Abstract
Staphylococcus aureus strains that cause human diseases produce a large family of pyrogenic toxin superantigens (SAgs). These include toxic shock syndrome toxin-1 (TSST-1), the staphylococcal enterotoxins (SEs), and the SE-like proteins; to date 23 staphylococcal SAgs have been described. Among the SAgs, three have been highly associated with human diseases (TSST-1, SEB, and SEC), likely because they are produced in high concentrations compared to other SAgs. Another major family of exotoxins produced by S. aureus is the cytolysins, particularly α-, β-, γ- and δ-toxins, phenol soluble modulins, and leukocidins. This review discusses the association of SAgs with human diseases, and particularly the “outside-in” signaling mechanism that leads to SAg-associated diseases. We discuss SAg interactions with three host immune cell receptors, including variable regions of the β-chain of the T cell receptor, MHC II α- and/or β-chains, and an epithelial/endothelial cell receptor that may include CD40. To a lesser extent we discuss the role of cytolysins in facilitating disease production by SAgs.
Keywords: Superantigen, TSST-1, Toxic shock syndrome toxin, menstrual toxic shock
Introduction
Staphylococcus aureus is a gram-positive, non-motile bacterium. It has the classical coccus shape, forming into “grape-like” clusters. S. aureus has been shown to play a role in various relatively mild diseases including superficial skin and soft tissue infections, such as furuncles, and food poisoning, and multiple life-threatening diseases such as, toxic shock syndrome (TSS), pneumonia, infective endocarditis (IE), and sepsis [1–8]. Antibiotic resistance has become a major problem in managing S. aureus infections, with the high occurrence of methicillin-resistant S. aureus (MRSA).
A wide variety of virulence factors are produced by S. aureus contributing to disease causation [1]. During exponential-phase growth, the organism produces cell surface virulence factors such as protein A, fibronectin-binding protein, clumping factor, as well as other microbial surface components recognizing adhesive matrix molecules [9]. These factors facilitate microbial colonization of the host and contribute to immune evasion. During post-exponential and early stationary phase, the cell-surface factors are no longer produced, but instead, secreted virulence factors are produced, including the major superantigens (SAgs), cytolysins, and other exoenzymes. This complex array of virulence factors has allowed S. aureus to become the leading cause of both healthcare- and community- associated serious, life-threatening infections in the U.S. and around the world [10].
This review will focus on research performed in our laboratory related to the mechanisms by which S. aureus uses its secreted virulence factors, notably SAgs and cytolysins, to cause serious human diseases. These infections typically begin at skin and mucosal surfaces with colonization of up to 40% of humans [1]. Subsequent to colonization, the organism uses its SAg and cytolysin secreted virulence factors to induce mucosal or skin inflammation, followed by SAg barrier penetration and massive activation of T-cells and antigen-presenting cells (APCs) to cause overt disease.
Families of Secreted Virulence Factors Causing Immune Dysfunction
Superantigens
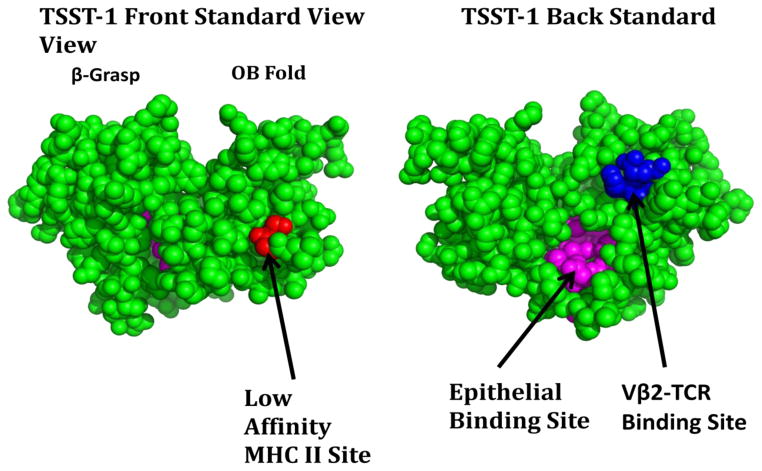
SAgs are a family of secreted virulence factors that formerly were categorized as pyrogenic toxins until renamed [11]. All pathogenic strains of S. aureus secrete SAgs. These non-glycosylated, exoproteins are relatively low molecular weight, ranging from 19,000–30,000 daltons [5]. SAgs are highly resistant to heat denaturation, proteolysis, acid denaturation, and desiccation [5, 12]. Twenty-three serologically distinct S. aureus SAgs exist including: TSS toxin-1 (TSST-1), staphylococcal enterotoxins (SEs), and SE-like (SE-l) superantigens [4, 5, 12]. SAgs contain an amino-terminal oligosaccharide/oligonucleotide binding (OB) fold and a carboxy-terminal β-grasp domain (Figure 1). In a groove between these folds, on the top front (standard view) for most SAgs, and the top back (for TSST-1) lies a variable region, β-chain T cell receptor (Vβ-TCR) binding motif, and in the OB fold lies a low-affinity major histocompatibility complex class II (MHC II) binding site. In addition, a higher-affinity MHC II site may be located in the β-grasp domain in many SAgs. A dodecapeptide region is also present, usually on the backside of the central α-helix domain, thought to be important for epithelial and endothelial cell binding. This site has been suggested to bind to immune co-stimulatory molecules CD28 and CD40, as well as one or more unknown host receptors [13, 14]. SEs, which are known to cause vomiting and diarrhea and thus staphylococcal food poisoning, also have a cystine loop structure in the top of the OB fold which is responsible for emetic activity [15]. Depending on which of these host cell binding sites are present, staphylococcal SAgs are classified into four groups [12].
Figure 1.
Three dimensional structure of TSST-1 as representative of the SAg family. Red amino acids identify two residues within the MHC II contact site; blue amino acids identify three residues within the Vβ2-TCR contact site; purple amino acids identify the dodecapeptide epithelial cells binding site.
SAgs, except SE-l X, are encoded on variable genetic DNA elements. Their expression is controlled by global regulatory systems including multiple staphylococcal two-component and quorum sensing systems. The major regulator systems include SrrA/B, AgrA/B, and SaeR/S [16–18, 14].
In addition to being pyrogenic by inducing production of interleukin (IL) 1β and IL-6 from APCs, combined with direct action on the fever response control center of the hypothalamus, all SAgs enhance susceptibility to gram-negative lipopolysaccharide (LPS) up to 106-fold [19, 20]. This mechanism, although incompletely proven, has been suggested to explain TSS development in humans but not in mice and non-human primates [14]. Humans have many more LPS-containing gram-negative bacteria in their mucosal microbiomes than do mice and non-human primates [21]. It has been hypothesized that TSST-1 (and other SAgs) inactivation of liver LPS clearance function, combined with small amounts of LPS leakage across the gastrointestinal tract and vagina in women, leads to synergy in immune cell production of tumor necrosis factor (TNF) α and β [22, 14]. These latter two factors cause capillary leak, the severest symptom of TSS.
Today, the most well-known activity of SAgs is their superantigenicity. SAgs massively activate T-cells and APCs. For example in TSS patients, Vβ2-TCR+ T-cells may expand from being 10% rising up to 70% of all T-cells during acute infection with TSST-1 producing S. aureus. This superantigenicity is achieved with by cross-bridging Vβ-TCRs with α- and/or β-chains of MHCII molecules on APCs [5, 23, 24]. Each SAg interacts with a relatively unique set of Vβ-TCRs. The interactions induce significant activation and proliferation of T-cells and APC activation. A typical antigen stimulates approximately 1/10,000 T-cells, whereas SAgs stimulate up to 50% of T-cells. The massive production of cytokines by the T-cells and APCs results in the cytokine storm that typifies TSS.
Cytolysins and PSMs
Another group of secreted S. aureus virulence factors are the cytolysins, which damage host plasma membranes. The cytolysins include the sphingomyelinase, β-toxin, phenol soluble modulins (PSMs), and the pore-forming toxins: α-toxin, γ-toxin, Panton Valentine leukocidin (PVL), LukED, and LukGH/AB [25]. These toxins are known to be hemolytic to red blood cells, with susceptibility depending on the source of the blood cells. However, this probably is not their main functions, but instead, they function to kill immune cells influxing into sites of infection. All pathogenic S. aureus strains encode for one or more cytolysins [25]; it appears that pathogenic strains must produce at least one major SAg and one major cytolysin to colonize and cause disease in humans [9].
β-toxin is a neutral sphingomyelinase, and as a member of the DNase I superfamily, folds into a four-layer sandwich, at the center of which are two β-sheets [26]. The sphingomyelinase activity allows it to be a “hot-cold” cytolysin capable of lysing blood cells when incubated at 4 °C after cleaving sphingomyelin at 37 °C. The toxin cleaves phosphodiesterase bonds, digesting sphingomyelin into ceramide and phosphorylcholine, along with causing the release of the chemotactic molecule sphingosine-1-phosphate [25]. The reorganization that β-toxin causes within cell membranes suggests it may be causing cell death by modifying membrane fluidity, and destabilizing bi-layer structure. A possible alternative is that the formation of large ceramide-rich signaling platforms results in cell death. In addition to having sphingomyelinase activity, β-toxin also has a biofilm ligase activity. The biofilm ligase activity is defined by its ability to cross-link in the presence of DNA [27]. β-toxin has been shown to affect a variety of cell types and is cytotoxic to monocytes, polymorphonuclear leukocytes (PMNs), resting lymphocytes, and proliferating lymphocytes [25, 26].
The cytolysin, α-toxin, has a molecular weight of 33,000 daltons and is primarily made up of β-sheets. A prepore α-toxin complex is comprised of a group of monomers that assemble into a homo-heptamer which then matures into a β-barrel transmembrane pore. Formation of this pore on host cells leads to necrotic cell death [25]. α-toxin is cytolytic at high concentrations (microgram amounts) and pro-inflammatory at low concentrations (nanogram amounts) to a variety of mammalian cells such as erythrocytes, monocytes, epithelial, and endothelial cells [28]; it is the predominant cytolysin produced by S. aureus. α-toxin is considered to be a critical virulence factor in skin infections, such as furuncles and soft tissue abscesses in humans, and has been demonstrated to be a key virulence factor in mouse skin infection and pneumonia models [29]. α-toxin’s expression is increased upon interaction with epithelial cells and infection in vivo [30].
γ-toxin and leukocidins are bicomponent toxins, made up of two polypeptides each, slow (S) and fast (F), so named based on electrophoretic mobility. HlgB, the F component, and either HlgA or HlgC S components, combined together form γ-toxin. PVL is made from the combination of LukS-PV and LukF-PV, encoded on a bacteriophage. The mature pore of these pore forming toxins is formed from four S components alternatively arranged with four F components, maturing into a hetero-octamer to form a β-barrel transmembrane pore. Human neutrophils are highly susceptible to the cytotoxic effects of the pore forming cytolysins, γ-toxin and PVL. However, to be cytotoxic to human neutrophils, LukAB/GH must be present at 100 times the concentration as γ-toxin or PVL [25].
δ-toxin, like other PSMs, is a small amphipathic peptide with an α-helical structure. Its cytotoxic effects are thought to occur through the formation of transmembrane pores, plasma membrane destabilization following binding and aggregation at the host cell surface, or as a detergent at high concentrations to solubilize membranes [31]. Not only is δ-toxin cytotoxic to erythrocytes and causes membrane damage to a variety of mammalian cells, but it is also capable to lysing organelles and bacterial protoplasts and spheroplasts [31, 12]. Additionally, PSMs, have pro-inflammatory and chemotactic activities [32]. PSMs significantly contribute to the virulence of CA-MRSA, and have been shown to enhance PVL induced lysis of human neutrophils [29].
Outside-In Mechanism of Disease Production
As noted in the introduction, S. aureus causes human diseases, most often initiating disease production across mucosal and skin barriers. These two barriers are the most important immune defense mechanisms to prevent most microbes from causing diseases. Pathogens, such as S. aureus, have developed complex mechanisms to penetrate these barriers, mechanisms we collectively refer to as “outside-in signaling”. The principal feature of these mechanisms is interaction with non-standard immune cells, epithelial cells (outside), which begin a cascade of cytokine/chemokine signals that attract and activate standard immune cells (in) to disrupt the permeability barrier and facilitate disease production. This mechanism will be illustrated across the vaginal mucosal barrier as a model (Figure 2), but the same model exists to explain S. aureus disease across any mucosal or skin barrier. It is important to note that S. aureus strains must produce much higher levels of cytolysins for disease initiation across the difficult skin barrier, compared amounts of cytolysins for disease initiation across mucosal surfaces. The important non-immune barrier functions of mucosae require that they be permeable to an extent not required of highly-impenetrable skin barriers.
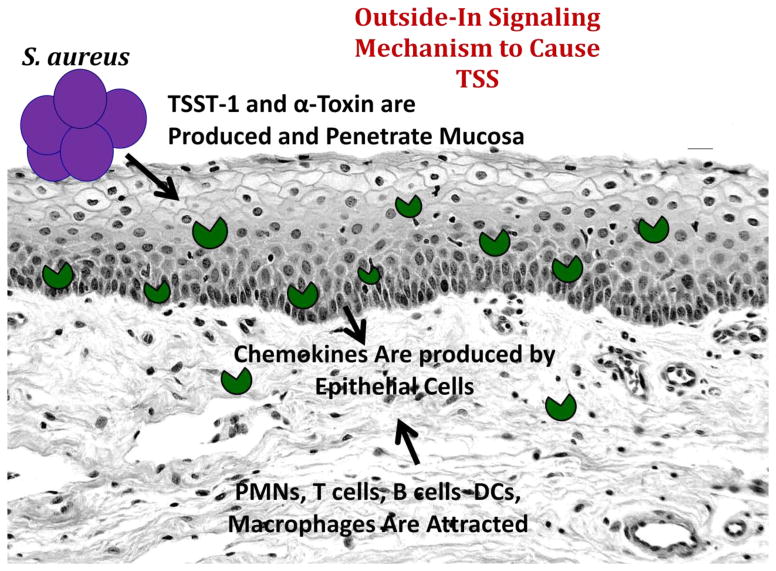
Figure 2.
“Outside-In signaling mechanism for S. aureus (purple) to cause menstrual, vaginal TSS. S. aureus produces TSST-1 and α-toxin (green pacmen) that are pro-inflammatory for human vaginal epithelial cells, causing them to produce pro-inflammatory chemokines. The chemokines attract components of the innate and adaptive immune systems into the submucosa, disrupting mucosal barrier integrity. Barrier disruption allows TSST-1 to penetrate and massively stimulate CD4+ T cell and macrophage proliferation. This leads to production of a cytokine storm, including TNF-α and β, IL-1β, IL-2, IL-6, and interferon γ. These cytokines are critical in producing the defining criteria of TSS (fever, hypotension, rash, skin peeling upon recovery, and a variable multi-organ component.
TSST-1 was first recognized to interact with epithelial cells to elicit a response through the outside-in signaling mechanism. The SAg binds through its dodecapeptide region to human vaginal epithelial cells, possible CD40 or another unknown receptor, stimulating the production of pro-inflammatory chemokines. Small amounts of cytolysins, particularly α-toxin, are required to facilitate this process through combinations of their cytotoxic and pro-inflammatory properties [33, 34]. The chemokines, including IL-8 and MIP-3α, then attract cells of the innate and adaptive immune systems into the submucosa. These immune cells become highly-activated, further enhance inflammation, and cause barrier disruption. Indeed in menstrual TSS, the human vaginal mucosal barrier becomes completely disrupted, and permeable to TSST-1 during menstrual TSS. At the same time, as TSST-1 penetrates the barrier into the submucosa, the SAg interacts potently with T-cells and macrophages, generating the cytokine response that manifests as TSS [35]. Our recent studies show, through use of TSST-1 mutants altered in mucosal permeability, that the SAg must penetrate the mucosal barrier to cause disease, but it appears likely that submucosal SAg activities, rather than systemic activities, are sufficient for TSS production. It is also important to note that epithelial cells interact with TSST-1 less effectively than T-cells and APCs. This reduced interaction likely explains why TSS does not occur in all persons, but is primarily restricted to those individuals with a vaginal mucosal epithelial receptor for TSST-1, who encounter large amounts of TSST-1, and who lack neutralizing antibodies to the SAg [36–38].
Subsequent to our studies of the outside-in signaling mechanism in development of staphylococcal TSS, we have proposed a similar mechanism to explain heterosexual transmission and production of AIDS in women [39]. In this instance, low-level inflammation induced by HIV and seminal fluid factors leads to chemokine production by vaginal epithelial cells. This in turn attracts and activates components of the innate and adaptive immune systems that lead to barrier disruption and HIV transmission. The presence of attracted and activated CD4+ T-cells in the vaginal submucosa provide the cells that become infected with HIV.
It is our ultimate hypothesis that all or nearly all pathogens that cause disease across mucosal and skin barriers must induce some degree of inflammation through inducing epithelial cells to produce pro-inflammatory chemokines. Thus, these cells should be considered components of the innate immune system, where their intent may be to slow disease production, but counterintuitively, are subverted actually by pathogens to participate in disease production.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Que YA, Moreillon P. Infective endocarditis. Nature Revi Cardiol. 2011;8:322–36. doi: 10.1038/nrcardio.2011.43. [DOI] [PubMed] [Google Scholar]
- 3.Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–21. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 4.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–72. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 5.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143(4):509–16. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 7.Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis. 2009;199:201–8. doi: 10.1086/595738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarre WW, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010;125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 12.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26:422–47. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovde CJ, Marr JC, Hoffmann ML, Hackett SP, Chi YI, Crum KK, et al. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol Microbiol. 1994;13:897–909. doi: 10.1111/j.1365-2958.1994.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 16.Yarwood JM, McCormick JK, Schlievert PM. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol. 2001;183:1113–23. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–49. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 18.Pragman AA, Schlievert PM. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol Med Microbiol. 2004;42:147–54. doi: 10.1016/j.femsim.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim YB, Watson DW. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970;131:611–22. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlievert PM, Watson DW. Group A streptococcal pyrogenic exotoxin: pyrogenicity, alteration of blood-brain barrier, and separation of sites for pyrogenicity and enhancement of lethal endotoxin shock. Infect Immun. 1978;21:753–63. doi: 10.1128/iai.21.3.753-763.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlievert PM. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis. 2009;200:676–8. doi: 10.1086/605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–72. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotzin BL, Leung DY, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Ann Rev Immunol. 1999;17:435–66. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 25.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Frontiers Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, et al. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–26. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huseby MJ, Kruse AC, Digre J, Kohler PL, Vocke JA, Mann EE, et al. Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc NBatl Acad Sci USA. 2010;107:14407–12. doi: 10.1073/pnas.0911032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YC, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, et al. Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry. 2011;50:7157–67. doi: 10.1021/bi200435n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong SY, Chen LF, Fowler VG., Jr Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: what is the clinical relevance? Sem Immunopathol. 2012;34:185–200. doi: 10.1007/s00281-011-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker D, Prince A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Sem Immunopathol. 2012;34:281–97. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ythier M, Entenza JM, Bille J, Vandenesch F, Bes M, Moreillon P, et al. Natural variability of in vitro adherence to fibrinogen and fibronectin does not correlate with in vivo infectivity of Staphylococcus aureus. Infect Immun. 2010;78:1711–6. doi: 10.1128/IAI.01274-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clinics North America. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis CC, Kremer MJ, Schlievert PM, Squier CA. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am J Obstet Gynecol. 2003;189:1785–91. doi: 10.1016/s0002-9378(03)00873-1. [DOI] [PubMed] [Google Scholar]
- 34.Peterson ML, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, et al. The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1. Infect Immun. 2005;73:2164–74. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larkin SM, Williams DN, Osterholm MT, Tofte RW, Posalaky Z. Toxic shock syndrome: clinical, laboratory, and pathologic findings in nine fatal cases. Ann Intern Med. 1982;96(Pt 2):858–64. doi: 10.7326/0003-4819-96-6-858. [DOI] [PubMed] [Google Scholar]
- 36.Schlievert PM, Nemeth KA, Davis CC, Peterson ML, Jones BE. Staphylococcus aureus exotoxins are present in vivo in tampons. Clin Vaccine Immunol. 2010;17(5):722–7. doi: 10.1128/CVI.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, et al. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46:14349–58. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol. 2005;43:4628–34. doi: 10.1128/JCM.43.9.4628-4634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]