DM, Bangham CR, Ciminale V. Kinetics and intracellular compartmentalization of HTLV-1 gene expression: nuclear retention of HBZ mRNAs. Blood. 2011;117:4855–4859. doi: 10.1182/blood-2010-11-316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin M, Sahin E, Koksoy S. Regulatory T cells in cancer: an overview and perspectives on cyclooxygenase-2 and Foxp3 DNA methylation. Human immunology. 2013;74:1061–1068. doi: 10.1016/j.humimm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Lluis A, Illi S, Layland L, Olek S, von Mutius E, Schaub B. T regulatory cells in cord blood--FOXP3 demethylation as reliable quantitative marker. PloS one. 2010;5:e13267. doi: 10.1371/journal.pone.0013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukae H, Kohno S, Morikawa N, Kadota J, Matsukura S, Hara K. Increase in T-cells bearing CD25 in bronchoalveolar lavage fluid from HAM/TSP patients and HTLV-I carriers. Microbiology and immunology. 1994;38:55–62. doi: 10.1111/j.1348-0421.1994.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 34.Oh U, Grant C, Griffith C, Fugo K, Takenouchi N, Jacobson S. Reduced Foxp3 protein expression is associated with inflammatory disease during human t lymphotropic virus type 1 Infection. The Journal of infectious diseases. 2006;193:1557–1566. doi: 10.1086/503874. [DOI] [PubMed] [Google Scholar]
- 35.Yamano Y, Araya N, Sato T, Utsunomiya A, Azakami K, Hasegawa D, Izumi T, Fujita H, Aratani S, Yagishita N, Fujii R, Nishioka K, Jacobson S, Nakajima T. Abnormally high levels of virus-infected IFN-gamma+ CCR4+ CD4+ CD25+ T cells in a retrovirus-associated neuroinflammatory disorder. PloS one. 2009;4:e6517. doi: 10.1371/journal.pone.0006517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. International immunology. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 37.Janson PC, Winerdal ME, Marits P, Thorn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PloS one. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant C, Oh U, Yao K, Yamano Y, Jacobson S. Dysregulation of TGF-beta signaling and regulatory and effector T-cell function in virus-induced neuroinflammatory disease. Blood. 2008;111:5601–5609. doi: 10.1182/blood-2007-11-123430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satou Y, Yasunaga J, Zhao T, Yoshida M, Miyazato P, Takai K, Shimizu K, Ohshima K, Green PL, Ohkura N, Yamaguchi T, Ono M, Sakaguchi S, Matsuoka M. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS pathogens. 2011;7:e1001274. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currer R, Van Duyne R, Jaworski E, Guendel I, Sampey G, Das R, Narayanan A, Kashanchi F. HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Frontiers in microbiology. 2012;3:406. doi: 10.3389/fmicb.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. Journal of neurovirology. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 43.Nishiura Y, Nakamura T, Ichinose K, Shirabe S, Tsujino A, Goto H, Furuya T, Nagataki S. Increased production of inflammatory cytokines in cultured CD4+ cells from patients with HTLV-I-associated myelopathy. The Tohoku journal of experimental medicine. 1996;179:227–233. doi: 10.1620/tjem.179.227. [DOI] [PubMed] [Google Scholar]
- 44.Yano H, Ishida T, Inagaki A, Ishii T, Kusumoto S, Komatsu H, Iida S, Utsunomiya A, Ueda R. Regulatory T-cell function of adult T-cell leukemia/lymphoma cells. International journal of cancer. Journal international du cancer. 2007;120:2052–2057. doi: 10.1002/ijc.22536. [DOI] [PubMed] [Google Scholar]
- 45.d'Hennezel E, Yurchenko E, Sgouroudis E, Hay V, Piccirillo CA. Single-cell analysis of the human T regulatory population uncovers functional heterogeneity and instability within FOXP3+ cells. J Immunol. 2011;186:6788–6797. doi: 10.4049/jimmunol.1100269. [DOI] [PubMed] [Google Scholar]
- 46.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. European journal of immunology. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 47.Barzaghi F, Passerini L, Gambineri E, Ciullini Mannurita S, Cornu T, Kang ES, Choe YH, Cancrini C, Corrente S, Ciccocioppo R, Cecconi M, Zuin G, Discepolo V, Sartirana C, Schmidtko J, Ikinciogullari A, Ambrosi A, Roncarolo MG, Olek S, Bacchetta R. Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. Journal of autoimmunity. 2012;38:49–58. doi: 10.1016/j.jaut.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ukena SN, Hopting M, Velaga S, Ivanyi P, Grosse J, Baron U, Ganser A, Franzke A. Isolation strategies of regulatory T cells for clinical trials: phenotype, function, stability, and expansion capacity. Experimental hematology. 2011;39:1152–1160. doi: 10.1016/j.exphem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Zheng Q, Xu Y, Liu Y, Zhang B, Li X, Guo F, Zhao Y. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J Mol Med (Berl) 2009;87:1191–1205. doi: 10.1007/s00109-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 50.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossio S, Hernandez-Campo P, Diez-Campelo M, Herrero-Sanchez C, Rodriguez-Serrano C, Santamaria C, Sanchez-Guijo FM, Del Canizo C, San Miguel JF. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94:975–983. doi: 10.3324/haematol.2008.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belrose G, Gross A, Olindo S, Lezin A, Dueymes M, Komla-Soukha I, Smadja D, Tanaka Y, Willems L, Mesnard JM, Peloponese JM, Jr., Cesaire R. Effects of valproate on Tax and HBZ expression in HTLV-1 and HAM/TSP T lymphocytes. Blood. 2011;118:2483–2491. doi: 10.1182/blood-2010-11-321364. [DOI] [PubMed] [Google Scholar]
- 52.Diamantopoulos PT, Michael M, Benopoulou O, Bazanis E, Tzeletas G, Meletis J, Vayopoulos G, Viniou NA. Antiretroviral activity of 5-azacytidine during treatment of a HTLV-1 positive myelodysplastic syndrome with autoimmune manifestations. Virology journal. 2012;9:1. doi: 10.1186/1743-422X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]