Abstract
Genetic information typically remains constant in all cells throughout the life cycle of most organisms. However, there are exceptions where DNA elimination is an integral, developmental program for some organisms, associated with generating distinct germline vs. somatic genomes. Programmed DNA elimination occurs in unicellular ciliates and diverse metazoa ranging from nematodes to vertebrates. DNA elimination can occur through chromosome breakage and selective loss of chromosome regions or the elimination of individual chromosomes. Recent studies provide compelling evidence that DNA elimination is a novel form of gene silencing, dosage compensation, and sex determination. Further identification of the eliminated sequences, genome changes, and in depth characterization of this phenomenon in diverse metazoan is needed to shed new light on the functions and mechanisms of this regulated process.
Introduction
In multicellular organisms, germ cells maintain the genetic information and ensure its integrity for the next generation, while somatic cells undergo differentiation and specialization. The genetic makeup of the germline and somatic cells is typically the same throughout the organism’s life cycle. However, there are exceptions to the general genome constancy observed in most organisms. During the development of some organisms, major genome changes can occur in various cell types [1,2]. One well-known example is the recombination events in the vertebrate immune system that generates diversity in antibodies and receptors in B and T cells, respectively [3]. Another major developmental genome change is programmed DNA elimination where specific DNA sequences, up to ~90% of the genome in some cases, are eliminated from somatic lineages. Since its discovery in 1887 [4], programmed DNA elimination in animals has been the subject of much interest and speculation [5–7]. The best-studied examples of programmed DNA elimination in eukaryotes are those present in the single-cell ciliates (see recent reviews [8–10]). Recently, high-throughput sequencing has been used in multicellular organisms to comprehensively examine genome changes that occur during programmed DNA elimination. Here, we review the broad range of organisms that demonstrate this phenomenon, and what is known regarding the function(s) and molecular mechanism(s) of programmed DNA elimination in metazoa.
Distribution and identification of programmed DNA elimination
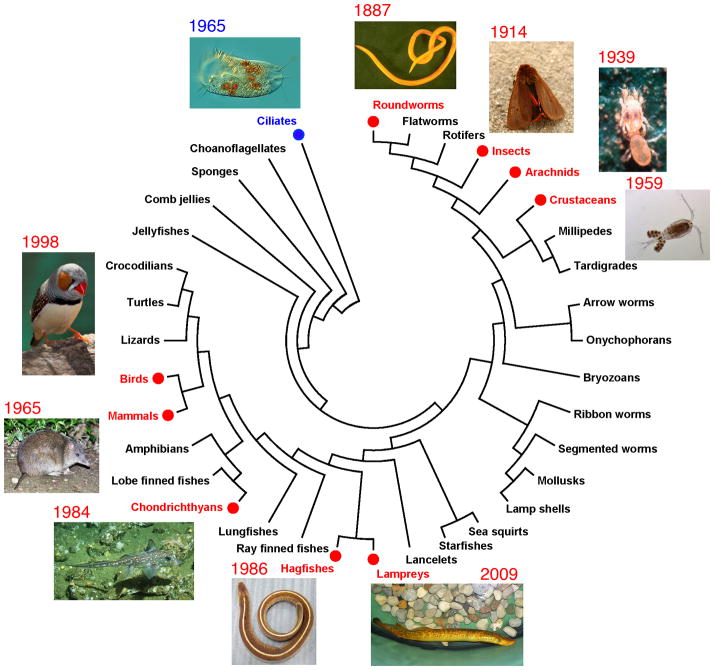
Programmed DNA elimination has been described in single-cell ciliates and a diversity of multicellular animals including more than 100 species from nine major taxonomic groups (Fig. 1 and Table 1). In most cases, programmed DNA elimination is associated with either differentiation of somatic cells or sex determination [1,6]. Two types of programmed DNA elimination, chromatin diminution and chromosome elimination, have been described (see Table 1). In chromatin diminution, chromosomes break and regions of the chromosomes are lost. Diminution occurs in ciliates and some parasitic nematodes, copepods, spotted ratfish, hagfish, and lampreys. In chromosome elimination, entire chromosomes are lost. This elimination occurs in some nematodes, insects, mites, finches, and bandicoots, as well as in some hagfish [11]. Given its wide phylogenetic distribution, programmed DNA elimination likely has arisen independently in these different lineages [6]. Outstanding questions remain including what the selective pressure for this process is, whether this pressure is the same in different organisms, and whether elimination serves the same function in diverse organisms?
Fig. 1. Programmed DNA elimination in multicellular organisms.
Organisms known to undergo DNA elimination are illustrated on a phylogenetic tree. The tree was constructed from 18S ribosomal RNA sequences using MEGA (v5.22) [55]. Common names are used for the groups. The tree is rooted on ciliates. Photo credits: Antonio Guillen from Water Project, Spain (ciliate S. mytilus), Colin Johnstone (nematode P. univalens), Entomart (moth P. fuliginosa), wiley library (mite M. occidentalis), James Haney (copepod M. edax), Jeremiah Smith (Sea lamprey P. marinus), Kinya G. Ota and Shigeru Kuratani (hagfish E. burgeri), wikipedia.org (Spotted ratfish H. colliei and Zebra finch T. guttata), and Joseph McKenna (bandicoot I. macrourus). The year that DNA elimination was discovered in each group of organisms is noted.
Table 1.
Organisms with programmed DNA elimination.
| Organism | First discovered (Year; Organism) | Common name | Other representative organisms | Known species* | D/E *** | References |
|---|---|---|---|---|---|---|
| Nematodes | 1887; Parascaris univalens | Roundworm | Ascaris suum; Strongyloides papillosus | 11 | D/E | [4,35] |
| Insects | 1914; Phragmatobia fuliginosa | Moth | Sciara ocellaris; S. coprophila | 65 | E | [27,56] |
| Arachnids | 1939; Pediculopis graminum | Grass mite | Metaseiulus occidentalis | 2 | E | [57,58] |
| Crustaceans | 1959; Cyclops strenuus | Copepod | Cyclops kolensis; Mesocyclops edax | 17 | D | [59,60] |
|
| ||||||
| Ciliates | 1965; Stylonychia mytilus | Ciliate | Tetrahymena thermophila; Oxytricha trifallax; Paramecium tetraurelia | 4,500** | D | [8,61] |
|
| ||||||
| Mammals | 1965; Isoodon macrourus | Bandicoot | Perameles nasuta | 10 | E | [62,63] |
| Chondrichthyans | 1984; Hydrolagus colliei | Spotted ratfish | Chimaera monstrosa | 4 | D | [64] |
| Hagfishes | 1986; Eptatretus burgeri | Inshore hagfish | Myxine glutinosa | 10 | D/E | [11,65] |
| Birds | 1998; Taeniopygia guttata | Zebra finch | Lonchura domestica | 2 | E | [36,46] |
| Lampreys | 2009; Petromyzon marinus | Sea lamprey | - | 1 | D | [14] |
Minimum number of known species
Ciliates exhibit nuclear dimorphism
D: Chromatin Diminution; E: Chromosome Elimination
Programmed DNA elimination typically has been identified through careful cytological studies of chromosome behavior during development. Theodor Boveri first discovered the diminution process by studying the chromosome segregation behavior in the horse parasitic nematode, Parascaris univalens [4]. Boveri’s analysis contributed to the establishment of chromosome theory of heredity and the first nematode cell lineages [12,13]. The single, large germline chromosome pair, a large increase in somatic chromosome number, and elimination of over 85% of the germline genome in somatic cells enabled Boveri to readily observe and describe chromatin diminution (Fig. 2). Soon thereafter, DNA elimination was described in several other nematodes including the related nematode Ascaris suum in 1895 (see Fig. 2), and then in insects and other organisms (Fig. 1 and Table 1, see review [6]). In the most recent discovery of chromatin diminution, Smith et al. followed a repetitive germline-specific DNA marker, germ1, in the germline and somatic tissue of lamprey to find that germ1 is eliminated in somatic tissues [14].
Fig. 2. Chromatin diminution in Parascaris and Ascaris.
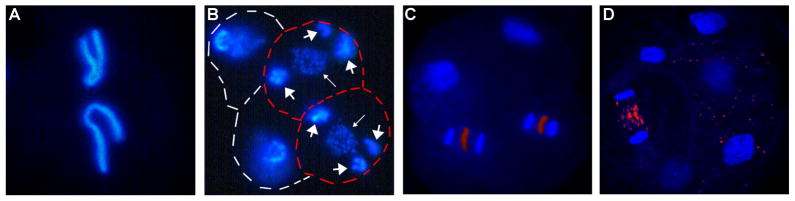
A and B. P. univalens embryos. A. 1-cell embryo showing the single pair of germline chromosomes. B. 4-cell embryo with two cells (outlined in red) undergoing diminution. The retained portions of the germline chromosomes are fragmented into many smaller chromosomes (small arrows). The heterochromatic arms that will be eliminated (big arrows) remain visible. C and D. A. suum embryos. C. 4-cell embryo with two cells undergoing chromatin diminution. D. 6-cell embryo with one cell undergoing chromatin diminution. Note that DNA to be eliminated is present as fragments (artificially colored red) between chromosomes segregating in early anaphase (C); DNA fragments (red) derived from a previous cell diminution can be seen in the cytoplasm of cells to the right (D).
The historical identification of DNA elimination using cytological methods has been serendipitous, and only large-scale genome changes are likely to be discovered by these approaches. The current broad use of high-throughput sequencing in diverse organisms, such as the Genome 10K Project [15], and single-cell sequencing may lead to the identification of additional examples of DNA elimination. These studies will likely contribute to our understanding of the breadth and frequency of DNA elimination in different metazoa, as well as whether genome differences might be present within different cells in mammals.
Identification of eliminated sequences provides insights into the function of DNA elimination
A key to understanding DNA elimination is defining the organization of chromosomes and their eliminated sequences. Early studies using DNA reassociation kinetics demonstrated that significant amounts of repetitive DNA were eliminated from the parasitic nematode A. suum; subsequent studies demonstrated that the major eliminated repeat was a 121 bp tandemly repeated satellite [16]. Later, seminal studies demonstrated that some transposon elements [17] and three single-copy genes were eliminated in A. suum [18–21]. Furthermore, by comparing the genomic sequences around chromosomal breakage regions, Muller et al. demonstrated that new telomeres were added at the DNA breaks and several break sites were conserved between the nematodes P. univalens and A. suum [22,23].
More recently, a comprehensive genomic approach was used to compare the genome differences between the germline (spermatids) and somatic cells (intestine) of a single male A. suum [24]. Wang et al. sequenced, de novo assembled, and compared the germline and somatic genomes from a male A. suum and found that ~43 Mb (~13%) of DNA was eliminated from the intestinal genome. Seventy percent of the eliminated DNA was repetitive sequences consisting predominantly of the previously described 121 bp tandem repeat. Surprisingly, the other eliminated sequences (~12.7 Mb) were single-copy sequences corresponding to ~700 protein-coding genes that are exclusively expressed in the germline and early embryos. A major group of the eliminated genes is associated with translation, demonstrating that the translation machinery may be very different between the germline and soma, supporting and extending earlier observations made by Muller et al. [18,19]. Notably, ~50 eliminated genes are orthologs to well-characterized genes in C. elegans whose loss is associated with clear phenotypes in germline formation, gametogenesis, and early embryogenesis. This large-scale elimination of germline genes suggests that DNA elimination may be an extreme and permanent mechanism for germline gene regulation in A. suum, deleting rather than repressing their expression in somatic cells. Wang et al. also identified ~50 breakpoints where chromosome regions were lost and telomere addition occurred on the retained chromosomes in the somatic cells, but no DNA fusions or rearrangements were observed. This genomic study significantly extends our understanding of the eliminated sequences and DNA breakpoints in A. suum, however, the current genome assemblies do not enable large-scale characterization of changes at the chromosomal level. Improved genome assemblies and additional studies are now needed to provide an overall view of the organization of the chromosomes and their alterations during diminution. High-throughput analysis of chromatin diminution in the related nematode P. univalens demonstrated that many of the break sites are conserved between the two nematodes as previously suggested [23], and also indicates that the genes eliminated are similar to those observed in A. suum (Wang, J. and Davis, R.E., unpublished data). This further supports the idea that diminution is a highly regulated and conserved process in these related parasitic nematodes.
Recent studies identified chromatin diminution in the sea lamprey and also demonstrated the elimination of both repetitive and single-copy sequences. Smith et al. used flow cytometry to measure the DNA content in lamprey testes and blood and found that ~20% (~500 Mb) of the lamprey germline genome is eliminated in somatic cells [14]. Further comparisons between sperm and liver DNA using array comparative genomic hybridization and genome survey sequences indicated that the eliminated DNA consists not only of repetitive sequences, but strikingly, also a few thousand genes [25]. The eliminated genes include homologs of vertebrate genes that function in either the development or maintenance of the germline. Given that a large number of germline-associated genes are eliminated in these divergent organisms, nematodes and lampreys, this suggests a possible common function of chromatin diminution. It will be interesting to see if loss of single-copy germline-associated genes is a common feature within other metazoa that undergo diminution such as copepods, where ribosomal RNA gene copy number can be regulated by diminution [26].
Although DNA elimination events are often associated with germ-soma differentiation, others are associated with sex determination. In sciarid flies, the elimination of one or two paternal X chromosomes in the pre-somatic cells determines the sex of the embryo (see reviews [27,28]). In a recent study on chromatin diminution in the parasitic nematode of sheep, Strongyloides papillosus [29], Nemetschke et al. used genetic crosses to determine that one of the two copies of a whole section of a chromosome undergoes DNA elimination by chromatin diminution. The region eliminated corresponds to a sex chromosome that is entirely eliminated in the closely related parasitic nematode of rats, S. ratti. This demonstrated that diminution provides a means to restore the sex chromosome ratio in males, and thus functions in the sex-determination system in this organism. Additional analyses suggest that chromatin diminution in S. papillosus is a derived state in Strongyloides species, evolved as a consequence of an X chromosome and autosome fusion that requires chromatin diminution to generate S. papillosus males [30].
A common theme in organisms that exhibit DNA elimination is the elimination of large amounts of repetitive sequences (see Table 2). In chromatin diminution, the eliminated repeats are typically tandem repeats that vary from 2 – 172 bp. Recent observations in zebra finch show that repetitive sequences are eliminated during chromosome elimination [31]. The conserved elimination of repetitive sequences in somatic cells raises the key question: why is it that the eliminated sequences are primarily repetitive? Clearly, repetitive sequences play key roles in genome evolution, recombination, and meiosis. They may also play additional roles in germline development and maintenance. A recent study in copepods suggests chromatin diminution in somatic cells may be necessary to reduce the ongoing repeat expansion and load in the germline [32]. A difficult but important goal will be to determine the location and organization of the repeats on chromosomes undergoing diminution. Such studies might provide important insights into the function of simple repeats in the germline, as well as perhaps their potential role in contributing to the process of diminution.
Table 2.
DNA elimination removes primarily repetitive sequences.
| Organism | % Genome eliminated | % Repeat in eliminated sequence | Eliminated repetitive sequence | References |
|---|---|---|---|---|
| Nematode | ||||
| Ascaris suum | 13 | 70 | 121 bp tandem repeats | [16,24] |
| Parascaris univalens | 88 | 98 | 5- and 10-bp tandem repeats | [66,67] |
| Lamprey and hagfish | ||||
| Petromyzon marinus | 20 | ~35% are Germ1 | Germ1, 200bp tandem repeats, others. | [14,25,68] |
| Eptatretus cirrhatus | 35 | Majority* | 4 tandem repeats, from 54 to 172 bp | [69] |
| Copepod | ||||
| Cyclops kolensis | 94 | Majority* | Tandem repeat with 10–30 bp motifs | [70] |
| Mesocyclops edax | 90 | Majority* | 2-, 8-, or 9-bp tandem repeats and other | [32,71,72] |
All known sequences are repeats
A variety of theories/hypotheses have been proposed to explain the biological significance of programmed DNA elimination [1,6,7,27,33–35] including mechanisms for 1) gene silencing, 2) dosage compensation, 3) sex determination, 4) position-effects for gene expression, 5) germline development and meiosis, and 6) germline and soma differentiation. The recent studies on Ascaris and the lamprey, where significant numbers of germline and early embryonic genes are eliminated and thus silenced in the somatic cells, provides strong support for a role in gene silencing. Recent studies also suggest it is a mechanism for dosage compensation in Ascaris, where many eliminated genes have undergone duplication, and sex determination in S. papillosus [29], flies [27], and birds [36]. The association of DNA elimination with germ-soma differentiation also poses the interesting question of whether somatic DNA elimination contributes to the differentiation of specific cell lineages. Wang et al. [24] compared DNA elimination in different cell lineages in A. suum, that exhibit deterministic cleavage similar to that observed in C. elegans, and found that the overall genomic content and the breakpoints are the same in all five precursor somatic cells undergoing diminution. In the sea lamprey, flow cytometry data indicate there might be subtle variation in the somatic genome size in different cells, although all markers assayed thus far exhibit uniform loss across different somatic tissues [14,25]. Thus, while current data suggest that the sequences lost from diminution are overall the same in all cells, it remains to be determined whether variations in diminution or the resulting chromosomal position effects might have functional significance that contributes to the differentiation of various cell lineages.
Molecular mechanisms of DNA elimination
Early mechanistic studies in Parascaris and Ascaris focused on the role of cytoplasmic determinants and the germ plasm in diminution (see reviews [1,5,6]). Using a variety of methods including doubly fertilized eggs, centrifugation, ultraviolet irradiation, and chemical induction [37], these studies suggested that cytoplasmic factors play a key role in chromatin diminution and may be segregated between the germline and soma. No specific factors have yet been identified that contribute to diminution [38–40]. Studies in ciliates have shown that small RNAs (piRNAs) and domesticated transposons are involved in programmed DNA rearrangement and elimination [10]. The piRNAs target sequences for retention or elimination in different types of ciliates [8,41] whereas the transposons lead to DNA breaks [42]. A recent study used high-throughput sequencing to examine total small RNA profiles during A. suum diminution; however, no correlation between small RNAs and diminution was observed [24,43]. Additional studies are required to determine whether specific Argonaute proteins and small RNAs contribute to DNA elimination in metazoa.
How cells define the breakpoints and what cellular machinery acts on them is likely to provide important insights into the mechanism of chromatin diminution. The sites for chromosomal breakage are conserved in each generation in parasitic nematodes and the sea lamprey. In the sea lamprey, distinct short palindromic sequences at three independent breakpoint regions were observed, suggesting site-specific recombination might facilitate DNA elimination [25]. Analysis of the 50 breakpoints identified in Ascaris demonstrated high fidelity of the break sites at the chromosomal level. However, the break sites can be heterogeneous (ranging over 200–2000 bp at a site) [22,24], and no conserved sequence motifs or other characteristics were identified 5 kb on either side of the DNA breakpoint regions [24]. It also remains to be determined whether DNA destined for elimination or retention in Ascaris and lampreys undergoes large-scale chromatin reorganization that could be involved in the mechanisms of diminution. In the sea lamprey, recent observations identified extra-nuclear aggregations of repressive chromatin (Herdy, J.R. III and Smith, J.J., personal communication), similar to those observed during elimination in ciliates and finch [44–46], suggesting an interrelationship between epigenetic silencing and loss. Studies on chromosome elimination in insects and finches indicate that a number of epigenetic modifications are associated with elimination of chromosomes including changes in histone H3/H4 acetylation, H3S10 phosphorylation, and DNA methylation ([44,46–50] and see recent review [28]).
A key question in DNA elimination is how chromosomes or portions of chromosomes are selectively lost and thus not segregated during cell division (Fig. 2). Loss or alterations in centromeres, kinetochore assembly, microtubule attachment, or chromosome segregation could lead to DNA elimination. Studies on chromosome elimination in insects suggest that chromosome loss is most likely a function of a segregation defect in the metaphase/anaphase transition [48,51]. In sciarid flies, reduction in the dephosphorylation of H3S10P is associated with a failure or retardation in sister chromatid separation [48]. In contrast, chromosome elimination in finches may be associated with a defect in kinetochore–microtubule interactions [47]. In chromatin diminution, once DNA breaks occur in monocentric chromosomes, regions that retain the centromeres would likely be properly segregated, whereas those regions that lack them would not and thus be eliminated. Genomic regions without centromeres could also fuse with other chromosome regions that retain their centromeres and thus be faithfully segregated as observed in copepods [52]. Nematodes such as Ascaris and Parascaris have holocentric chromosomes; kinetochore activity and microtubule attachment sites extend along the length of holocentric chromosomes. The location of centromeres is typically constant on most chromosomes. However, recent data in C. elegans suggest that centromere deposition can be dynamic [53]. In addition, unpublished studies in Ascaris indicate that the centromeric histone H3 variant Cenp-A marks chromosomes that will be retained, but is greatly reduced or absent on chromosomes that will be lost in diminution mitoses (Wang, J. and Davis, R.E., unpublished data). This is consistent with data from Parascaris that a kinetochore plate is absent in chromosome regions that will be lost [54] and suggests that centromere deposition may play an important role in determining chromosomal regions that will be retained or lost.
Perspective
Programmed DNA elimination occurs in ciliates and diverse multicellular organisms. Recently, chromatin diminution was described in the sea lamprey, a jawless vertebrate, extending the distribution of diminution into vertebrate lineages. New findings indicate that in addition to the loss of repetitive sequences, many protein-coding genes are lost in chromatin diminution, suggesting that diminution serves as a mechanism for gene regulation and silencing. Programmed DNA elimination is a complex biological process that requires the identification of sequences to be eliminated and a mechanism for their elimination. Additional studies are needed to define the mechanism(s) for selective loss of chromosomes or chromosome regions, breakage of chromosomes, and chromatin organizational changes associated with DNA elimination. Analysis of DNA elimination in different systems is likely to give new insight into the permanent gene silencing, the genome dynamics, the evolution of genomes, the role of repetitive sequences, and perhaps also information on genome alterations in cancer and other diseases.
Box. Outstanding questions in programmed DNA elimination.
Does chromatin diminution serve the same function in diverse organisms?
How are the sites for DNA breaks in chromatin diminution identified, made, and processed? Is this process the same in the divergent organisms that undergo diminution?
What are the molecular mechanisms that alter normal chromosome segregation leading to the elimination of portions of chromosomes or whole chromosomes?
Does retained versus eliminated DNA undergo specific chromatin and chromosomal organization changes that contribute to DNA retention or elimination?
Why are the majority of the sequences eliminated in chromatin diminution repetitive? What is the function of eliminating germline repetitive sequences in somatic cells? Do repetitive sequences contribute to the elimination process?
Does chromatin diminution contribute to cell lineage determination or differentiation?
Do small RNAs play a role in programmed DNA elimination as observed in ciliates?
Does programmed DNA elimination contribute to some genomic mosaicism in vertebrates?
Are processes associated with DNA elimination involved in pathological conditions such as cancer, disease, or other developmental abnormalities?
Acknowledgments
We apologize to those authors whose work was not included due to space limitations. We thank Jeremiah Smith for sharing unpublished data, and Clara Goday, Jeremiah Smith, and Adrian Streit for critical comments on the manuscript. We thank the members of the Davis laboratory for discussions and editorial suggestions. Work in the Davis lab is supported by Grants from the National Institutes of Health (AI0149558 and AI098421).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Kloc M, Zagrodzinska B. Chromatin elimination--an oddity or a common mechanism in differentiation and development? Differentiation. 2001;68:84–91. doi: 10.1046/j.1432-0436.2001.680202.x. [DOI] [PubMed] [Google Scholar]
- 2.Zufall RA, Robinson T, Katz LA. Evolution of developmentally regulated genome rearrangements in eukaryotes. Journal of experimental zoology Part B, Molecular and developmental evolution. 2005;304:448–455. doi: 10.1002/jez.b.21056. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annual review of immunology. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 4**.Boveri T. Ueber Differenzierung der Zellkerne wahrend der Furchung des Eies von Ascaris megalocephala. Anat Anz. 1887;2:688–693. The first discovery and description of a DNA elimination, chromatin diminution, in the parasitic nematode Parascaris. [Google Scholar]
- 5.Beams HW, Kessel RG. The problem of germ cell determinants. International review of cytology. 1974;39:413–479. doi: 10.1016/s0074-7696(08)60944-4. [DOI] [PubMed] [Google Scholar]
- 6.Tobler H. The differentiation of germ and somatic cell lines in nematodes. Results and problems in cell differentiation. 1986;13:1–69. doi: 10.1007/978-3-540-39838-7_1. [DOI] [PubMed] [Google Scholar]
- 7.Muller F, Tobler H. Chromatin diminution in the parasitic nematodes ascaris suum and parascaris univalens. International journal for parasitology. 2000;30:391–399. doi: 10.1016/s0020-7519(99)00199-x. [DOI] [PubMed] [Google Scholar]
- 8.Bracht JR, Fang W, Goldman AD, Dolzhenko E, Stein EM, Landweber LF. Genomes on the edge: programmed genome instability in ciliates. Cell. 2013;152:406–416. doi: 10.1016/j.cell.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mochizuki K. Developmentally programmed, RNA-directed genome rearrangement in Tetrahymena. Development, growth & differentiation. 2011 doi: 10.1111/j.1440-169X.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalker DL, Yao MC. DNA elimination in ciliates: transposon domestication and genome surveillance. Annual review of genetics. 2011;45:227–246. doi: 10.1146/annurev-genet-110410-132432. [DOI] [PubMed] [Google Scholar]
- 11.Kohno S, Kubota S, Nakai Y. The Biology of Hagfishes. Chapman and Hall; London: 1998. Chromatin diminution and chromosome elimination in hagfish species; pp. 81–100. [Google Scholar]
- 12.Maderspacher F. Theodor Boveri and the natural experiment. Current biology: CB. 2008;18:R279–286. doi: 10.1016/j.cub.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 13.Satzinger H. Theodor and Marcella Boveri: chromosomes and cytoplasm in heredity and development. Nature reviews Genetics. 2008;9:231–238. doi: 10.1038/nrg2311. [DOI] [PubMed] [Google Scholar]
- 14**.Smith JJ, Antonacci F, Eichler EE, Amemiya CT. Programmed loss of millions of base pairs from a vertebrate genome. Proceedings of the National Academy of Sciences. 2009;106:11212–11217. doi: 10.1073/pnas.0902358106. The recent discovery of chromatin diminution in a jawless vertebrate expanded the distribution of organisms with chromatin diminution to vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.G10KCOS. Genome 10K: a proposal to obtain whole-genome sequence for 10,000 vertebrate species. The Journal of heredity. 2009;100:659–674. doi: 10.1093/jhered/esp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Muller F, Walker P, Aeby P, Neuhaus H, Felder H, Back E, Tobler H. Nucleotide sequence of satellite DNA contained in the eliminated genome of Ascaris lumbricoides. Nucleic acids research. 1982;10:7493–7510. doi: 10.1093/nar/10.23.7493. The first cloning and comprehensive characterization of an eliminated DNA sequence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Aeby P, Spicher A, de Chastonay Y, Muller F, Tobler H. Structure and genomic organization of proretrovirus-like elements partially eliminated from the somatic genome of Ascaris lumbricoides. The EMBO journal. 1986;5:3353–3360. doi: 10.1002/j.1460-2075.1986.tb04650.x. Identification, cloning, and comprehensive characterization of an eliminated mobile element from the parasitic nematode, A. suum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Etter A, Aboutanos M, Tobler H, Muller F. Eliminated chromatin of Ascaris contains a gene that encodes a putative ribosomal protein. Proceedings of the National Academy of Sciences. 1991;88:1593–1596. doi: 10.1073/pnas.88.5.1593. This paper first demonstrated that single-copy genes are eliminated during chromatin diminution in the parasitic nematode, A. suum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Etter A, Bernard V, Kenzelmann M, Tobler H, Muller F. Ribosomal heterogeneity from chromatin diminution in Ascaris lumbricoides. Science. 1994;265:954–956. doi: 10.1126/science.8052853. This paper observed that different ribosomal protein genes were expressed in the germline and somatic tissue of the nematode, A. suum, and that DNA elimination altered their expression. [DOI] [PubMed] [Google Scholar]
- 20.Spicher A, Etter A, Bernard V, Tobler H, Muller F. Extremely stable transcripts may compensate for the elimination of the gene fert-1 from all Ascaris lumbricoides somatic cells. Dev Biol. 1994;164:72–86. doi: 10.1006/dbio.1994.1181. [DOI] [PubMed] [Google Scholar]
- 21.Huang YJ, Stoffel R, Tobler H, Mueller F. A newly formed telomere in Ascaris suum does not exert a telomere position effect on a nearby gene. Molecular and Cellular Biology. 1996;16:130–134. doi: 10.1128/mcb.16.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Muller F, Wicky C, Spicher A, Tobler H. New telomere formation after developmentally regulated chromosomal breakage during the process of chromatin diminution in Ascaris lumbricoides. Cell. 1991;67:815–822. doi: 10.1016/0092-8674(91)90076-b. This paper identified DNA breakpoints for chromatin diminution and demonstrated that the ends are healed by new telomere addition in the parasitic nematode, Ascaris. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann-Waldmann C, Jentsch S, Tobler H, Muller F. Chromatin diminution leads to rapid evolutionary changes in the organization of the germ line genomes of the parasitic nematodes A. suum and P. univalens. Molecular and biochemical parasitology. 2004;134:53–64. doi: 10.1016/j.molbiopara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24**.Wang J, Mitreva M, Berriman M, Thorne A, Magrini V, Koutsovoulos G, Kumar S, Blaxter ML, Davis RE. Silencing of germline-expressed genes by DNA elimination in somatic cells. Developmental cell. 2012;23:1072–1080. doi: 10.1016/j.devcel.2012.09.020. This comprehensive genome study identified germline sequences eliminated in somatic cells of the nematode, A. suum, described the elimination of ~700 genes, and suggested that diminution is a mechansims of germline gene silencing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Smith JJ, Baker C, Eichler EE, Amemiya CT. Genetic consequences of programmed genome rearrangement. Current biology: CB. 2012;22:1524–1529. doi: 10.1016/j.cub.2012.06.028. This study demonstrated that thousands of genes are eliminated during sea lamprey diminution, suggesting that diminution serves as a gene silencing mechanism in vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagoskin MV, Marshak TL, Mukha DV, Grishanin AK. Chromatin Diminution Process Regulates rRNA Gene Copy Number in Freshwater Copepods. Acta naturae. 2010;2:52–57. [PMC free article] [PubMed] [Google Scholar]
- 27.Goday C, Esteban MR. Chromosome elimination in sciarid flies. BioEssays. 2001;23:242–250. doi: 10.1002/1521-1878(200103)23:3<242::AID-BIES1034>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez L. Sex-Determining Mechanisms in Insects Based on Imprinting and Elimination of Chromosomes. Sexual development: genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2013 doi: 10.1159/000356709. [DOI] [PubMed] [Google Scholar]
- 29**.Nemetschke L, Eberhardt AG, Hertzberg H, Streit A. Genetics, chromatin diminution, and sex chromosome evolution in the parasitic nematode genus Strongyloides. Current biology. 2010;20:1687–1696. doi: 10.1016/j.cub.2010.08.014. This study demonstrated that chromatin diminution in the nematode, Strongyloides papillosus, contributes to sex determination. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni A, Dyka A, Nemetschke L, Grant WN, Streit A. Parastrongyloides trichosuri suggests that XX/XO sex determination is ancestral in Strongyloididae (Nematoda) Parasitology. 2013:1–9. doi: 10.1017/S0031182013001315. [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Kampf K, Pigozzi MI, Arnold AP. Molecular cloning and characterization of the germline-restricted chromosome sequence in the zebra finch. Chromosoma. 2009;118:527–536. doi: 10.1007/s00412-009-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Sun C, Wyngaard G, Walton DB, Wichman HA, Mueller RL. Billions of basepairs of recently expanded, repetitive sequences are eliminated from the somatic genome during copepod development. BMC genomics. 2014;15:186. doi: 10.1186/1471-2164-15-186. This paper provides a comparative analysis of ~1% of the somatic (3 Gb) and germline genomes (15 Gb) of the copepod, Mesocyclops edax. The data demonstrate that many repetitive sequences including mobile elements are eliminated. The authors suggest that ongoing germline expansion of repetitive elements requires somatic chromatin diminution to reduce and control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobler H, Muller F, Back E, Aeby P. Germ line - soma differentiation in Ascaris: A molecular approach. Experentia. 1985;41:1311–1319. [Google Scholar]
- 34.Pimpinelli S, Goday C. Unusual kinetochores and chromatin diminution in Parascaris. Trends in genetics. 1989;5:310–315. doi: 10.1016/0168-9525(89)90114-5. [DOI] [PubMed] [Google Scholar]
- 35.Tobler H, Etter A, Muller F. Chromatin diminution in nematode development. Trends in genetics. 1992;8:427–432. doi: 10.1016/0168-9525(92)90326-y. [DOI] [PubMed] [Google Scholar]
- 36*.Pigozzi MI, Solari AJ. Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 1998;6:105–113. doi: 10.1023/a:1009234912307. The identification of chromosome elimination in a bird contributing to sex determination. [DOI] [PubMed] [Google Scholar]
- 37.Esteban MR, Giovinazzo G, Goday C. Chromatin diminution is strictly correlated to somatic cell behavior in early development of the nematode Parascaris univalens. Journal of cell science. 1995;108 (Pt 6):2393–2404. doi: 10.1242/jcs.108.6.2393. [DOI] [PubMed] [Google Scholar]
- 38.Seidl C, Moritz KB. A novel UV-damaged DNA binding protein emerges during the chromatin-eliminating cleavage period in Ascaris suum. Nucleic Acids Research. 1998;26:768–777. doi: 10.1093/nar/26.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidl C, Moritz KB. Protein kinase activities during early development of Ascaris suum. Die Naturwissenschaften. 1990;77:482–485. doi: 10.1007/BF01135927. [DOI] [PubMed] [Google Scholar]
- 40.Jansen P, Moritz KB. Ascaris DNA topoisomerase I binds preferentially to the germ-line- limited DNA. Die Naturwissenschaften. 1986;73:739–741. doi: 10.1007/BF00399247. [DOI] [PubMed] [Google Scholar]
- 41.Fang W, Wang X, Bracht JR, Nowacki M, Landweber LF. Piwi-interacting RNAs protect DNA against loss during Oxytricha genome rearrangement. Cell. 2012;151:1243–1255. doi: 10.1016/j.cell.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogt A, Goldman AD, Mochizuki K, Landweber LF. Transposon domestication versus mutualism in ciliate genome rearrangements. PLoS genetics. 2013;9:e1003659. doi: 10.1371/journal.pgen.1003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Czech B, Crunk A, Wallace A, Mitreva M, Hannon GJ, Davis RE. Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome research. 2011;21:1462–1477. doi: 10.1101/gr.121426.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goday C, Pigozzi MI. Heterochromatin and histone modifications in the germline-restricted chromosome of the zebra finch undergoing elimination during spermatogenesis. Chromosoma. 2010;119:325–336. doi: 10.1007/s00412-010-0260-2. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Mochizuki K, Gorovsky MA. Histone H3 lysine 9 methylation is required for DNA elimination in developing macronuclei in Tetrahymena. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1679–1684. doi: 10.1073/pnas.0305421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Priore L, Pigozzi MI. Histone modifications related to chromosome silencing and elimination during male meiosis in Bengalese finch. Chromosoma. 2014 doi: 10.1007/s00412-014-0451-3. [DOI] [PubMed] [Google Scholar]
- 47.Schoenmakers S, Wassenaar E, Laven JS, Grootegoed JA, Baarends WM. Meiotic silencing and fragmentation of the male germline restricted chromosome in zebra finch. Chromosoma. 2010;119:311–324. doi: 10.1007/s00412-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escriba MC, Goday C. Histone H3 phosphorylation and elimination of paternal X chromosomes at early cleavages in sciarid flies. Journal of cell science. 2013;126:3214–3222. doi: 10.1242/jcs.128900. [DOI] [PubMed] [Google Scholar]
- 49*.Goday C, Ruiz MF. Differential acetylation of histones H3 and H4 in paternal and maternal germline chromosomes during development of sciarid flies. Journal of cell science. 2002;115:4765–4775. doi: 10.1242/jcs.00172. The first investigation and demonstration of specific histone modification changes associated with chromosome elimination. [DOI] [PubMed] [Google Scholar]
- 50.Greciano PG, Goday C. Methylation of histone H3 at Lys4 differs between paternal and maternal chromosomes in Sciara ocellaris germline development. Journal of cell science. 2006;119:4667–4677. doi: 10.1242/jcs.03279. [DOI] [PubMed] [Google Scholar]
- 51.Escriba MC, Giardini MC, Goday C. Histone H3 phosphorylation and non-disjunction of the maternal X chromosome during male meiosis in sciarid flies. Journal of cell science. 2011;124:1715–1725. doi: 10.1242/jcs.083022. [DOI] [PubMed] [Google Scholar]
- 52.Beermann S. The diminution of Heterochromatic chromosomal segments in Cyclops (Crustacea, Copepoda) Chromosoma. 1977;60:297–344. doi: 10.1007/BF00292858. [DOI] [PubMed] [Google Scholar]
- 53.Gassmann R, Rechtsteiner A, Yuen KW, Muroyama A, Egelhofer T, Gaydos L, Barron F, Maddox P, Essex A, Monen J, et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature. 2012;484:534–537. doi: 10.1038/nature10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Goday C, Gonzalez-Garcia JM, Esteban MR, Giovinazzo G, Pimpinelli S. Kinetochores and chromatin diminution in early embryos of Parascaris univalens. The Journal of cell biology. 1992;118:23–32. doi: 10.1083/jcb.118.1.23. This paper and previous work describe kinetochore/centromeric regions on Parascaris holocentric chromosomes and provides evidence for dynamic changes in its organization in different parts of the germline and the loss of the kinetochore/centromeric organization in eliminated chromosome regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fogg LC. A study of chromatin diminution in Ascaris and Ephestia. Journal of morphology. 1930;50:413–451. [Google Scholar]
- 57.Copper KW. The nuclear cytology of the grass mite, pediculopsis graminum (Reut), with special reference to Karyomerokinesis. Chromosoma. 1939;1:51–103. [Google Scholar]
- 58.Nelson-Rees WA, Hoy MA, Roush RT. Heterochromatinization, chromatin elimination and haploidization in the parahaploid mite Metaseiulus occidentalis (Nesbitt) (Acarina: Phytoseiidae) Chromosoma. 1980;77:263–276. doi: 10.1007/BF00286052. [DOI] [PubMed] [Google Scholar]
- 59.Beermann S. [Chromatin diminution in copepods] Chromosoma. 1959;10:504–514. doi: 10.1007/BF00396586. [DOI] [PubMed] [Google Scholar]
- 60.Bron JE, Frisch D, Goetze E, Johnson SC, Lee CE, Wyngaard GA. Observing copepods through a genomic lens. Frontiers in zoology. 2011;8:22. doi: 10.1186/1742-9994-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao MC, Gorovsky MA. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma. 1974;48:1–18. doi: 10.1007/BF00284863. [DOI] [PubMed] [Google Scholar]
- 62*.Hayman DL, Martin PG. Sex chromosome mosaicism in the marsupial genera Isoodon and Perameles. Genetics. 1965;52:1201–1206. doi: 10.1093/genetics/52.6.1201. The identification of chromosome elimination in marsupials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Close RL. Rates of sex chromosome loss during development in different tissues of the bandicoots Perameles nasuta and Isoodon macrourus (Marsupialia: Peramelidae) Australian Journal of Biological Sciences. 1984;37:53–61. [Google Scholar]
- 64.Stanley HP, Kasinsky HE, Bols NC. Meiotic chromatin diminution in a vertebrate, the holocephalan fish Hydrolagus collie (Chondrichthyes, Holocephali) Tissue & cell. 1984;16:203–215. doi: 10.1016/0040-8166(84)90045-4. [DOI] [PubMed] [Google Scholar]
- 65*.Kohno S, Nakai Y, Satoh S, Yoshida M, Kobayashi H. Chromosome elimination in the Japanese hagfish, Eptatretus burgeri (Agnatha, Cyclostomata) Cytogenetics and cell genetics. 1986;41:209–214. doi: 10.1159/000132231. The identification of chromosome elimination in hagfish. [DOI] [PubMed] [Google Scholar]
- 66*.Niedermaier J, Moritz KB. Organization and dynamics of satellite and telomere DNAs in Ascaris: implications for formation and programmed breakdown of compound chromosomes. Chromosoma. 2000;109:439–452. doi: 10.1007/s004120000104. Comprehensive characterization of the chromosomal localization and elimination of satellite repeats and addition of telomeric repeats to break sites using FISH on chromosomes in parasitic nematodes. [DOI] [PubMed] [Google Scholar]
- 67.Teschke C, Solleder G, Moritz KB. The highly variable pentameric repeats of the AT-rich germline limited DNA in Parascaris univalens are the telomeric repeats of somatic chromosomes. Nucleic Acids Research. 1991;19:2677–2684. doi: 10.1093/nar/19.10.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith JJ, Stuart AB, Sauka-Spengler T, Clifton SW, Amemiya CT. Development and analysis of a germline BAC resource for the sea lamprey, a vertebrate that undergoes substantial chromatin diminution. Chromosoma. 2010;119:381–389. doi: 10.1007/s00412-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goto Y, Kubota S, Kohno S. Highly repetitive DNA sequences that are restricted to the germ line in the hagfish Eptatretus cirrhatus: a mosaic of eliminated elements. Chromosoma. 1998;107:17–32. doi: 10.1007/s004120050278. [DOI] [PubMed] [Google Scholar]
- 70.Degtyarev S, Boykova T, Grishanin A, Belyakin S, Rubtsov N, Karamysheva T, Makarevich G, Akifyev A, Zhimulev I. The molecular structure of the DNA fragments eliminated during chromatin diminution in Cyclops kolensis. Genome research. 2004;14:2287–2294. doi: 10.1101/gr.2794604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drouin G. Chromatin diminution in the copepod Mesocyclops edax: diminution of tandemly repeated DNA families from somatic cells. Genome/National Research Council Canada = Genome/Conseil national de recherches Canada. 2006;49:657–665. doi: 10.1139/g06-022. [DOI] [PubMed] [Google Scholar]
- 72.McKinnon C, Drouin G. Chromatin diminution in the copepod Mesocyclops edax: elimination of both highly repetitive and nonhighly repetitive DNA. Genome/National Research Council Canada = Genome/Conseil national de recherches Canada. 2013;56:1–8. doi: 10.1139/gen-2012-0097. [DOI] [PubMed] [Google Scholar]