Abstract
Highly potent broadly neutralizing human monoclonal antibodies hold promise for HIV prophylaxis and treatment. We used the SCID-hu Thy/Liv and BLT humanized mouse models to study the efficacy of these antibodies, primarily PG16, against HIV-1 clade A, B, and C. PG16 targets a conserved epitope in the V1/V2 region of gp120 common to 70–80% of HIV-1 isolates from multiple clades and has extremely potent in vitro activity against HIVJR-CSF. PG16 was highly efficacious in SCID-hu mice as a single intraperitoneal administration the day before inoculation of R5-tropic HIV-1 directly into their Thy/Liv implants and demonstrated even greater efficacy if PG16 administration was continued after Thy/Liv implant HIV-1 infection. However, PG16 as monotherapy had no activity in humanized mice with established R5-tropic HIV-1 infection. These results provide evidence of tissue penetration of the antibodies, which could aid in their ability to prevent infection if virus crosses the mucosal barrier.
Introduction
Human monoclonal antibodies that potently neutralize a broad range of HIV isolates hold promise for the prevention of HIV infection. The anti-gp120 broadly neutralizing monoclonal antibodies 2G12 and b12 and anti-gp41 antibodies 4E10 and 2F5 block diverse HIV variants because they target conserved, functionally important Env epitopes (Muster et al., 1994; Roben et al., 1994; Sagar et al., 2012; Stiegler et al., 2001; Trkola et al., 1996). Importantly, passive transfer of these antibodies can protect against intravenous (Mascola et al., 1999) and mucosal (Burton et al., 2011; Hessell et al., 2009a; Hessell et al., 2009b; Hessell et al., 2010; Mascola et al., 2000; Parren et al., 2001) challenge in macaque models of simian/HIV (SHIV) infection. In recent years, several extraordinarily potent neutralizing antibodies with activity against a wide range of HIV clades have been discovered, including the somatically related antibodies PG9 and PG16 (Davenport et al., 2011; Pancera et al., 2010; Walker et al., 2009); VRC01 and VRC07 (Wu et al., 2010; Zhou et al., 2010); CH01-CH04 (Bonsignori et al., 2011); and 3BNC117, NIH45–46, PGV04, and PGT121 and PGT128 (Diskin et al., 2013; Diskin et al., 2011; Falkowska et al., 2012; Scheid et al., 2011; Walker et al., 2011; Wu et al., 2011). Sterilizing protection against vaginal mucosal SHIV challenge has been achieved in macaques with PGT121 (IC50 of 0.005 µg/ml against SHIVSF162P3) by passive intravenous transfer of as little as 0.2 mg/kg, corresponding to a “single-digit” serum concentration of 1.8 µg/ml at the time of virus challenge (Moldt et al., 2012).
Encouraged by the highly potent neutralizing activity of PG16 against HIVJR-CSF in vitro (IC50 of 0.001 µg/ml), we sought to determine whether PG16 would be effective as a prophylactic modality against HIV challenge in humanized SCID-hu Thy/Liv mice. PG16 targets the V1/V2 loop region at residues 160 and 162, corresponding to a potential N-linked glycosylation site that may form the PG16 epitope (McLellan et al., 2011; Pejchal et al., 2010; Walker et al., 2009). The crystal structure of the antigen-binding fragment (Fab) of PG16 revealed that the antibody is sulfated and has a unique complementarity determining region (CDR) H3 subdomain structure with a stable stalk mediating extensive H3 protrusion from the combining site and two interconnected loops (Pejchal et al., 2010).
The SCID-hu Thy/Liv mouse model of HIV infection is a useful platform for the preclinical evaluation of antiviral efficacy in vivo. The human thymus implant in these mice supports long-term differentiation of human T cells, and the model has been standardized and validated with four classes of licensed antiretrovirals for the evaluation of antiviral drugs against HIV (Rabin et al., 1996; Stoddart et al., 2007). One important advantage of SCID-hu Thy/Liv mice for studies of HIV prophylaxis is their high (essentially 100%) susceptibility to HIV infection after injection of the virus directly into the thymus/liver implant. In previously reported humanized mouse studies, b12 antibody completely protected hu-PBL-SCID mice from intraperitoneal (i.p.) challenge with HIVJR-CSF but only when administered at very high dosage levels (50 mg/kg) (Gauduin et al., 1997). We hypothesized that PG16 would protect against HIVJR-CSF infection at much lower dosage levels because it is >200 times more potent than b12 (IC50 of 0.001 versus 0.210 µg/ml) (Walker et al., 2009), and higher in vitro neutralization potency of PGT-121 against SHIVSF162P3 has been shown to translate into enhanced protection against virus challenge in macaques (Moldt et al., 2012). In addition to HIVJR-CSF, we assessed the prophylactic activity of PG16 against four other clade B and non-clade B viruses in SCID-hu Thy/Liv mice and also explored the potential for PG16 in treating established HIVJR-CSF infection.
Results
PG16 half-life in SCID-hu Thy/Liv mice
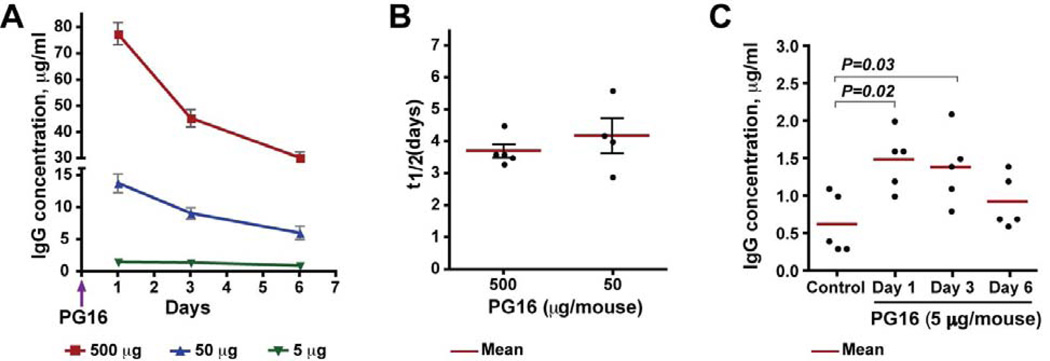
To determine the frequency of PG16 administration, we determined the half-life (t1/2) of PG16 in a separate pharmacokinetics study performed in uninfected SCID-hu Thy/Liv mice. Mice were treated with various doses of PG16 (5, 50, and 500 µg per mouse) by i.p. injection, and the level of human IgG was measured by ELISA in mouse serum collected 1, 3, and 6 days after treatment (Fig. 1A). When administered at the highest dose (500 µg), PG16 exhibited an initial rapid decline during the first 3 days, which could be the result of a combination of IgG concentration-dependent catabolism and distribution to extravascular spaces (Lobo et al., 2004). Consistent with this explanation, the more gradual decline from days 3 to 6 was similar for the 500-µg and 50-µg doses. The PG16 t1/2 was 3.7 days for the 500-µg dose and 4.2 days for the 50-µg dose (Fig. 1B). Importantly, the day after PG16 administration (corresponding to the time of HIV challenge in the protection studies), the mean level of human IgG in mouse circulation was 78 µg/ml, 14 µg/ml, and <1.5 µg/ml for 500 µg, 50 µg and 5 µg PG16, respectively (Fig. 1A).
Fig. 1. PG16 serum half-life after a single administration of 5, 50, or 500 µg in SCID-hu Thy/Liv mice.
(A) Mice were treated with PG16 by i.p. injection, and the level of human IgG was measured by pan-human IgG ELISA in mouse serum collected 1, 3, and 6 days after treatment. (B) PG16 mean t1/2 was 3.7 days for the 500-µg dose and 4.2 days for the 50-µg dose. (C) Untreated SCID-hu Thy/Liv mice (control) had low levels (mean of 0.6 µg/ml) of human IgG in their serum, so the t1/2 for the 5-µg PG16 dose could not be accurately determined. On the day after treatment with 5 µg PG16, the mean human IgG concentration was 1.5 µg/ml, a portion of which (0.3–1.1 µg/ml) was nonspecific human IgG, as demonstrated by the low levels in serum from untreated control SCID-hu Thy/Liv mice.
Untreated SCID-hu Thy/Liv mice (but not unengrafted CB17-scid mice) had low levels (mean of 0.6 µg/ml) of human IgG in their serum, likely resulting from the presence of small numbers of human B cells (0.2–2.5% of implant cells) in the implants of these mice (Namikawa, et al., 1990; Dittmer et al., 1999). On the day after treatment with 5 µg PG16, the mean human IgG concentration was 1.5 µg/ml, a portion of which (0.3–1.1 µg/ml) was nonspecific human IgG (Fig. 1C). Determination of the t1/2 for the 5 µg PG16 dose was therefore not possible because the pan-human IgG ELISA cannot discriminate PG16 from endogenously produced human IgG. Taking into account the results of the pharmacokinetics experiments, we elected to give the antibody i.p. to the mice three times per week (i.e., every other day) for studies involving repeated administration of PG16.
Selection of HIV for SCID-hu Thy/Liv mouse protection studies
Because protection in vivo is generally highly correlated with neutralization in vitro (Burton et al., 2011; Moldt et al., 2012), before initiating our studies we evaluated PG16 in both pseudovirus and PBMC neutralization assays against several HIV isolates that have been previously characterized in SCID-hu Thy/Liv mice (Stoddart, et. al., 2007 and unpublished observations). The data shown in Table 1 confirm the extreme sensitivity of HIVJR-CSF and lower susceptibility of HIVNL4-3 to PG16 (Walker et al., 2009). Of the other six HIV clade B isolates in our SCID-hu Thy/Liv panel, HIVJD was the most sensitive to PG16 neutralization with an IC50 of 0.008 µg/ml in the pseudovirus assay and 0.1 µg/ml with PBMC. The PG16 resistance exhibited by four of these six clade B HIV isolates in our SCID-hu panel (HIVPD, HIVEW, HIVEF, and HIVGV) was unexpected given the reported broadly neutralizing activity (~80% of 162 pseudoviruses with IC50 <50 µg/ml) of this antibody. We found that both HIVPD and HIVEW have the N160K mutation in gp120 (data not shown), which explains the PG16 resistance of these primary isolates. However, no known PG16-resistance mutations in the C1 through C2 regions of gp120 were identified for the other two PG16-resistant isolates (HIVEF and HIVGV). We also tested two non-clade B HIV isolates with the greatest reported sensitivity to PG16 neutralization in the Walker et al. (2009) pseudovirus assay, clade A HIV92/RW/008 (IC50 0.002 µg/ml) and clade C HIV98/IN/022 (IC50 0.003 µg/ml). Except for HIVNL4-3, the IC50 values for all viruses were substantially higher in PBMC than in the pseudovirus assay. The two assays have previously been reported to differ in assay sensitivity attributable to greater envelope spike density and stability of pseudoviruses compared to primary isolates, thus accounting for a higher sensitivity to neutralization by pseudoviruses (Fenyo et al., 2009; Heyndrickx et al., 2012).
Table 1.
HIV neutralization by P16 and PG9 in a pseudovirus reporter gene assay and with PBMC
| Virus | Coreceptor usage |
Antibody | Pseudovirus (luciferase) assay |
PBMC assay | ||
|---|---|---|---|---|---|---|
| IC50 (µg/ml) |
IC90 (µg/ml) |
IC50 (µg/ml) |
IC90 (µg/ml) |
|||
| HIVJR-CSF | R5 | PG16 | 0.001 | 0.012 | 0.049 | 0.249 |
| PG9 | 0.003 | 0.025 | NDc | ND | ||
| HIVJD | R5X4 | PG16 | 0.008 | 0.490 | 0.105 | 2.3 |
| PG9 | 0.074 | 2.3 | ND | ND | ||
| HIVPD (N160K) | X4 | PG16 | >50 | >50 | 2.3 | >10 |
| PG9 | >50 | >50 | ND | ND | ||
| HIVEW(N160K) | X4 | PG16 | >50 | >50 | >10 | >10 |
| PG9 | >50 | >50 | ND | ND | ||
| HIVF | X4 | PG16 | >50 | >50 | >10 | >10 |
| PG9 | >50 | >50 | ND | ND | ||
| HIVJW | R5 | PG16 | 0.12 | >50 | >10 | >10 |
| PG9 | 11 | >50 | ND | ND | ||
| HIVGV | X4 | PG16 | >50 | >50 | >10 | >10 |
| PG9 | >50 | >50 | ND | ND | ||
| HIVNL4-3 | X4 | PG16 | 0.23 | >50 | 0.093 | 0.714 |
| PG9 | 8.9 | >50 | ND | ND | ||
| HIV92/RW/008(clade A) | R5 | PG16 | 0.002a | NRb | 0.046 | 0.520 |
| PG9 | 0.01a | NR | ND | ND | ||
| HIV98/IN/022(clade C) a | R5 | PG16 | 0.003a | NR | 0.071 | 0.443 |
| PG9 | 0.006a | NR | ND | ND | ||
Data from Walker et al. (2009). Number of replicates not specified.
Not reported.
Not determined.
IC50 and IC90 values represent the average of two separate assays for both pseudovirus and PBMC assays.
Rationale and study design for in vivo protection studies
The first set of experiments was performed in mice inoculated with HIVJR-CSF, a molecular clone reported by Walker et al. (2009) to be highly sensitive (IC50: 0.001 µg/ml) to PG16 neutralization in vitro. The second set was performed with HIVJD, a dual/mixed primary isolate in our SCID-hu mouse panel that is also highly sensitive to PG16 in vitro, and a third set with HIVNL4-3, which is less sensitive to PG16 with a plateau in dose response at <100% neutralization. The fourth set of experiments was performed with clade A and clade C isolates, and a final set was carried out in mice with established HIVJR-CSF infection to assess the potential of PG16 for HIV therapy. In each study, a range of PG16 dosage levels was used to establish a dose-response effect. The dosage range was very large (0.05–500 µg) across the studies for two main reasons: 1) very high doses were used in an attempt to produce sterilizing protection in the implants (which could rarely be achieved at 500 µg), and 2) very low doses were necessary to establish a no-effect level in the mice for this extremely potent antibody. We included in each study a positive control group treated with an antiretroviral regimen (either 3TC or Truvada) known to have reproducible efficacy in the model.
Highly potent protection by PG16 against challenge with HIVJR-CSF
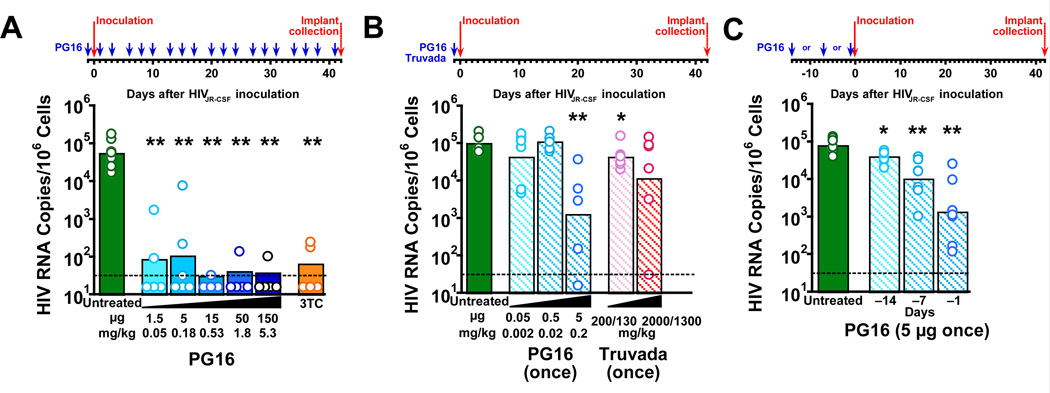
For studies with HIVJR-CSF, implants from SCID-hu Thy/Liv mice were collected 42 days after inoculation, a time point when HIVJR-CSF replication typically peaks in the implants, and assayed for cell count, HIV RNA, and p24. In the first study, mice were injected i.p. with varying doses of PG16 starting the day before inoculation and repeating every other day until Thy/Liv implant collection. Specifically, groups of 5 or 6 mice each were given a wide range of PG16 doses from 1.5 to 150 µg and challenged with 1,000 50% tissue culture infectious doses (TCID50) of HIVJR-CSF by direct injection of 50 µl virus into the implants of anesthetized mice. In mice treated with as little as 1.5 µg (0.05 mg/kg) PG16, we observed a 630-fold reduction in HIV RNA (from a mean of 104.7 HIV RNA copies per 106 cells in untreated mice to 101.9 copies in PG16-treated mice) (Fig. 2A, Supplementary Table 1). In fact, three of the five mice treated with 1.5 µg had no detectable viral RNA 42 days after inoculation, and all but one PG16-treated mouse (in the 5 µg group) had no detectable p24 (<5 pg per 106 cells) in their implants. Mice in the positive antiviral control group treated twice daily with 3TC (30 mg/kg/day) by i.p. injection had similarly large reductions in viral RNA (from a mean of 104.7 to 101.8 copies per 106 cells) relative to untreated mice.
Fig. 2. PG16 protected SCID-hu Thy/Liv mice from infection with HIVJR-CSF in three independent challenge studies.
(A) HIV RNA was reduced to <102.0 copies per 106 implant cells in mice treated i.p. with 1.5–150 µg PG16 (blue arrows) three times per week beginning the day before inoculation and continuing until implant collection at 42 days. Similar reductions in HIV RNA were observed in mice treated i.p. with 30 mg/kg 3TC once daily beginning the day before inoculation until implant collection. (B) HIV RNA was reduced to a mean of 103.0 copies per 106 cells in mice treated with a single administration of 5 µg PG16 the day before inoculation, which was a greater reduction than observed in mice treated by oral gavage with a single administration of high doses of Truvada (200 mg/kg TDF plus 130 mg/kg FTC or 2,000 mg/kg TDF plus 1,300 mg/kg FTC). (C) Statistically significant reductions in HIV RNA occurred in mice treated with a single administration of 5 µg PG16 at 1, 7, and 14 days before inoculation. The columns represent means, and the open circles represent individual mice. **
P<0.01 and *P<0.05 compared to untreated HIV-infected mice by the Mann-Whitney U test. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells).
In the second study, we treated groups of 6 mice each with a single prophylactic administration of 0.05, 0.5, or 5 µg PG16 or a single administration of oral Truvada (200 mg/kg tenofovir disoproxil fumarate [TDF] and 130 mg/kg emtricitabine [FTC] or 2,000 mg/kg TDF and 1,300 mg/kg FTC the day before HIVJR-CSF challenge (Fig. 2B, Supplementary Table 2). In a previous report, we showed that a single administration of Truvada the day before inoculation had minimally protective activity against HIVNL4-3 challenge in the mice (Stoddart et al., 2012), unlike the much more potent activity we reported for multiple licensed antiretroviral drugs when administered continually once or twice a day until implant collection (Stoddart et al., 2007). We found that 5 µg (0.18 mg/kg) PG16 reduced HIV RNA at 42 days by 79-fold (from a mean of 105.0 to 103.1 copies per 106 cells) with no statistically significant reductions at the lower doses (Fig. 2B, Supplementary Table 2). Despite the high dose, a single prophylactic administration of Truvada resulted in reductions in HIV RNA that were small (from 105.0 to 104.6 copies per 106 cells) but statistically significant at the lower dose and not statistically significant (because of higher sample variance) at the higher dose 42 days after inoculation (Fig. 2B). In the third study, we treated mice with a single administration of 5 µg PG16 at 1, 7, and 14 days before HIVJR-CSF inoculation and observed statistically significant reductions in HIV RNA for all three prophylactic time points (Fig. 2C, Supplementary Table 3).
Potent protection by PG16 against challenge with HIVJD
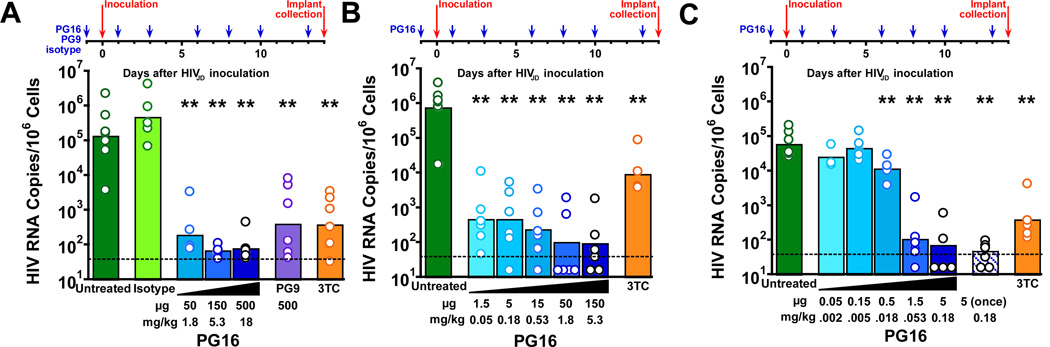
Similar to the studies described above in SCID-hu Thy/Liv mice inoculated with HIVJR-CSF, we found that PG16 also had potent activity in mice inoculated with HIVJD. Mice were injected i.p. with varying doses of PG16 starting one day before HIVJD injection and repeating three times per week until peak virus replication and implant collection 14 days after inoculation for cell count, HIV RNA, and p24. We observed a 1,600-fold reduction in HIV RNA in mice given 500 µg PG16, a 2,000-fold reduction in mice given 150 µg PG16, and a 630-fold reduction in those given 50 µg PG16 relative to untreated mice (Fig. 3A, Supplementary Table 4). A human IgG1 isotype control antibody had no activity at the highest dose of 500 µg given three times per week. In this same study (Fig. 3A), we compared the activity of PG9, a somatically related antibody, and PG16 at the 500-µg dose level and found somewhat less protective activity for PG9 (320-fold reduction in HIV RNA) compared to PG16 (1,600-fold reduction). This difference was also reflected in the lack of detectable p24 in PG16-treated mice while 2 of 7 PG9-treated mice had 38 and 42 pg p24 per 106 implant cells, respectively (Supplementary Table 4). The greater protective activity of PG16 compared to PG9 against HIVJD challenge is also consistent with the 9-fold lower pseudovirus neutralization IC50 for PG16 (0.008 µg/ml) compared to PG9 (0.074 µg/ml) (Table 1).
Fig. 3. PG16 protected SCID-hu Thy/Liv mice from infection with HIVJD in three independent challenge studies with progressively lower antibody dose ranges.
(A) Mean HIV RNA was reduced to <102.5 copies per 106 implant cells in mice treated i.p. with 50–500 µg PG16 three times per week beginning the day before inoculation and continuing until implant collection at 14 days. Similar reductions in HIV RNA were observed in mice treated i.p. with 500 µg PG9 under the same regimen as well as treatment with 30 mg/kg 3TC once daily beginning the day before inoculation until implant collection. No reductions occurred in mice treated with 500 µg isotype control mAb under the same regimen as PG16 and PG9. (B) Mean HIV RNA was reduced to ≤102.5 copies per 106 implant cells in mice treated i.p. with 1.5–150 µg PG16 three times per week beginning the day before inoculation and continuing until implant collection at 14 days. (C) Statistically significant reductions in HIV RNA occurred in mice starting with a dose of 0.5 µg PG16 three times per week beginning the day before inoculation, and HIV RNA was undetectable in 2 of 5 mice treated with a single administration of 5 µg PG16 the day before inoculation. The columns represent means, and the open circles represent individual mice. **P<0.01 compared to untreated HIV-infected mice by the Mann-Whitney U test. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells).
We performed two additional studies with progressively lower doses to determine a minimally protective dose for PG16 against HIVJD challenge. In the first study, administration of as little as 1.5 µg PG16 three times per week for 14 days beginning the day before virus inoculation resulted in a 1,600-fold reduction in HIV RNA (from a mean of 105.9 to 102.7 copies per 106 cells) and reduced HIV p24 to undetectable levels in 5 of 6 mice (Fig. 3B, Supplementary Table 5). In the subsequent study, the amount of antibody was further reduced to determine the dose at which PG16 had no measurable effect on HIV replication (Fig. 3C, Supplementary Table 6). Here we determined the no-effect level of PG16 to be 0.15 µg three times per week (Fig. 3C). When administered as a single prophylactic dose of 5 µg, PG16 was highly protective against HIVJD challenge with a 1,600-fold reduction in HIV RNA (from a mean of 104.9 to 101.7 copies per 106 cells) (Fig. 3C), which was substantially more effective than the 79-fold reduction observed for HIVJR-CSF (Fig. 2B).
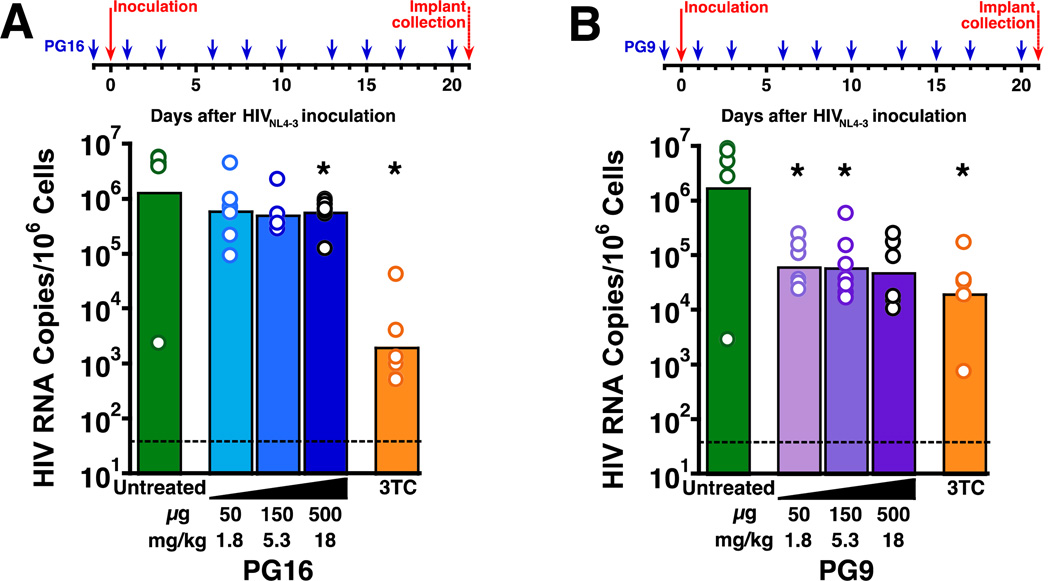
Protection by PG16 against challenge with HIVNL4-3
We next evaluated the prophylactic efficacy of PG16 against HIVNL4-3, which is less susceptible to PG16 neutralization in vitro (Table 1). SCID-hu Thy/Liv mice were injected i.p. with 50, 150, or 500 µg PG16 starting one day before virus inoculation and repeating three times per week until peak virus replication and implant collection on day 21. In contrast to our findings with HIVJR-CSF and HIVJD, high-dose (500 µg) PG16 had very low (2-fold reduction in HIV RNA) protective activity against HIVNL4-3 (Fig. 4A, Supplementary Table 7), consistent with the less potent neutralization of HIVNL4-3 by PG16 observed in vitro (Table 1). In a separate study, treatment of the mice with PG9 showed somewhat higher protective activity (25-fold reduction in HIV RNA for 50 and 150 µg PG16) against HIVNL4-3 challenge (Fig. 4B, Supplementary Table 8).
Fig. 4. PG16 and PG9 exhibited minimal protective activity in SCID-hu Thy/Liv mice challenged with HIVNL4-3.
(A) Statistically significant reductions in HIV RNA occurred in mice treated i.p. with 500 µg PG16 three times per week beginning the day before inoculation and continuing until implant collection at 21 days. Much larger (~3 log10) reductions in HIV RNA were observed in mice treated i.p. with 30 mg/kg 3TC once daily beginning the day before inoculation until implant collection. (B) Statistically significant reductions of >1 log10 in HIV RNA occurred in mice treated i.p. with 50 and 150 µg PG9 three times per week beginning the day before inoculation and continuing until implant collection at 21 days (P=0.055 for 500 µg PG9). Comparable reductions in HIV RNA were observed in mice treated i.p. with 30 mg/kg/day 3TC once daily beginning the day before inoculation until implant collection. The columns represent means, and the open circles represent individual mice. *P<0.05 compared to untreated HIV-infected mice by the Mann-Whitney U test. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells).
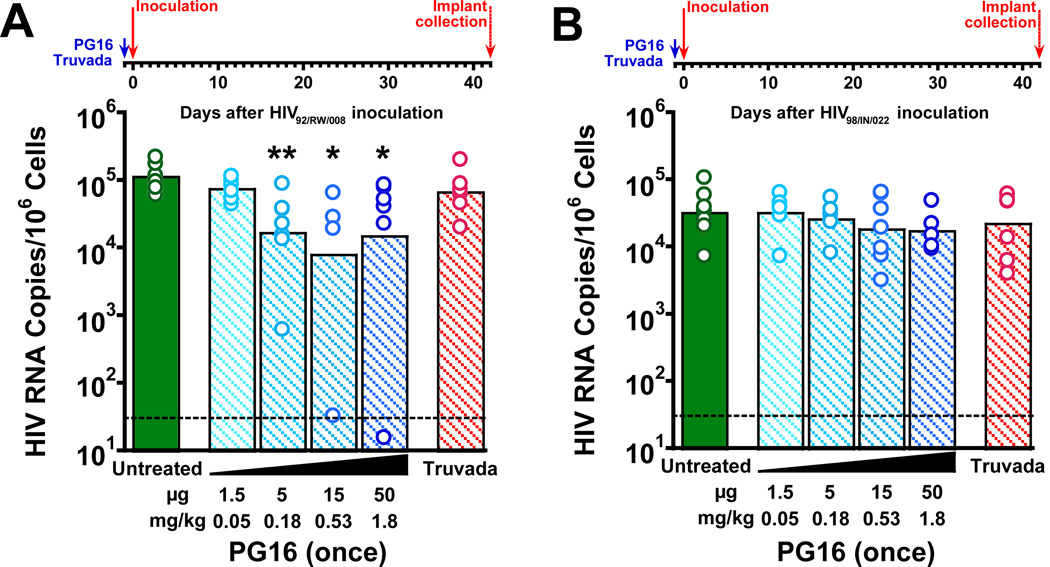
Protective effects of a single administration of PG16 against challenge with clade A HIV92/RW/008
The non-clade B HIV isolates reported by Walker et al. to have the greatest sensitivity to PG16 neutralization, clade A HIV92/RW/008 and clade C HIV98/IN/022, were also evaluated in SCID-hu Thy/Liv mice. There were statistically significant reductions (8–16-fold) in HIV RNA 42 days after inoculation in mice treated with a single prophylactic administration of 5, 15, and 50 µg PG16 the day before HIV92/RW/008 inoculation, but no protective effect was detected for 1.5 µg (Fig. 5A, Supplementary Table 9). Similarly to what we observed for HIVJR-CSF (Fig. 2A), there was no statistically significant protective effect of a very high single oral administration of Truvada given the day before HIV92/RW/008 inoculation. In contrast to the moderate protective effects observed for HIV92/RW/008, no significant protective effect was observed after PG16 treatment of mice inoculated with clade C HIV98/IN/022 (Fig. 5B, Supplementary Table 10) despite the high in vitro sensitivity of this strain to PG16.
Fig. 5. A single administration of PG16 protected SCID-hu Thy/Liv mice from challenge with clade A HIV92/RW/008 but not clade C HIV98/IN/022.
(A) HIV RNA was reduced by ~1 log10 in mice treated with a single administration of 5–50 µg PG16 the day before inoculation with HIV92/RW/008, unlike mice treated once by oral gavage with high-dose Truvada (2,000 mg/kg TDF plus 1,300 mg/kg FTC), which had no reductions in viral RNA 42 days after inoculation. (B) No reductions in HIV RNA were observed in mice treated with a single administration of 1.5–50 µg PG16 the day before inoculation with HIV98/IN/022. The columns represent means, and the open circles represent individual mice. **P<0.01, *P<0.05 compared to untreated HIV-infected mice by the Mann-Whitney U test. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells).
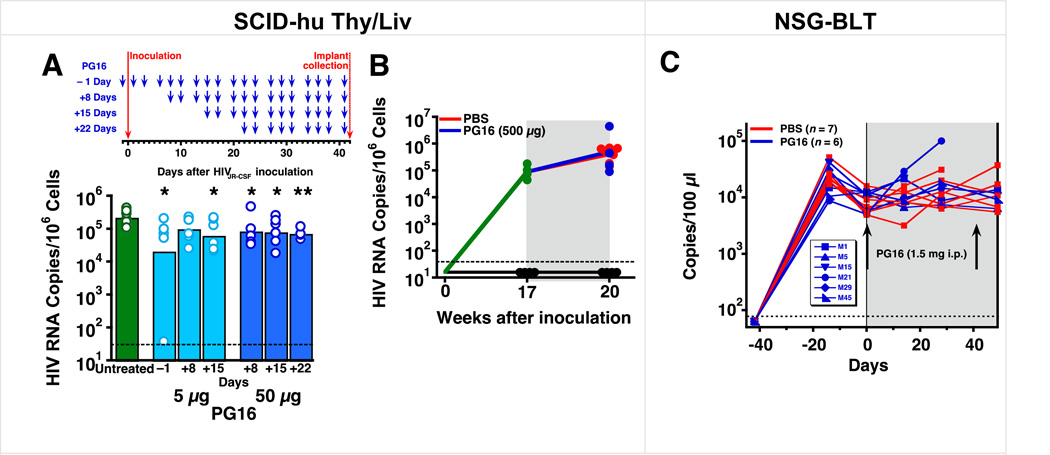
Substantially reduced activity of PG16 when administered after HIVJR-CSF challenge
We next evaluated the therapeutic activity of PG16 in HIVJR-CSF-inoculated mice. Mice were treated with 5 µg PG16 three times per week starting 1 day before or 8 or 15 days after HIVJR-CSF challenge and with 50 µg PG16 three times per week starting 8, 15, or 22 days after HIVJR-CSf challenge. In comparison to starting PG16 treatment the day before inoculation, which showed the expected protective effect with 5 µg PG16, delay of treatment initiation to 8 days after inoculation resulted in only 2.5–3-fold HIV-inhibitory activity (from a mean of 105.3 HIV RNA copies per 106 cells in untreated mice to 104.8–4.9 copies in all groups treated after inoculation (Fig. 6A, Supplementary Table 11). In a separate study where SCID-hu Thy/Liv mice with established HIVJR-CSF infection were treated 17 weeks after inoculation with 500 µg PG16 administered three times per week for 3 weeks, no protection was observed (Fig. 6B, Supplementary Table 12).
Fig. 6. PG16 had substantially reduced activity in SCID-hu Thy/Liv mice when treatment was initiated 8 days or more after HIVJR-CSF inoculation and had no significant activity in both SCID-hu Thy/Liv mice and NSG-BLT mice with established HIVJR-CSF infection.
(A) HIV RNA was reduced by 1 log10 in SCID-hu Thy/Liv mice treated i.p. with 5 µg PG16 three times per week beginning the day before inoculation and continuing until implant collection at 42 days. Smaller reductions in HIV RNA were observed when treatment was delayed until 8 or more days after inoculation. The columns represent means, and the open circles represent individual mice. **P<0.01 and *P<0.05 compared to untreated HIV-infected mice by the Mann-Whitney U test. (B) No reduction in HIV RNA in SCID-hu Thy/Liv mice treated i.p. with high-dose (500 µg) PG16 or PBS three times per week for 3 weeks beginning 17 weeks after HIVJR-CSF inoculation. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells). (C) Viremic NSG-BLT mice were treated with 1.5 mg PG16 at 6 and 12 weeks after intravaginal HIVJR-CSF inoculation. Each line represents an individual mouse, and sequence analysis of viral RNA from the spleens of PG16-treated mouse #5 and #29 (Supplementary Table 13) revealed Env mutation T162N (data not shown). Mouse #15 died and mouse #21 was euthanized with clinical signs consistent with graft-versus-host disease.
It is difficult to achieve significant and sustained antiviral activity in SCID-hu Thy/Liv mice with established HIV infection even with high-dose combination therapy including a protease inhibitor (Amado, et al., 1999). Mindful of this potential limitation with the SCID-hu Thy/Liv model, we also treated NOD-scid IL-2Rγ−/− (NSG) BLT mice (NSG-BLT) mice with established HIV infection and stable viremia. In the NSG-BLT model, Thy/Liv implantation is supplemented by the injection of CD34+ hematopoietic stem/progenitor cells (HSPC) isolated from the autologous fetal liver. The Thy/Liv implant allows for positive and negative selection of human T cells to occur in autologous human thymus tissue, while the injected HSPC populate the mouse bone marrow to reconstitute and maintain human hematopoiesis. This approach leads to the most comprehensive reconstitution of the human immune system in a mouse model yet reported, with high levels of multilineage human cell engraftment and sustained HIV plasma viremia after parenteral and mucosal HIV exposure.
We treated groups of 6–7 HIV-viremic NSG-BLT mice with a very high dose of PG16 (1.5 mg) or PBS vehicle 6 and 12 weeks after i.p. HIVJR-CSF inoculation and observed no reduction in plasma HIV RNA after the first administration and up to 7 days after the second administration (Fig. 6C, Supplementary Table 13). Plasma viral load increased dramatically in one mouse after the first PG16 treatment, but this mouse had evidence of graft-versus host disease necessitating euthanasia before the second PG16 treatment. To determine whether viral escape from PG16 had occurred in the mice, we sequenced gp120 RNA obtained from spleens 1 week after the second PG16 administration and observed a mutation at residue 162 (T162N) in two of the six treated mice. Outgrowth of T162N was also reported in the previous work in PG16-treated humanized NRG mice along with other substitutions at positions T162 and N160, and these mutants were found to be highly resistant to PG16 neutralization in vitro (Klein et al., 2012). It is unlikely, however, that the lack of protective activity we observed was the result of viral escape because we detected PG16-resistance mutations in only 2 of 6 mice with established HIVJR-CSF infection.
Discussion
The broadly HIV-neutralizing antibodies PG9 and PG16 were isolated from an African clade A-infected individual, who ranked in the top 5% of 1,800 HIV-infected donors screened for potent anti-HIV serum neutralizing activity in an international effort named Protocol G (Walker et al., 2009). From a panel of 162 HIV isolates, PG9 neutralized 127 and PG16 neutralized 119 of derived pseudoviruses with potencies ~1 log10 greater than broadly neutralizing antibodies 2G12, b12, 2F5, and 4E10 (Doores and Burton, 2010) and comparable to that of VRC01 (Wu et al., 2010).
In the present study, we evaluated both the prophylactic and therapeutic activities of PG16 against HIV challenge in humanized mice. We used five different challenge isolates that were sensitive to PG16 neutralization in vitro (Table 1), including a clade A (HIV92/RW008) and a clade C (HIV98/IN/022) isolate (Table 1). The IC50 values for PG16 ranged from 0.001 µg/ml for HIVJR-CSF to 0.23 µg/ml for HIVNL4-3 in the in vitro pseudovirus assay. It is notable that four of our primary isolates (HIVPD, HIVEW, HIVEF, and HIVGV) were resistant to PG9 and PG16 (IC50 >50 µg/ml) and that they were all X4 tropic whereas the sensitive viruses were either R5 (HIVJR-CSF, HIVJW, HIV92/RW008, and HIV98/IN/022) or R5X4 (HIVJD). This unusual pattern of neutralization sensitivity may be limited to our small sample size.
In our prophylaxis studies, we treated SCID-hu Thy/Liv mice with either a single prophylactic administration the day before HIV inoculation or repeated treatment three times per week beginning the day before inoculation until implant collection 14–42 days after inoculation, depending on peak virus replication for the respective challenge virus (14 days for R5X4 HIVJD, 21 days for X4 HIVNL4–3, and 42 days for R5 strains HIVJR-CSF, HIV92/RW008, and HIV98/IN/022). We chose the intrathymic HIV exposure route because injection of HIV directly into the human target tissue provides a more stringent assessment of the efficacy of the test agent under various study designs compared to the mucosal or intravenous routes, for which exposure of the virus to target organs is less direct.
In an initial dose-ranging study with the most PG16-sensitive isolate, HIVJR-CSF, we observed a 630-fold reduction in HIV RNA in mice treated with the lowest PG16 dose evaluated, 1.5 µg (0.05 mg/kg), starting the day before virus inoculation and repeating three times per week for 42 days. In a second study, we gave the mice a single administration of PG16 the day before HIVJR-CSF challenge and found that 5 µg (0.2 mg/kg) reduced HIV RNA by 79-fold. The latter results are comparable to those reported by Gauduin et al., (1997) where 80% (actual number not specified) of hu-PBL-SCID mice were protected by a single administration of 1 mg/kg b12 antibody 1 h before i.p. inoculation with HIVLAI. In our SCID-hu Thy/Liv mouse model, mice treated with 5 µg PG16 had an antibody serum concentration of <1.5 µg/ml the day after treatment, indicating a protective serum concentration for PG16 that is in the single-digit µg/ml range, similar to that recently reported for PGT121 in macaques protected from mucosal SHIV challenge (Moldt et al., 2012). The t1/2 of 3.7 days we obtained corresponds well to the 2.5 days reported for a 500-µg dose of PG16 by Klein et al. (2012) in humanized NOD Rag1−/−IL2Rγ−/− (NRG) mice reconstituted with human fetal liver-derived CD34+ cells at birth.
Compared to a single administration of 0.2 mg/kg (5 µg) PG16, a single very large dose of Truvada (2,000 mg/kg TDF and 1,300 mg/kg FTC) resulted in only relatively small reductions in HIV RNA in HIVJR-CSF-challenged mice (Fig. 2B). We previously reported similarly small reductions in HIV RNA after a single preexposure administration of Truvada in SCID-hu Thy/Liv mice inoculated with HIVNL4–3 (Stoddart et al., 2012). The potent activity of a single administration of PG16 observed in the present study is reminiscent of the sustained activity obtained for an albumin-conjugated C34 peptide fusion inhibitor with prolonged plasma half-life (~20 h rats) in that previous report (Stoddart et al., 2012). Moreover, we showed that a single treatment with PG16 had sustained, although lower, activity when HIVJR-CSF challenge was delayed by up to 7 or 14 days.
PG16 was also highly protective against HIVJD challenge, with a 1,600-fold reduction in HIV RNA and lack of detectable p24 in 5 of 6 mice treated with 1.5 µg three times per week for 14 days and a 1,300-fold reduction in HIV RNA after a single prophylactic administration of 5 µg. In contrast, the somatically related PG9 antibody was somewhat less protective than PG16 at the 500-µg dose level. The greater protective activity of PG16 compared to PG9 against HIVJD challenge is consistent with the 9-fold lower pseudovirus neutralization IC50 for PG16 (0.008 µg/ml) compared to PG9 (0.074 µg/ml).
In contrast to our findings with HIVJR-CSF and HIVJD, high-dose (500 µg) PG16 had minimal protective activity against HIVNL4-3 when administered three times per week, which is consistent with the less potent neutralization of HIVNL4-3 by PG16 observed in vitro. It should be noted that, unlike for other viruses, the PG9 and PG16 pseudovirus neutralization curves for HIVNL4-3 plateaued at <100% neutralization (Walker et al., 2009), and this was confirmed in our study. The PG16 dose-response curves for HIVNL4-3 in the PBMC assay did not plateau with a relatively low IC90 value of 0.7 µg/ml (Table 1). This incomplete in vitro neutralization appears to be reflected in the plateaued dose responses we obtained for PG9 and PG16 in HIVNL4-3-challenged mice.
Contrary to predictions from in vitro neutralization potency, a single prophylactic administration of up to 50 µg PG16 had 1 log10 lower protective activity against challenge with clade A HIV92/RW/008 than against HIVJR-CSF. No detectable activity against clade C HIV98/IN/022 was observed. Similar to HIVJR-CSF, both of these isolates have the greatest in vitro sensitivity to PG16 neutralization (IC50 0.002–0.003 µg/ml), so the difference in in vivo protection against these non-clade B isolates was unexpected. While a higher dosage of antibody or repeated treatment during the infection period may have resulted in more potent protection from HIV92/RW/008 and HIV98/IN/022 challenge, it remains unclear whether the lack of greater protection with a single administration is associated with differences in their infection behavior in SCID-hu Thy/Liv mice despite the fact that they have the same tropism.
We compared the prophylactic and therapeutic activities of PG16 in a series of experiments. First, we treated SCID-hu Thy/Liv mice with 5 µg PG16 three times per week starting 1 day before or 8 or 15 days after HIVJR-CSF challenge and with 50 µg PG16 three times per week starting 8, 15, or 22 days after HIVJR-CSF challenge. Although repeated dosing of 5 µg PG16 starting the day before inoculation had the expected protective effect, delay of treatment initiation after inoculation resulted in little protective activity. Since it remained possible that a higher repeated dosage of PG16 would lead to reductions in HIV RNA in the implants, we further evaluated the therapeutic activity of high-dose PG16 in established HIV infection in two separate studies. In one study, a high repeat-dose PG16 treatment of SCID-hu Thy/Liv mice at 500 µg (18 mg/kg) for 3 weeks starting 17 weeks after HIVJR-CSF inoculation had no effect on HIV RNA levels in the implants 3 weeks after treatment. This limited efficacy in established HIV infection is consistent with results reported previously for b12, 2G12, 2F5, or their combination using hu-PBL-SCID mice (Poignard et al., 1999).
In the second study using HIV-viremic NSG-BLT mice, a very high dose of PG16 (1.5 mg or 54 mg/kg) at 6 and 12 weeks after i.p. HIVJR-CSF challenge resulted in no reduction in plasma HIV RNA measured 2 and 4 weeks after the first treatment and 1 week after the second treatment. In both of these models, the lack of therapeutic efficacy by PG16 might be the result of using antibody monotherapy. This possibility is supported by the results from a recent report where PG16 was evaluated in an established infection model in humanized NOD Rag1−/−Il2rγnull (NRG) mice that were reconstituted with human fetal liver-derived CD34+ cells at birth (Klein et al., 2012). In that report, mice were given 500 µg (20 mg/kg) PG16 once or twice a week after infection was established by i.p. challenge with HIVYU-2, a clone of HIVNL4-3 carrying the envelope of YU-2, and only a transient reduction of HIV RNA was detected before virus rebound. Moreover, unlike the NRG mice, in which nearly all rebound virus contained escape mutations at N160 or N162, we detected viral escape in the rebound virus population after two administrations of 1.5 mg (54 mg/kg) PG16 in only two of the six SCID-hu Thy/Liv mice. Overall, the observed effect of PG16 treatment in these two models of established JR-CSF infection was limited by the antibody monotherapy regimen we used. Recently, a combination of PG16 with an anti-CD4 binding sites and an anti-V1/V2 loop antibody administered at 1 mg each (40 mg/kg) twice a week rapidly suppressed plasma viral RNA in NRG mice with established HIVYU-2 infection and demonstrated the protective activity of PG16 and its therapeutic potential in the context of combination therapy (Horwitz et al., 2013).
The current study confirms the usefulness of the SCID-hu Thy/Liv mouse model for evaluation of in vivo preexposure prophylaxis of human HIV-specific monoclonal antibodies and demonstrates the utility of in vitro characterization of challenge viruses prior to in vivo experimentation. The high (essentially 100%) HIV susceptibility of SCID-hu Thy/Liv mice across many cohorts makes such prophylaxis experiments feasible because it increases confidence that the observed protection is not the result of poor susceptibility to infection.
A major advantage of the BLT mouse model is the establishment of systemic HIV infection and plasma viremia after HIV challenge by multiple routes; the model’s major drawbacks are variability between mice in HIV susceptibility (Long and Stoddart, 2012) and a high incidence (35% by 22 weeks) of GvHD (Greenblatt et al., 2012; Covassin et al., 2013), which might have perturbed the efficacy of PG16 in the BLT mice. Indeed, we show in Fig. 6C a spike in HIV viremia in a PG16-treated mouse experiencing signs of GvHD and surmise that systemic immune activation driven by the GvHD disease process may have led to greater HIV expression. According to Greenblatt et al., GvHD in BLT mice is associated with the infiltration of human CD4+ T cells into the skin and a shift towards Th1 cytokine production. GvHD also induced a mixed M1/M2 polarization phenotype in a dermal murine macrophage population that is CD11b+ and MHC class II+. GVHD mice displayed robust expression of human IFNγ and the profibrotic mediators human IL13 and human CCL2. The presence of xenogeneic GvHD in BLT mice presents both a major obstacle in the use of humanized mice and an opportunity to conduct preclinical studies on GvHD in a humanized model.
In summary, our results demonstrate the ability of PG16 to penetrate and protect primary lymphoid tissues from HIV infection and that antibodies can work in central immune sites, not just at the mucosal surface. This feature could add to the broadly neutralizing monoclonal antibodies’ ability to prevent infection if HIV crosses the mucosal barrier. Overall, these findings suggest that this antibody or similar agents with high potency and sustained activity may hold promise as a single intervention modality or in cocktail combinations (to prevent viral escape) for targeting early infection events after HIV exposure. The potent protective efficacy we observed for a single preexposure administration supports further preclinical and clinical evaluation of this promising passive immunization strategy.
Materials and methods
Viruses
The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV molecular clones pYK-JRCSF (R5) from Dr. Irvin SY Chen and Dr. Yoshio Koyanagi (Cann et al., 1990; Haltiner et al., 1985; Koyanagi et al., 1987), pNL4-3 (X4) from Dr. Malcolm Martin (Adachi et al., 1986), and HIV-1 92RW008 (clade A) and 98IN022 (clade C) (from The UNAIDS Network for HIV Isolation and Characterization). Primary HIV isolates HIVJD (Kovalev et al., 1999; Stoddart et al., 2007; Su et al., 1995), HIVEW (Kovalev et al., 1999; Rabin et al., 1996; Su et al., 1995), HIVPD, HIVEF, HIVJW, and HIVGV were obtained from Dr. J. M. McCune. Working stocks of the molecular clones were prepared in HEK 293T cells by lipofectamine 2000 transfection, and primary isolates were expanded in phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells. Stock virus titers (50% tissue culture infectious doses; TCID50) were determined in PHA-activated PBMC by 50% endpoint dilution and assessment of supernatant p24 by ELISA after 7 days.
Antibodies and drugs
PG16 and PG9 were provided by Theraclone Sciences and were purified chromatographically from cultures of CHO-S1 cells cotransduced with PG16 or PG9 heavy and light chain genes (Bleck et al., 2012). Lamivudine (3TC), tenofovir disoproxil fumarate (TDF), and emtricitabine (FTC) were kindly provided by the NIH AIDS Research and Reference Reagent Program. PG16 in mouse serum was measured by ELISA for human IgG (Bethyl Laboratories).
In vitro neutralization assays
Pseudoviruses were produced by cotransfection of HEK 293 cells with a subgenomic plasmid, pHIV-1luc∆u3, that incorporates a firefly luciferase indicator gene and a second plasmid, pCXAS, that expresses HIV-1 Env libraries or clones. Following transfection, pseudoviruses were harvested and used to infect U87 cell lines expressing either CCR5 or CXCR4 (Richman et al., 2003).
PHA-activated PBMCs pooled from six donors were inoculated with HIV-1 at an MOI of 0.001 for 2 h at 37°C, and triplicate wells of round-bottom 96-well plates containing 100,000 cells in 100 µl were treated with 100 µl of serially diluted antibody or medium alone and cultured for 7 days. Supernatants were collected and assayed for p24 antigen at 1:800 dilution in HIV p24 antibody-coated microplates (Perkin-Elmer) by quantitative ELISA using the p24 standard supplied by the manufacturer. IC50 values were determined by a 4-parameter fit model (SOFTmax PRO 3.0, Molecular Devices). At day 7, untreated virus control wells had mean p24 concentrations of 5–20 ng/ml.
SCID-hu Thy/Liv mice
Male C.B-17 SCID (model #CB17SC-M, homozygous, C.B-Igh-1b/IcrTac-Prkdcscid) mice were obtained at 6–8 weeks of age from Taconic and coimplanted with 1-mm3 pieces of human fetal thymus and liver under the kidney capsule to generate SCID-hu Thy/Liv mice as described previously (Rabin et al., 1996; Stoddart et al., 2007). Cohorts of 50–60 mice each were generated from the tissues of one donor, and implants were inoculated 18 weeks after implantation with 50 µl of stock virus (1,000 TCID50) or RPMI 1640 medium (mock infection) by direct injection into the implants of anesthetized mice. Each experiment was performed in a separate SCID-hu Thy/Liv mouse cohort, and details for the twelve cohorts are shown in Supplementary Tables 1–12. Of the 559 mice included in the studies, 20 (3.6%) mice died during the course of the experiment, and 22 (3.9%) mice had abnormal implants and were excluded from analysis.
Antibodies were administered i.p. to the mice (5–7 mice per group) at the indicated dosages beginning, in most experiments, the day before inoculation of the Thy/Liv implants. Thy/Liv implants were collected from euthanized mice 14 days after inoculation with HIVJD inoculation, 21 days after HIVNL4-3, and 42 days after HIVJR-CSF, HIV92RW008, and HIV98IN022 when virus replication peaks in the implants with these isolates. Animal protocols were approved by the UCSF Institutional Animal Care and Use Committee.
NSG-BLT mice
One cohort of humanized NOD-scid IL-2Rγ−/− (NSG) BLT mice (NSG-BLT) mice was used to study PG16 treatment of established HIV infection. NSG-BLT mice were produced as described previously (Lan et al., 2006; Long and Stoddart, 2012; Melkus et al., 2006) by coimplanting human fetal liver and thymus under the kidney capsule of NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Jackson Laboratories). Human CD34+ hematopoietic stem progenitor cells were purified from fetal liver by magnetic bead selection and cryopreserved until tail vein injection (815,000 cells per mouse) 3 weeks after Thy/Liv implantation and 30 h after conditioning with 225 cGy gamma irradiation. Of the cells injected, 917 were CD45+, CD34+, Lin-1neg, CD38neg, C-kit+, CD90+, and CD45RAneg human hematopoietic stem cells (HSC) (Long and Stoddart, 2012). NSG-BLT mice were inoculated intravaginally with HIVJR-CSF (8,000 TCID50) 12 weeks after CD34+ cell injection.
Thy/Liv implant processing and assay
Single-cell suspensions were made by placing the implant into a sterile nylon mesh bag, submerging the bag in phosphate-buffered saline (PBS)/2% fetal bovine serum (FBS) in a 60-mm tissue culture dish, and dispersing the tissue between the nylon layers with forceps, as described previously (Rabin et al., 1996; Stoddart et al., 2007; Stoddart et al., 2000). The cells were counted with a Coulter counter to determine total implant cellularity. For the bDNA assay, dry pellets of 5 × 106 implant cells were frozen and stored at −80°C. Cells were disrupted with sterile disposable pestles and a cordless motor grinder (Kontes) in 8 M guanidine HCl with 0.5% sodium N-lauroylsarcosine. The RNA was extracted with 0.5 ml 100% ethanol and pelleted at 12,000 × g for 20 min at 4°C. Supernatants were aspirated to remove DNA, and RNA pellets were washed with 0.5 ml 70% ethanol, placed on dry ice, and digested with reagents supplied by the manufacturer (VERSANT™ HIV-1 RNA 3.0 Assay, Siemens Healthcare Diagnostics). Implant HIV RNA is expressed as copies per 106 implant thymocytes, and the log10 values were used for calculation of geometric means. The limit of detection was 101.48 RNA copies per 106 cells, and this lower-limit value was used for calculation of means for implants with undetectable viral RNA. For p24 ELISA, pellets of 2.5 × 106 cells were resuspended in 400 µl of p24 lysing buffer (1% Triton X-100, 0.5% sodium deoxycholate, 5 mM EDTA, 25 mM Tris Cl, 250 mM NaCl, and 1% aprotinin), rotated overnight at 4°C, and stored at −20°C. Thawed samples were transferred into HIV p24 antibody-coated microplates (PerkinElmer Life Sciences) for quantitative ELISA. A standard curve was generated with the kit-supplied standards, and the results were calculated as pg p24 per 106 cells. Implant cells were also stained with antibodies to CD3, CD4, and CD8 for analysis of T-cell subsets by multiparameter flow cytometry (Supplementary Material).
Statistical analysis
Results are expressed as the mean ± SEM for each mouse group. Nonparametric statistical analyses were performed by use of the Mann-Whitney U test. Data for mice in each group were compared to those for untreated infected mice, and P values <0.05 were considered statistically significant.
Supplementary Material
Highlights.
We tested potent broadly HIV-neutralizing human monoclonal antibodies in humanized mice.
We studied PG16 in the SCID-hu Thy/Liv and BLT models against HIV clade A, B, and C.
PG16 was efficacious in SCID-hu mice as a single dose the day before inoculation.
PG16 as monotherapy had no activity in humanized mice with established HIV infection.
These results show tissue penetration of the antibodies, which could prevent infection.
Acknowledgments
This work was supported in part by Federal funds from NIAID, NIH, under Contract no. HHSN266200700002C/N01-AI-70002 (Contracting Officer Representative: Dr. Brigitte Sanders). This work was also supported in part by the AIDS Research Institute at UCSF and the Harvey V. Berneking Living Trust. We dedicate this work in memory of Dr. Paul L. Black (DAIDS, NIAID, NIH), who provided key input into the design of the PG16 efficacy studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado RG, Jamieson BD, Cortado R, Cole SW, Zack JA. Reconstitution of human thymic implants is limited by human immunodeficiency virus breakthrough during antiretroviral therapy. J. Virol. 1999;73:6361–6369. doi: 10.1128/jvi.73.8.6361-6369.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleck GT. Consistent production of genetically stable mammalian cell lines. BioPharm. Int. 2012;25:56–59. [Google Scholar]
- Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O’Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Nat. Acad. Sci. U.S.A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann AJ, Zack JA, Go AS, Arrigo SJ, Koyanagi Y, Green PL, Pang S, Chen IS. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J. Virol. 1990;64:4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, Brehm MA. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rY(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 2013;174:372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport TM, Friend D, Ellingson K, Xu H, Caldwell Z, Sellhorn G, Kraft Z, Strong RK, Stamatatos L. Binding interactions between soluble HIV envelope glycoproteins and quaternary-structure-specific monoclonal antibodies PG9 and PG16. J. Virol. 2011;85:7095–7107. doi: 10.1128/JVI.00411-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin R, Klein F, Horwitz JA, Halper-Stromberg A, Sather DN, Marcovecchio PM, Lee T, West AP, Jr., Gao H, Seaman MS, Stamatatos L, Nussenzweig MC, Bjorkman PJ. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J. Exp. Med. 2013;210:1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr., Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 1999;190:1857–1868. doi: 10.1084/jem.190.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E, Ramos A, Feng Y, Zhou T, Moquin S, Walker LM, Wu X, Seaman MS, Wrin T, Kwong PD, Wyatt RT, Mascola JR, Poignard P, Burton DR. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J. Virol. 2012;86:4394–4403. doi: 10.1128/JVI.06973-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyo EM, Heath A, Dispinseri S, Holmes H, Lusso P, Zolla-Pazner S, Donners H, Heyndrickx L, Alcami J, Bongertz V, Jassoy C, Malnati M, Montefiori D, Moog C, Morris L, Osmanov S, Polonis V, Sattentau Q, Schuitemaker H, Sutthent R, Wrin T, Scarlatti G. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One. 2009;4:e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Vrbanac V, Tivey T, Tsang K, Tager AM, Aliprantis AO. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS One. 2012;7:e44664. doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiner M, Kempe T, Tjian R. A novel strategy for constructing clustered point mutations. Nucleic Acids Res. 1985;13:1015–1025. doi: 10.1093/nar/13.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009a;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009b;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyndrickx L, Heath A, Sheik-Khalil E, Alcami J, Bongertz V, Jansson M, Malnati M, Montefiori D, Moog C, Morris L, Osmanov S, Polonis V, Ramaswamy M, Sattentau Q, Tolazzi M, Schuitemaker H, Willems B, Wrin T, Fenyo EM, Scarlatti G. International network for comparison of HIV neutralization assays: the NeutNet report II. PLoS One. 2012;7:e36438. doi: 10.1371/journal.pone.0036438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Büning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. U.S.A. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev G, Duus K, Wang L, Lee R, Bonyhadi M, Ho D, McCune JM, Kaneshima H, Su L. Induction of MHC class I expression on immature thymocytes in HIV-1-infected SCID-hu Thy/Liv mice: evidence of indirect mechanisms. J. Immunol. 1999;162:7555–7562. [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- Long BR, Stoddart CA. Alpha interferon and HIV infection cause activation of human T cells in NSG-BLT mice. J. Virol. 2012;86:3327–3336. doi: 10.1128/JVI.06676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. Highly potent HIVspecific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Nat. Acad. Sci. U.S.A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, McLellan JS, Wu X, Zhu J, Changela A, Schmidt SD, Yang Y, Zhou T, Phogat S, Mascola JR, Kwong PD. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J. Virol. 2010;84:8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc. Nat. Acad. Sci. U.S.A. 2010;107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poignard P, Sabbe R, Picchio GR, Wang M, Gulizia RJ, Katinger H, Parren PW, Mosier DE, Burton DR. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- Rabin L, Hincenbergs M, Moreno MB, Warren S, Linquist V, Datema R, Charpiot B, Seifert J, Kaneshima H, McCune JM. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob. Agents Chemother. 1996;40:755–762. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Nat. Acad. Sci. U.S.A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roben P, Moore JP, Thali M, Sodroski J, Barbas CF, 3rd, Burton DR. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J. Infect. Dis. 2012;205:1248–1257. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- Stoddart CA, Bales CA, Bare JC, Chkhenkeli G, Galkina SA, Kinkade AN, Moreno ME, Rivera JM, Ronquillo RE, Sloan B, Black PL. Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals. PLoS One. 2007;2:e655. doi: 10.1371/journal.pone.0000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart CA, Moreno ME, Linquist-Stepps VD, Bare C, Bogan MR, Gobbi A, Buckheit RW, Jr., Bedard J, Rando RF, McCune JM. Antiviral activity of 2’-deoxy-3’-oxa-4’-thiocytidine (BCH-10652) against lamivudine-resistant human immunodeficiency virus type 1 in SCID-hu Thy/Liv mice. Antimicrob. Agents Chemother. 2000;44:783–786. doi: 10.1128/aac.44.3.783-786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart CA, Nault G, Galkina SA, Bousquet-Gagnon N, Bridon D, Quraishi O. Preexposure prophylaxis with albumin-conjugated C34 peptide HIV-1 fusion inhibitor in SCID-hu Thy/Liv mice. Antimicrob. Agents Chemother. 2012;56:2162–2165. doi: 10.1128/AAC.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune JM. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol GPI, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O’Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.