Abstract
After decades focusing on the molecular and genetic aspects of organogenesis, researchers are showing renewed interest in the physical mechanisms that create organs. This review deals with the mechanical processes involved in constructing the heart and brain, concentrating primarily on cardiac looping, shaping of the primitive brain tube, and folding of the cerebral cortex. Recent studies suggest that differential growth drives large-scale shape changes in all three problems, causing the heart and brain tubes to bend and the cerebral cortex to buckle. Relatively local changes in form involve other mechanisms such as differential contraction. Understanding the mechanics of organogenesis is central to determining the link between genetics and the biophysical creation of form and structure.
Introduction
During embryonic development, many organs begin as simple tubes. Some of these organs (e.g., lungs and kidneys) eventually become a network of branched tubes, while others (e.g., heart and brain) develop into complex structures that no longer bear much resemblance to tubes. Although much is now known about the physical mechanisms that drive many of the fundamental processes of morphogenesis [1,2,3], how specific processes are integrated to create specific organs remains poorly understood. The importance of proper organ formation is clear, as without properly functioning organs, the embryo usually does not survive.
This review focuses on mechanical aspects of heart and brain development. Both of these organs are initially simple tubes that bend, twist, and remodel into their mature forms. Branching morphogenesis, which is central to the development of organs such as the lungs and kidneys, is not considered here. After providing a brief background for each problem, we discuss current thinking on each topic, as well as some of the remaining unanswered questions. We emphasize similarities in heart and brain development, as nature may use comparable means to create other organs.
It is important to note that, during the past few decades, most work has focused on molecular and genetic aspects of development. Hence, the current state of knowledge is several years old for some of the topics discussed herein. One objective of this review is to stimulate new interest in these important and challenging problems of organ morphomechanics.
Cardiac Morphogenesis
The heart has long fascinated developmental biologists. The heart is initially a relatively straight tubular structure comprised of three layers: an inner endothelium (endoderm); a relatively thick middle layer of extracellular matrix (cardiac jelly, CJ); and a two-cell-thick outer layer of myocardium [4]. During the fourth week of development in human or days 2–3 in chick, the heart tube (HT) loops into a curved tube that subsequently divides (septates) into four chambers [5,6]. The heart also undergoes changes in internal structure, including the formation of valves and highly organized myofibrils, allowing it to pump increasing amounts of blood to the rapidly growing embryo [7,8].
Cardiac Looping
Looping of the heart represents the first large-scale morphogenetic event that breaks left-right symmetry in the vertebrate embryo. During this process, the HT first becomes c-shaped (c-looping) and then s-shaped (s-looping) [5].
During c-looping, the HT simultaneously bends ventrally and twists rightward [5] (Fig. 1A). In a remarkable master’s thesis written more than 60 years ago, Butler (JK Butler, M.S. Thesis, University of Texas, 1952) showed that bending is caused by forces generated within the HT. He also speculated that torsion depends “on factors associated with the attachment of the heart to the body of the embryo.” Recent studies generally support these early observations.
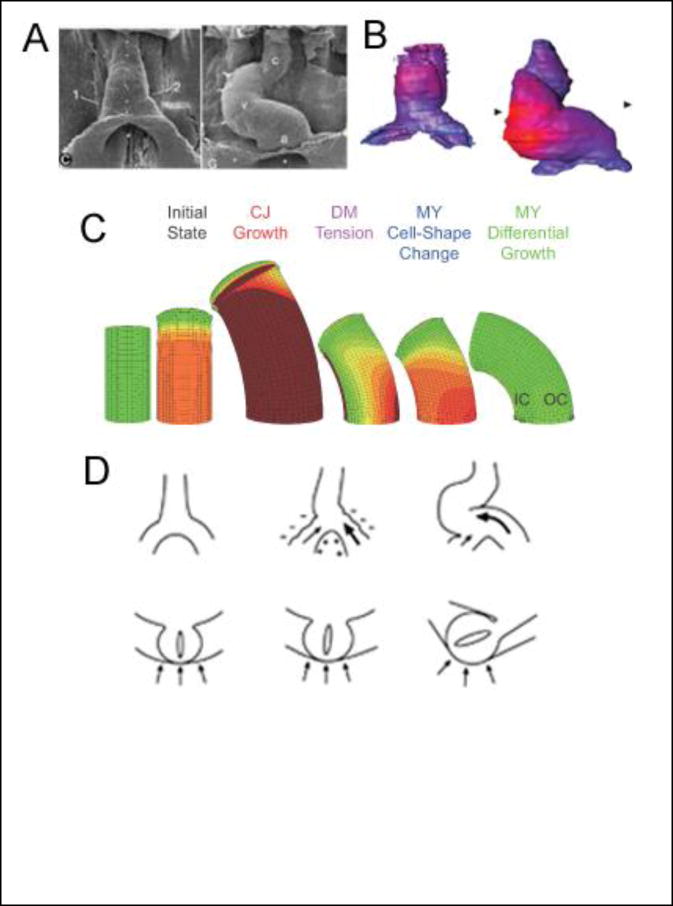
Fig 1. Cardiac looping.
(A) Scanning electron micrographs of embryonic chick heart at beginning (left) and end (right) of c-looping (ventral view, stages 10 and 12 of Hamburger and Hamilton [88]). Small dots along the ventral midline of the heart tube move to the outer curvature, illustrating that c-looping consists of ventral bending and rightward torsion. (c = conotruncus; v = ventricle; a = primitive atrium) From [5]. (B) Maps of myocardial cell size at similar stages of development as in (A) (blue = small cells; red = large cells). Dorsal-ventral (inner to outer curvature) gradient in cell size is consistent with a differential growth mechanism for cardiac bending. From [16**]. (C) Computational models for the bending component of c-looping. From the initial state including cardiac jelly (CJ) swelling, the models simulate the following mechanisms: dorsally constrained expansion of the heart tube as CJ continues to grow and swell, dorsal forces exerted on the heart by tension in the rupturing dorsal mesocardium (DM), active cell-shape changes in the myocardium (MY), and differential myocardial growth. Only differential growth yields a bending magnitude consistent with experiments. (IC = inner curvature; OC = outer curvature) From [18**]. (D) Schematic of mechanism for torsional component of c-looping (top row = ventral view; bottom row = cross-sectional view). Left: before looping; center: relatively large force exerted by left omphalomesenteric vein (bold arrow) pushes heart tube slightly rightward; right: compression exerted by splanchnopleure (arrows in cross section) enhances rotation of heart tube. From [22*].
As the HT bends, the original ventral and dorsal sides become the convex outer curvature (OC) and concave inner curvature (IC) of the curved tube, respectively. Investigators have proposed and tested numerous possible mechanisms for the bending component of c-looping [4]. These include buckling of the HT as it outgrows its allotted space [9], regionally constrained longitudinal stretching of the HT caused by CJ swelling [10], differential hyperplastic myocardial growth [11], active cell-shape changes in the myocardium [12,13,14], differential cytoskeletal contraction [15], and forces exerted on the HT by the rupturing dorsal mesocardium [15]. With the possible exception of active cell-shape change, none of these hypotheses have survived experimental scrutiny unscathed [4,13].
Recent studies suggest another possibility. Soufan et al. [16**] have found significant increases in myocardial cell size on the ventral side of the HT during c-looping (Fig. 1B), which would be consistent with bending driven by differential hypertrophic growth. This finding was unexpected, because it had been commonly thought that the heart grows primarily by hyperplasia before birth and hypertrophy after birth [17]. Motivated by these new results, Shi et al. [18**] reexamined the differential growth hypothesis using computational modeling and experiments on isolated chick hearts. Their model shows that the gradient in cardiomyocyte growth measured by Soufan et al. [16**] is capable of generating the degree of bending, as well as the changes in myocardial stress and strain distributions, observed experimentally (Fig. 1C).
In summary, it appears that differential hypertrophic growth is the primary cause of the bending component of cardiac c-looping, although the other mechanisms (active cell-shape change, dorsal myocardial tension, CJ swelling) may play secondary roles [18**] (Fig. 1C). In fact, looping may involve a combination of several different processes, some of which may be redundant and compensate when others fail [11,19]. Such backup mechanisms, which probably include modified gene expression as well, may explain why congenital defects are more rare than the number of developmental perturbations would suggest [20].
In contrast to bending, which is driven by internally generated forces, the torsional component of c-looping is caused mainly by external loads. In his thesis, Butler suggested that the main twist-causing force is provided by the left omphalomesenteric vein, which grows larger than the right vein and exerts a torque on the heart. Recent research supports this idea, as inducing the right vein to grow larger than the left leads to abnormal leftward looping [21*]. Other results suggest, however, that vein forces provide only a relatively small amount of torsion which determines looping direction, while the splanchnopleure supplies a surface load that pushes the HT into its fully twisted position (Fig. 1D) [22*,23].
This is not the full story, however; multiple redundant mechanisms also may be involved in torsion. For example, some data suggest that asymmetric cell proliferation in the dorsal mesocardium determines looping direction, as cells normally divide faster on the left side of this structure and push the HT rightward [24*]. In addition, Linask and colleagues have shown that a protein called flectin is expressed predominantly on the left side of the HT during rightward looping, while greater expression on the right side leads to abnormal leftward looping [25,26]. More recently, flectin has been identified as a form of myosin II [27], but its function in looping remains unknown. Interestingly, c-looping in the chick does not require contraction, while in zebrafish embryos inhibition of contraction apparently affects looping [28]. Here, it is important to note that significant morphological differences exist between zebrafish and chick (as well as human) hearts [29,30*].
The next phase of looping, termed s-looping, moves the primitive atria from their initial location caudal to the primary HT (future left ventricle) into their ultimate positions anterior to the ventricle [5]. The forces that drive s-looping apparently are exerted by external loads, including those supplied by the brain tube as it bends [31].
Effects of Blood Flow
Some researchers have speculated and continue to speculate that hemodynamic loads affect the early stages of looping [26,32], but most available evidence suggests otherwise. While some studies in zebrafish support a role for blood flow [32], the chick heart undergoes normal c-looping when the heartbeat is blocked [33,34].
On the other hand, studies have shown convincingly that blood flow affects later growth and remodeling of the heart. For example, the embryonic heart has the ability to adapt to changes in loading conditions in ways that parallel the mature heart, e.g., pressure overload triggers ventricular hypertrophy [35,36]. Moreover, perturbing pressure and flow leads to abnormalities in patterns of myocardial trabeculation, septation, and valve formation [7,30*,32,37,38,39,40]. Experiments have linked fluid shear (drag) to the regulation of these processes, possibly through changes in gene expression [41,42,43,44,45]. Relatively little is known about the corresponding morphogenetic mechanisms, but a recent computer model suggests that both fluid pressure and shear stress play major roles in molding the valves [46*].
Brain Morphogenesis
The embryonic brain begins to develop at approximately the same time as the heart.
Shaping of the Primitive Brain Tube
The anterior part of the neural tube expands to create the brain tube (BT), while the posterior portion of the neural tube becomes the spinal cord. Local circumferential constrictions next divide the neuroepithelium of the BT into three primary vesicles called the forebrain, midbrain, and hindbrain. In addition, bilateral evaginations from the forebrain create the optic vesicles, and a series of transient bulges called rhombomeres form along the hindbrain (Fig. 2A).
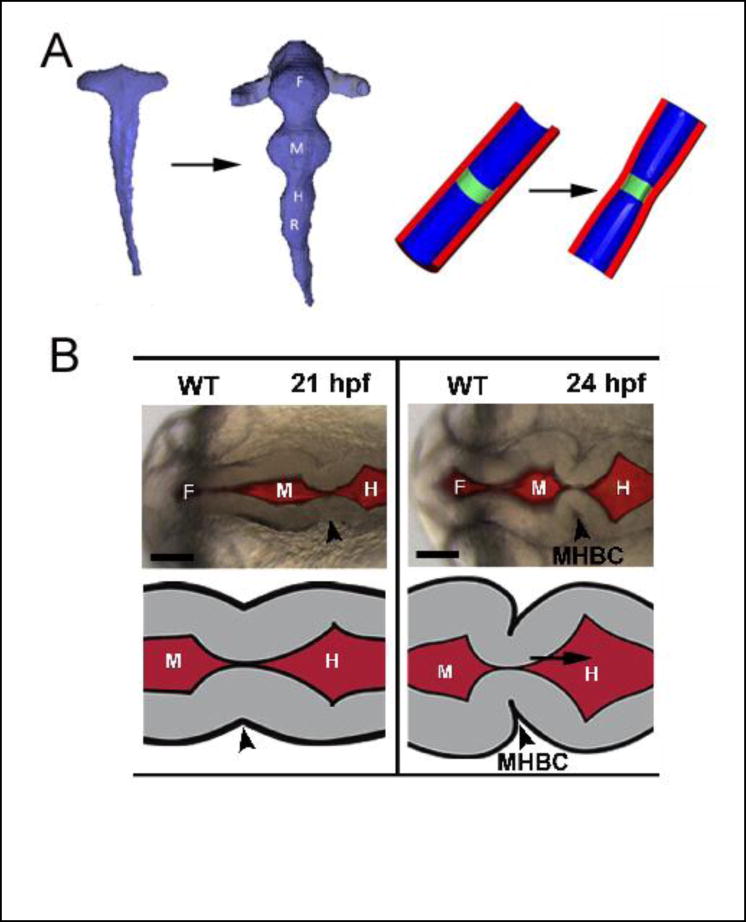
Fig 2. Formation of primary vesicles in brain tube.
(A) Chick embryo. Reconstructed brain lumen at stages 10 and 12 (left) and schematic of boundary formation (right). Boundaries between vesicles are created by circumferential actomyosin contraction (green region) at apical side of wall. From [47*]. (B) Zebrafish embryo. Boundary between midbrain and hindbrain (arrowhead) forms in two steps: radial shortening (left) and basal constriction (right) of neuroepithelial cells. (F= forebrain; M = midbrain; H = hindbrain; MHBC = midbrain-hindbrain boundary constriction) From [48*].
Recent studies have shown that the mechanism that creates the boundaries between vesicles is species dependent. The primary mechanism in the chick is localized circumferential contraction at the apical (inner) side of the wall, which decreases the BT circumference within the boundary regions (Fig. 2A) [47*]. In zebrafish, on the other hand, radial cellular shortening first generates a local circumferential groove that establishes the midbrain-hindbrain boundary, which is then sharpened by local laminin-dependent basal constriction [48*] (Fig. 2B). The specific biophysical mechanisms that drive these shape changes are not well understood, but actin intensity is highest on the basal side of the boundary region, suggesting that actomyosin contraction generates the constriction.
The reasons for these differences between zebrafish and chicken are unclear, but Filas et al. [49] speculate that interspecies differences in early BT morphology demand different mechanisms. For example, the lumen is initially closed in the zebrafish brain but open in chick and human. Localized contraction is required to open the zebrafish BT [50], which later relaxes so the BT can expand [51].
As in the early heart tube, fluid pressure inside the BT apparently plays a crucial role in growth but not morphogenesis. After the primary vesicles form, the ends of the BT seal and the brain expands rapidly as cerebrospinal fluid (CSF) accumulates in the lumen [52]. Experiments suggest that mitotic rates increase in response to wall stresses generated by rising CSF pressure [53,54]; growth is reduced considerably when the pressure is relieved [55]. However, although differential growth can deepen and sharpen the vesicle boundaries [48*], growth apparently plays a relatively minor role in primary vesicle formation [56].
Like the heart, the BT bends and (in some species) twists [57,58]. As in the heart, data suggest that differential growth drives bending [59,60,61], whereas forces imposed by extraembryonic membranes cause torsion [57,62]. Differential cell proliferation within the BT [63] and changes in somite shape [64,65] also may play a role in torsion. Interestingly, some investigators have speculated that the direction of cardiac looping determines the direction of brain torsion in the chick [66], as both structures almost always twist in the same direction [67]. However, the specific physical mechanisms of both bending and torsion remain poorly understood for the brain.
Later the forebrain undergoes further subdivision. A circumferential groove divides it into the telencephalon and diencephalon, followed by a longitudinal boundary that divides the anteriorlocated telencephalon into left and right sides that eventually become the cerebral hemispheres [68]. The mechanisms that create these boundaries are unknown but may involve both regional contraction and differential growth driven by the rising CSF pressure.
Cortical Folding
In most large mammals, the cerebral cortex (a thin outer layer of gray matter) develops a convoluted shape consisting of gyri (outward folds) and sulci (inward folds). This process, which occurs during the third trimester in humans, greatly increases the surface area of the cortex and is important for normal brain function [69,70]. While researchers have speculated about the mechanics of cortical folding for decades, interest in this problem has intensified during the last 15 years.
Two main theories have dominated thinking on this topic. According to the axon tension hypothesis, axons connecting related regions of the cortex generate tension that pulls these regions together, causing the surface to buckle outward [71**] (Fig. 3A). While this hypothesis has garnered considerable support [72,73], recent measurements of fiber architecture and tissue stress in ferret brains seem to contradict such a mechanism [74*].
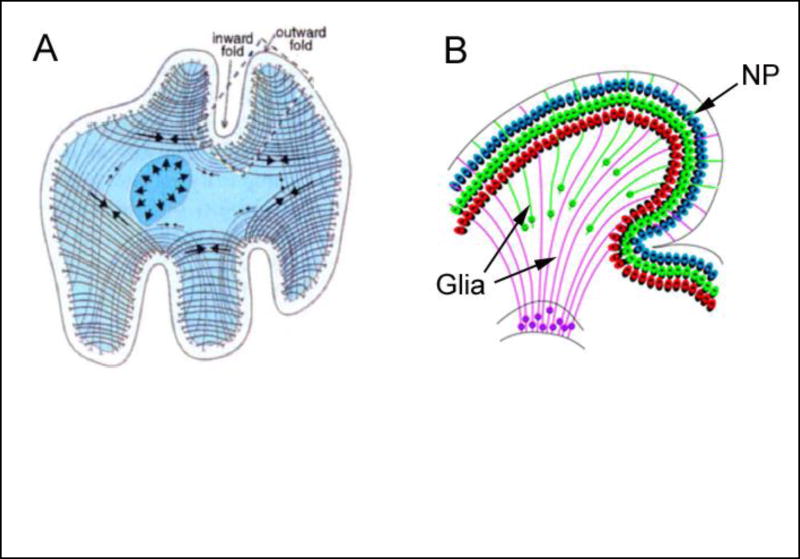
Fig 3. Hypotheses for folding of cerebral cortex.
(A) Axon tension. Tension generated by axons (arrows on curved lines) draws interconnected regions together, creating gyri (outward folds). From [71**]. (B) Differential growth. Neural progenitors (NP) migrate and spread out long fan-like glial fibers as they enter the cortex, expanding the surface area and creating a gyrus. From [77**].
In contrast, recent studies have provided compelling evidence supporting the differential growth hypothesis, whereby folding is driven by different growth rates between various regions and layers of the brain. According to this mechanism, tangential expansion of the cortex is restricted by slower growing subcortical layers, putting the cortex into a state of compression and causing it to buckle [74*,75]. Computer modeling has shown that this mechanism produces stress distributions that are consistent with experimental results [74*].
Currently available data suggest the following sequence of events. First, neuronal progenitors multiply within the ventricular zone of the brain at genetically determined rates that are higher under future gyri than sulci [76,77**]. Genetic regulation of proliferation ensures a consistent global folding pattern, which is highly conserved within a given species [69,76]. These progenitor cells migrate to the cortex along radially oriented glial fibers [78], which fan out toward the surface as new glia form between old fibers [77**] (Fig. 3B). These cells expand the cortex and generate relatively shallow surface bumps that are precursors to the primary gyri. These bumps grow larger during neuronal differentiation as cell bodies and dendrites grow and further expand the cortex [79**]. Finally, secondary folds develop with a more variable pattern influenced by local variations in geometry and mechanical properties [76].
This scheme is consistent with the following findings: (1) cortical folding occurs after neuronal proliferation and migration to the cortex is complete [79**,80,81]; (2) smooth (lissencephalic) brains lack fanning of glial fibers [77**]; (3) gyri undergo more rapid tangential growth than sulci [76,82]; and (4) experimentally accelerated cortical growth can cause normally lissencephalic brains to fold [83*,84,85]. Nevertheless, the differential growth hypothesis may not be consistent with all available data, and the mechanism of cortical folding continues to be debated [69,81].
Conclusions
Some common themes emerge from studies of early heart and brain morphogenesis. For both organs, differential growth and external loads play central roles in large-scale tissue shaping. Differential growth apparently causes most of the bending in both tubular structures, while external loads drive torsion. Differential growth also is prominent in generating local shape changes that occur during later development, such as myocardial trabeculation and cortical folding. Interestingly, the mechanisms that create these tubes in the first place, e.g., active contraction and cell intercalation [2], generally play more minor roles, with one exception being boundary formation between the primary brain vesicles.
These similarities extend to other organs that originate from epithelial tubes. For example, constraints imposed by the mesentery on the growing gut tube causes the gut tube to loop as it grows [86], while differential growth causes internal buckling that generates villi [87**].
Multiple backup mechanisms and complex 3-D changes in shape make studying the physical mechanisms of organogenesis an extremely challenging endeavor. Complete understanding of these problems will require the development of new molecular and genetic tools for targeting specific processes, as well as new image analysis techniques to measure morphogenetic changes in tissue strain and cell shapes in 4-D. Future work also is needed to investigate the role of mechanical feedback and the interactions between mechanics, gene expression, and morphogenesis. Despite the long history, studies of the mechanisms of organogenesis are in some ways just beginning.
Acknowledgments
This work was supported by NIH grant R01 NS070918. I thank Phil Bayly for providing comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*of special interest
**of outstanding interest
- 1.Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2010;341:114–125. doi: 10.1016/j.ydbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Lecuit T, Lenne PF, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu Rev Cell Dev Biol. 2011;27:157–184. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CM, Gleghorn JP. Sculpting organs: mechanical regulation of tissue development. Annu Rev Biomed Eng. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
- 4.Taber LA. Biophysical mechanisms of cardiac looping. International Journal of Developmental Biology. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- 5.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anatomical Record. 2000;259:248–262. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Kirby ML. Cardiac Development. Oxford: Oxford Univ Press; 2007. [Google Scholar]
- 7.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Patten BM. The Formation of the Cardiac Loop in the Chick. American Journal of Anatomy. 1922;30:373–397. [Google Scholar]
- 10.Manasek FJ, Kulikowski RR, Nakamura A, Nguyenphuc Q, Lacktis JW, Zak R. Early heart development: a new model of cardiac morphogenesis. In: Raven Press, editor. Growth of the Heart in Health and Disease. 1984. pp. 105–130. [Google Scholar]
- 11.Stalsberg H. Mechanism of dextral looping of the embryonic heart. American Journal of Cardiology. 1970;25:265–271. doi: 10.1016/s0002-9149(70)80002-9. [DOI] [PubMed] [Google Scholar]
- 12.Manasek FJ, Burnside MB, Waterman RE. Myocardial cell shape changes as a mechanism of embryonic heart looping. Developmental Biology. 1972;29:349–371. doi: 10.1016/0012-1606(72)90077-2. [DOI] [PubMed] [Google Scholar]
- 13.Latacha KS, Remond MC, Ramasubramanian A, Chen AY, Elson EL, Taber LA. The role of actin polymerization in bending of the early heart tube. Developmental Dynamics. 2005;233:1272–1286. doi: 10.1002/dvdy.20488. [DOI] [PubMed] [Google Scholar]
- 14.Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 2007;5:e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taber LA, Lin IE, Clark EB. Mechanics of cardiac looping. Developmental Dynamics. 1995;203:42–50. doi: 10.1002/aja.1002030105. [DOI] [PubMed] [Google Scholar]
- **16.Soufan AT, van den Berg G, Ruijter JM, de Boer PA, van den Hoff MJ, Moorman AF. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res. 2006;99:545–552. doi: 10.1161/01.RES.0000239407.45137.97. This paper presents quantitative measurements of changes in size and proliferation of cardiomyocytes in the looping chick heart. Among other important findings, a dorsal-ventral gradient in hypertrophy is identified, suggesting for the first time that regional changes in cell size may cause the looping heart tube to bend. [DOI] [PubMed] [Google Scholar]
- 17.Grossman W. Cardiac Hypertrophy: Useful Adaptation or Pathologic Process? American Journal of Medicine. 1980;69:576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- **18.Shi Y, Yao J, Xu G, Taber LA. Bending of the looping heart: differential growth revisited. J Biomech Eng. 2014 doi: 10.1115/1.4026645. (in press) This paper shows that differential hypertrophic growth likely provides the main driving force for bending of the heart tube during cardiac c-looping. Experiments on isolated embryonic chick hearts and computational modeling reveal that other mechanisms may play secondary roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Developmental Dynamics. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- 20.Winston JB, Erlich JM, Green CA, Aluko A, Kaiser KA, Takematsu M, Barlow RS, Sureka AO, LaPage MJ, Janss LL, et al. Heterogeneity of genetic modifiers ensures normal cardiac development. Circulation. 2010;121:1313–1321. doi: 10.1161/CIRCULATIONAHA.109.887687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Kidokoro H, Okabe M, Tamura K. Time-lapse analysis reveals local asymmetrical changes in C-looping heart tube. Dev Dyn. 2008;237:3545–3556. doi: 10.1002/dvdy.21662. This paper presents detailed observations of left-right morphological asymmetries in the looping chick heart. [DOI] [PubMed] [Google Scholar]
- *22.Voronov DA, Alford PW, Xu G, Taber LA. The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Developmental Biology. 2004;272:339–350. doi: 10.1016/j.ydbio.2004.04.033. This paper presents evidence that the torsional component of cardiac c-looping in chick embryos is driven by forces applied by tissues external to the heart tube, i.e., the splanchnopleure and omphalomesenteric veins. [DOI] [PubMed] [Google Scholar]
- 23.Voronov DA, Taber LA. Cardiac looping in experimental conditions: the effects of extraembryonic forces. Developmental Dynamics. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- *24.Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM. Cardiac morphogenesis: matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn. 2005;233:739–753. doi: 10.1002/dvdy.20377. In this paper, the authors inhibit matrix metalloproteinase (MMP) to perturb the left-right patterning of cell proliferation within the dorsal mesocardium, which initially attaches the embryonic chick heart to the foregut. The results show that the heart loops to the side opposite the side with the greatest proliferation, e.g., increasing cell division on the right side pushes the heart tube toward the left and causes abnormal leftward looping. [DOI] [PubMed] [Google Scholar]
- 25.Linask KK, Yu X, Chen Y, Han MD. Directionality of heart looping: effects of Pitx2c misexpression on flectin asymmetry and midline structures. Developmental Biology. 2002;246:407–417. doi: 10.1006/dbio.2002.0661. [DOI] [PubMed] [Google Scholar]
- 26.Linask KK, Vanauker M. A role for the cytoskeleton in heart looping. ScientificWorldJournal. 2007;7:280–298. doi: 10.1100/tsw.2007.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Seeholzer SH, Han M, Arnold AS, Serrano M, Garita B, Philp NJ, Farthing C, Steele P, Chen J, et al. Cellular nonmuscle myosins NMHC-IIA and NMHC-IIB and vertebrate heart looping. Dev Dyn. 2008;237:3577–3590. doi: 10.1002/dvdy.21645. [DOI] [PubMed] [Google Scholar]
- 28.Noel ES, Verhoeven M, Lagendijk AK, Tessadori F, Smith K, Choorapoikayil S, den Hertog J, Bakkers J. A Nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat Commun. 2013;4:2754. doi: 10.1038/ncomms3754. [DOI] [PubMed] [Google Scholar]
- 29.Aleksandrova A, Czirok A, Szabo A, Filla MB, Hossain MJ, Whelan PF, Lansford R, Rongish BJ. Convective tissue movements play a major role in avian endocardial morphogenesis. Dev Biol. 2012;363:348–361. doi: 10.1016/j.ydbio.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, Fraser SE. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 2009;7:e1000246. doi: 10.1371/journal.pbio.1000246. This paper presents evidence that morphogenesis of the heart valves in zebrafish is triggered by reversing fluid shear stress on the cardiac cushions (primordial valves). These stresses are caused by backflow before unidirectional flow has been established. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramasubramanian A, Chu-Lagraff QB, Buma T, Chico KT, Carnes ME, Burnett KR, Bradner SA, Gordon SS. On the role of intrinsic and extrinsic forces in early cardiac S-looping. Dev Dyn. 2013;242:801–816. doi: 10.1002/dvdy.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 33.Manasek FJ, Monroe RG. Early cardiac morphogenesis is independent of function. Developmental Biology. 1972;27:584–588. doi: 10.1016/0012-1606(72)90196-0. [DOI] [PubMed] [Google Scholar]
- 34.Remond MC, Fee JA, Elson EL, Taber LA. Myosin-based contraction is not necessary for cardiac c-looping in the chick embryo. Anat Embryol (Berl) 2006;211:443–454. doi: 10.1007/s00429-006-0094-0. [DOI] [PubMed] [Google Scholar]
- 35.Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ. Effect of Increased Pressure on Ventricular Growth in Stage 21 Chick Embryos. American Journal of Physiology. 1989;257:H55–H61. doi: 10.1152/ajpheart.1989.257.1.H55. [DOI] [PubMed] [Google Scholar]
- 36.Lin YF, Swinburne I, Yelon D. Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Dev Biol. 2012;362:242–253. doi: 10.1016/j.ydbio.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Sedmera D, Pexieder T, Hu N, Clark EB. A Quantitative Study of the Ventricular Myoarchitecture in the Stage 21–29 Chick Embryo Following Decreased Loading. European Journal of Morphology. 1998;36:105–119. doi: 10.1076/ejom.36.2.105.4775. [DOI] [PubMed] [Google Scholar]
- 39.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Developmental Dynamics. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 40.Goenezen S, Rennie MY, Rugonyi S. Biomechanics of early cardiac development. Biomech Model Mechanobiol. 2012;11:1187–1204. doi: 10.1007/s10237-012-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hierck BP, Van der Heiden K, Poelma C, Westerweel J, Poelmann RE. Fluid shear stress and inner curvature remodeling of the embryonic heart. Choosing the right lane! ScientificWorldJournal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res. 2005;96:1291–1298. doi: 10.1161/01.RES.0000171901.40952.0d. [DOI] [PubMed] [Google Scholar]
- 43.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res. 1997;80:473–481. doi: 10.1161/01.res.80.4.473. [DOI] [PubMed] [Google Scholar]
- 44.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morphogenesis. Circ Cardiovasc Genet. 2012;5:132–142. doi: 10.1161/CIRCGENETICS.111.961086. [DOI] [PubMed] [Google Scholar]
- 45.Tan H, Biechler S, Junor L, Yost MJ, Dean D, Li J, Potts JD, Goodwin RL. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev Biol. 2013;374:345–356. doi: 10.1016/j.ydbio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- *46.Buskohl PR, Jenkins JT, Butcher JT. Computational simulation of hemodynamic-driven growth and remodeling of embryonic atrioventricular valves. Biomech Model Mechanobiol. 2012;11:1205–1217. doi: 10.1007/s10237-012-0424-5. This paper presents a computational model for shaping of endocardial cushions into the primitive heart valve leaflets. With tissue growth assumed to depend on fluid pressure and shear stress through mechanical feedback laws, the model predicts realistic valve shapes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Filas BA, Oltean A, Majidi S, Bayly PV, Beebe DC, Taber LA. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys Biol. 2012;9:066007. doi: 10.1088/1478-3975/9/6/066007. The results in this paper indicate that the boundaries between the primary vesicles in the brain tube of the chick embryo are created by regional circumferential contraction at the luminal side of neuroepithelial wall. In contrast, longitudinal contraction between boundaries produces the bulging of rhombomeres in the hindbrain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Gutzman JH, Graeden EG, Lowery LA, Holley HS, Sive H. Formation of the zebrafish midbrain-hindbrain boundary constriction requires laminin-dependent basal constriction. Mech Dev. 2008;125:974–983. doi: 10.1016/j.mod.2008.07.004. This paper shows that two steps create the midbrain-hindbrain boundary in the zebrafish embryo. First, cells in the boundary region shorten radially, and then they become wedge-shaped via a laminin-dependent basal constriction at the outer wall (rather than the apical constriction that occurs in chick). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filas BA, Oltean A, Beebe DC, Okamoto RJ, Bayly PV, Taber LA. A potential role for differential contractility in early brain development and evolution. Biomech Model Mechanobiol. 2012;11:1251–1262. doi: 10.1007/s10237-012-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyholm MK, Abdelilah-Seyfried S, Grinblat Y. A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation. J Cell Sci. 2009;122:2137–2148. doi: 10.1242/jcs.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutzman JH, Sive H. Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development. 2010;137:795–804. doi: 10.1242/dev.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desmond ME, Levitan ML. Brain expansion in the chick embryo initiated by experimentally produced occlusion of the spinal neurocoel. Anatomical Record. 2002;268:147–159. doi: 10.1002/ar.10146. [DOI] [PubMed] [Google Scholar]
- 53.Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327:263–272. doi: 10.1016/j.ydbio.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 54.Desmond ME, Levitan ML, Haas AR. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat Rec A Discov Mol Cell Evol Biol. 2005;285:737–747. doi: 10.1002/ar.a.20211. [DOI] [PubMed] [Google Scholar]
- 55.Desmond ME, Jacobson AG. Embryonic brain enlargement requires cerebrospinal fluid pressure. Developmental Biology. 1977;57:188–198. doi: 10.1016/0012-1606(77)90364-5. [DOI] [PubMed] [Google Scholar]
- 56.Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. Bioessays. 2009;31:446–458. doi: 10.1002/bies.200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller SA, White RD. Right-left asymmetry of cell proliferation predominates in mouse embryos undergoing clockwise axial rotation. Anat Rec. 1998;250:103–108. doi: 10.1002/(SICI)1097-0185(199801)250:1<103::AID-AR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 58.Patten BM. Early Embryology of the Chick. New York: McGraw-Hill; 1951. [Google Scholar]
- 59.Pikalow AS, Flynn ME, Searls RL. Development of cranial flexure and Rathke’s pouch in the chick embryo. Anat Rec. 1994;238:407–414. doi: 10.1002/ar.1092380315. [DOI] [PubMed] [Google Scholar]
- 60.Takamatsu T, Fujita S. Growth of notochord and formation of cranial and mesencephalic flexures in chicken embryo. Development, growth \& differentiation. 1987;29:497–502. doi: 10.1111/j.1440-169X.1987.00497.x. [DOI] [PubMed] [Google Scholar]
- 61.Goodrum GR, Jacobson AG. Cephalic flexure formation in the chick embryo. J Exp Zool. 1981;216:399–408. doi: 10.1002/jez.1402160308. [DOI] [PubMed] [Google Scholar]
- 62.Deuchar EM, Parker FM. Further observations on axial rotation in rat embryos. Acta Embryol Exp (Palermo) 1975:55–68. [PubMed] [Google Scholar]
- 63.Poelmann RE, Mentink MM, van Leeuwen JL. Axial rotation of murine embryos, a study of asymmetric mitotic activity in the neural tube of somite stages. Anat Embryol (Berl) 1987;176:99–103. doi: 10.1007/BF00309757. [DOI] [PubMed] [Google Scholar]
- 64.Manca A, Capsoni S, Di Luzio A, Vignone D, Malerba F, Paoletti F, Brandi R, Arisi I, Cattaneo A, Levi-Montalcini R. Nerve growth factor regulates axial rotation during early stages of chick embryo development. Proc Natl Acad Sci U S A. 2012;109:2009–2014. doi: 10.1073/pnas.1121138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda M. Change of rat embryos from a ventrally concave U-shape to a ventrally convex C-shape. Development, Growth & Differentiation. 1991;33:117–122. doi: 10.1111/j.1440-169X.1991.00117.x. [DOI] [PubMed] [Google Scholar]
- 66.Waddington CH. The dependence of head curvature on the development of the heart in the chick embryo. Journal of Experimental Biology. 1937;14:229–231. [Google Scholar]
- 67.Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M. Cerberus regulates left-right asymmetry of the embryonic head and heart. Curr Biol. 1999;9:931–938. doi: 10.1016/s0960-9822(99)80419-9. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert SF. Developmental Biology. 9. Sunderland, MA: Sinauer Associates; 2010. [Google Scholar]
- 69.Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends in Neurosciences. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Bayly PV, Taber LA, Kroenke CD. Mechanical forces in cerebral cortical folding: A review of measurements and models. Journal of the mechanical behavior of biomedical materials. 2014;29:568–581. doi: 10.1016/j.jmbbm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **71.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. This paper presents the axon tension hypothesis for folding of the cerebral cortex, arguing that gyri form when tension generated in axons draws together interconnected regions of the cortex. [DOI] [PubMed] [Google Scholar]
- 72.Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity-driven white matter scaling and folding in primate cerebral cortex. Proc Natl Acad Sci U S A. 2010;107:19008–19013. doi: 10.1073/pnas.1012590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210:411–417. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- *74.Xu G, Knutsen AK, Dikranian K, Kroenke CD, Bayly PV, Taber LA. Axons pull on the brain, but tension does not drive cortical folding. J Biomech Eng. 2010;132:071013. doi: 10.1115/1.4001683. This paper uses experiments and computer modeling of cortical folding in the ferret brain to show that folding is likely driven by differential growth rather than axon tension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richman DP, Stewart RM, Hutchinson JW, Caviness VS., Jr Mechanical model of brain convolutional development. Science. 1975;189:18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- 76.Ronan L, Voets N, Rua C, Alexander-Bloch A, Hough M, Mackay C, Crow TJ, James A, Giedd JN, Fletcher PC. Differential Tangential Expansion as a Mechanism for Cortical Gyrification. Cereb Cortex. 2013 doi: 10.1093/cercor/bht082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **77.Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the Mammalian cerebral cortex. Cereb Cortex. 2010;21:1674–1694. doi: 10.1093/cercor/bhq238. This outstanding paper presents evidence that differential growth drives cortical folding. Observations show that radial glial fibers fan out as they near the cotex, facilitating the spread of neural progenitor cells as they migrate into the cortex. The authors show that this fanning is more pronounced in regions destined to become gyri and is not present in lissencephalic (smooth brain) species. [DOI] [PubMed] [Google Scholar]
- 78.Rakic P. A Small Step for the Cell, a Giant Leap for Mankind - a Hypothesis of Neocortical Expansion during Evolution. Trends in Neurosciences. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- **79.Neal J, Takahashi M, Silva M, Tiao G, Walsh CA, Sheen VL. Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J Anat. 2007;210:66–77. doi: 10.1111/j.1469-7580.2006.00674.x. This paper uses magnetic resonance imaging to follow the temporal and spatial pattern of neuronal migration, proliferation, and differentiation during cortical folding in the ferret brain. The results indicate that folding occurs largely after neuronal proliferation and migration are complete, suggesting that growth of the cortex during neural differentiation induces folding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman-Rakic PS, Rakic P. Experimental modification of gyral patterns. In: Geschwind N, Galaburda AM, editors. Cerebral dominance : the biological foundations. Harvard University Press; 1984. pp. 179–192. [Google Scholar]
- 81.Franze K. The mechanical control of nervous system development. Development. 2013;140:3069–3077. doi: 10.1242/dev.079145. [DOI] [PubMed] [Google Scholar]
- 82.Knutsen AK, Kroenke CD, Chang YV, Taber LA, Bayly PV. Spatial and temporal variations of cortical growth during gyrogenesis in the developing ferret brain. Cereb Cortex. 2013;23:488–498. doi: 10.1093/cercor/bhs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *83.Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, Sanz-Aquela JM, Beckers J, Blum R, Borrell V, et al. Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell. 2013;153:535–549. doi: 10.1016/j.cell.2013.03.027. This paper shows that experimentally increasing the proliferation of neural and glial progenitor cells causes the normally lissencephalic cerebral cortex of the mouse brain to fold. These results support the differential growth hypothesis for cortical folding. [DOI] [PubMed] [Google Scholar]
- 84.Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- 85.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 86.Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **87.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L. Villification: how the gut gets its villi. Science. 2013;342:212–218. doi: 10.1126/science.1238842. Although not a focus of the present review, this paper represents an outstanding example of how experiments and computer modeling can be used to uncover morphogenetic mechanisms. The authors show that intestinal villi are created by sequential buckling and folding of the lining of the gut tube caused by constrained growth as the layers of the wall differentiate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]