Abstract
A major focus of work in our laboratory concerns the molecular mechanisms and structural bases of Gram-negative bacterial endotoxin recognition by host (e.g., human) endotoxin-recognition proteins that mediate and/or regulate activation of Toll-Like Receptor (TLR) 4. Here we review studies of wild-type and variant monomeric endotoxin.MD-2 complexes, first produced and characterized in our laboratories. These purified complexes have provided unique experimental reagents, revealing both quantitative and qualitative determinants of TLR4 activation and antagonism. This review is dedicated to the memory of Dr. Theresa L. Gioannini (1949–2014) who played a central role in many of the studies and discoveries that are reviewed.
Keywords: Toll-Like Receptors, Lipopolysaccharides, MD-2, Radioiodination, Nuclear Magnetic Resonance Spectroscopy, Endotoxin recognition proteins
Introduction: endotoxin and innate immunity
Multicellular organisms are continuously challenged by intrusion of microorganisms from the surrounding environment. To meet this challenge, multicellular organisms have evolved highly efficient machinery to selectively recognize invading micro-organisms and to couple microbial recognition to the mobilization of host defense systems that can eliminate viable microbes and their remnants before appreciable microbial proliferation and dissemination ensue.
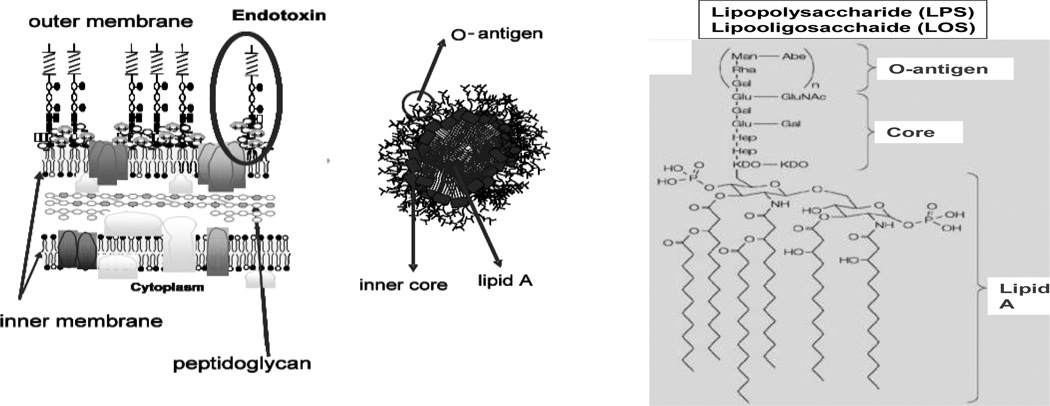
In many mammalian species including humans, recognition of endotoxins (E), unique and abundant surface glycolipids of Gram-negative bacteria (GNB), has provided a key strategy for defense against many GNB linked to induction of inflammation, targeting of GNB for elimination, and clearance of E itself (1–4). Endotoxins are amphipathic molecules, comprised of a relatively conserved lipid A region that contains a −1 6 linked disaccharide of N-acetylglucosamine linked by ester or amide bonds to 3-OH-fatty acids that may be further substituted with non-hydroxylated fatty acids in an acyloxyacyl linkage (Fig. 1) (5–7). Attached to the lipid A region is a carbohydrate chain of variable length and composition, including an acidic inner and less charged outer core oligosaccharide and, in many GNB, a distal strain-specific polymer of repeating tetra- or penta-saccharide units (O-antigen) (5). Endotoxins derived from GNB species that have the capacity to produce O-antigen (e.g. Escherichia coli) are called lipopolysaccharides (LPS); endotoxins from GNB species lacking the machinery to produce O-antigen (e.g., Neisseria meningitidis) are lipooligosaccharides (LOS).
Fig. 1. Schematics of: major structural and topological features of endotoxin.
Left) Integration of LPS, via lipid A fatty acyl chains, into the outer leaflet of the outer membrane of GNB; Center) organization of large E aggregates after extraction and purification o of E from GNB; Right) structure of E. coli LPS.
An important determinant of the outcome of many host-GNB interactions is the potency of E-induced host immune responses. Endotoxins can be extraordinarily potent, stimulating host responses to as few as 100 invading GNB, corresponding to femtamoles of E (1, 4, 8). This sensitivity facilitates prompt mobilization of host defenses before invading bacteria have time to multiply and potentially overwhelm mobilized host defenses. Variations in E structure may contribute to bacterial virulence (9–12) either by dampening early innate immune defense responses to infection, as exemplified by potential bioterror agents Yersinia pestis (11) and Francisella tularensis (12), or by exacerbating systemic inflammatory responses that ensue when local infection is not contained, as in sepsis (1, 8). Thus, knowledge of how recognition of E is translated into mobilization of pro-inflammatory responses is essential to understanding how the host normally eliminates many GNB invaders and how these same responses can be constrained either by a microbe to blunt host defense or by the host to reduce the likelihood of serious immuno-pathology.
Generally, the lipid A region is the most important structural determinant of the pro-inflammatory activity of E (1, 2, 6, 7). Due to the hydrophobic nature of lipid A, E is physically organized to shield lipid A from the aqueous environment. In GNB, lipid A is embedded in the outer leaflet of the outer membrane and, after extraction and purification, sequestered within large aggregates of E (13, 14). Given that physical organization, the sensitivity of human detection and response systems to many E species is remarkable, as is the ability of discrete variations in lipid A structure including differences in the number, structure and/or arrangement of fatty acids in lipid A to markedly alter the pro-inflammatory activity of E (6, 7, 9, 10, 15, 16).
Toll-like receptor (TLR) 4-dependent cell activation by endotoxin
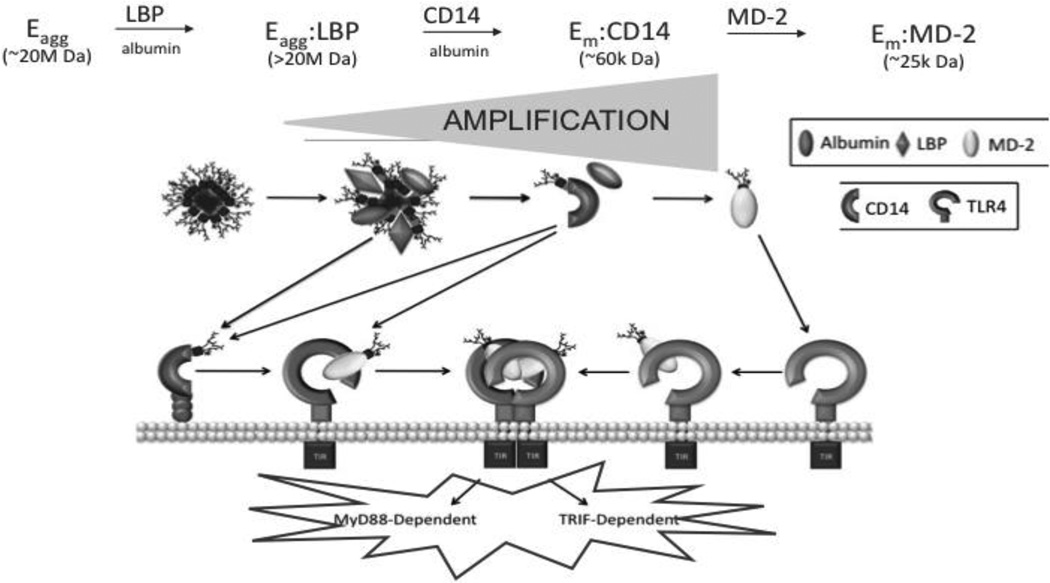
The Toll-like receptors (TLRs) are essential elements of innate immunity. These receptors couple molecular recognition of conserved and structurally unique microbial molecules to rapid mobilization of innate immune effector systems and later induction of adaptive immunity (1, 17). Among the various TLRs, TLR4 plays the major role in recognition and response to E and is unique in that its activation leads to both MyD88-dependent (e.g. NF B-mediated) and TRIF-dependent (e.g. interferon-β-mediated) cellular responses. Perhaps most remarkable is the ability of TLR4 to respond to minute (pM) concentrations of E. This does not reflect direct high affinity interaction of TLR4 with E but rather the ordered interactions of three extracellular and cell surface host proteins -- lipopolysaccharide-binding protein (LBP), soluble (s) and GPI-linked membrane (m)-associated forms of CD14, and secreted and TLR4-associated MD-2 – that act in concert with TLR4 (1–4, 18, 19). Together, LBP, CD14 and MD-2 dramatically alter the physical presentation of E, extracting individual E monomers from the outer membrane of GNB or from purified E aggregates to form monomeric E·protein complexes (E.CD14 and E.MD-2) (14, 20). These monomeric E. protein complexes alone have the ability, at pM concentrations, to engage and activate (or antagonize) TLR4, either indirectly (E·CD14) via MD-2 (MD-2·TLR4) or directly (E·MD-2) (21; Fig. 2). As a result of the combined action of LBP, CD14 (and MD-2), one GNB containing ca. 106 E molecules can yield 106 TLR4-activating monomeric E·protein complexes, sufficient to activate ~103–104 host cells and thus greatly amplifying host responsiveness to E. For each step leading to generation of monomeric E.MD-2(/TLR4) (Fig. 2), albumin is an essential co-factor (22). Albumin appears to stabilize otherwise transient topological re-arrangements of E within E.protein complexes (e.g. E-agg(LBPnE.CD14) that are likely needed for extraction and transfer of individual E molecules (monomers) from E-rich interfaces and between these extracellular and/or cell surface proteins. Albumin can also act as a CD14-independent E monomer acceptor/donor to MD-2 and MD-2/TLR4 (23). Extraction and transfer of E monomers from E aggregates to albumin is promoted by depletion of divalent cations, needed for dense packing of E monomers within E aggregates and the GNB outer membrane, but not by LBP-mediated modifications of E-rich interfaces that facilitate extraction of E monomers by CD14. Experiments are in progress to identify the physiological mechanism(s) for extraction and transfer of E monomers to albumin and the settings in which this mechanism may be important.
Fig. 2. Model for LBP/CD14/MD2-dependent transformation of E promoting TLR4 dependent cell activation by E.
Potency of E is amplified by interactions with E-binding proteins that modify the presentation of E to TLR4. See text for additional details. Note that TLR4-expressing cells include those that express TLR4 ± MD-2.
Development of novel assays to measure specific, high affinity interactions of endotoxin with the extracellular domain of TLR4 (TLR4ECD
Earlier studies (24–29) had estimated the affinity (KD) of interactions of E with MD-2 and MD-2/TLR4 as ranging from 3–65 nM, seemingly inconsistent with the ability of cells expressing TLR4 to be activated by pM E when LBP, CD14 and MD-2 were also present. We reasoned that these studies had under-estimated E interactions with MD-2 and MD-2/TLR4 by using experimental conditions in which the E added was presented as an undefined mixture of aggregates of E (± LBP ± sCD14) as well as monomeric E.protein complexes. To better define the molecular requirements for high affinity E-MD-2 and E-MD-2/TLR4 interactions, we compared the binding of purified E aggregates (E-agg), monomeric E.sCD14 and monomeric E.MD-2 complexes with conditioned insect cell culture medium ± secreted human TLR4ECD (21, 30), making use of epitope tags on FLAG-TLR4ECD and His6-MD-2 and appropriate adsorbing matrices to selectively cocapture E that had formed complexes with His6-MD-2 and/or FLAG-TLR4ECD. Precise quantitative measurement of these complexes was made possible by the creation and use of bacterial mutants that depend on dietary acetate for optimal growth (13). These bacterial mutants made possible uniform and virtually quantitative metabolic labeling of the acyl chains of E with [1,2-14C], [1-12C, 2-13C], or [3H] acetate with a specific radioactivity as high as 25,000 cpm/pmol. This made possible measurement of pM E-protein interactions, including E presented as an integral component of the GNB outer membrane, and analytical and more preparative identification, isolation, and compositional and functional characterization of the E.protein complexes formed (14, 20, 21, 23, 30–32). These studies showed specific, saturable, high affinity (KD ~200 pM) interaction of MD-2/TLR4ECD with E.sCD14 and of TLR4ECD with E.MD-2. Both interaction of MD-2/TLR4ECD with E.sCD14 and of TLR4ECD with E.MD-2 yielded a Mr 190,000 complex representing a dimer of the ternary complex ([3H]E.His6-MD-2.FLAG-TLR4ECD2. These interactions matched the molecular requirements for potent activation of MD-2/TLR4 and TLR4 by E and strongly suggested that the components of the recovered ternary complex (E, MD-2, and TLR4) are sufficient for receptor activation, focusing our subsequent studies on the structural properties of E, MD-2, and TLR4 determining TLR4 activation.
Production and characterization of radioiodinated E.MD-2[125I] to measure and relate cell surface TLR4 binding to TLR4 activation and/or antagonism
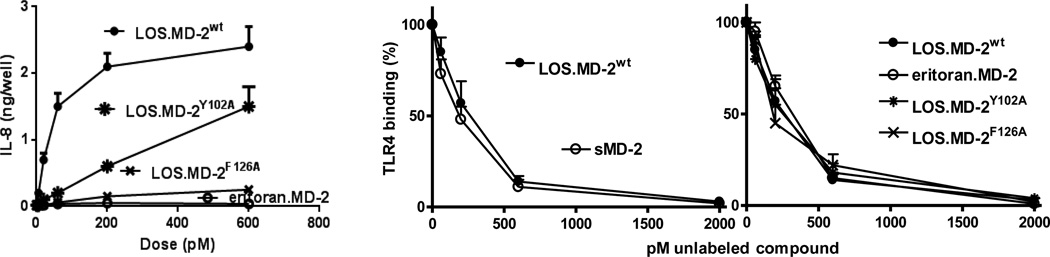
The remarkable stability of monomeric E.MD-2 complexes and presence of several surface-exposed tyrosine residues that could be individually mutated without loss of MD-2 function prompted us to examine the possibility of radioiodination of a purified E.MD-2 complex for even more sensitive measurement of cell surface E.MD-2-TLR4 binding. Under conditions of roughly 1 mol 125I incorporated/mol of E.MD-2, a specific radioactivity of the E.MD-2[125I] complex of ca. 500,000 cpm/pmol was achieved that showed specific, saturable binding to cell surface TLR4 and to secreted TLR4ECD with a KD of ~500 pM (33). The 20-fold increase in specific radioactivity (vs. metabolically labeled [3H]E.MD-2) made possible measurement of specific cell surface TLR4 binding at the very low doses of E.MD-2 (i.e., meningococcal LOS.MD-2; 2–6 pM) still sufficient for measurable TLR4-dependent cell activation. These experiments revealed that occupation of only 50–100 TLR4/cell (representing 1–3% of the total cell surface TLR4 pool) by this potent TLR4 agonist (representing <0.01% of the total endotoxin pool of one meningococcus) induced nearly 25% maximal TLR4-dependent cell activation, underscoring the remarkable sensitivity of TLR4 to meningococcal endotoxin when this endotoxin is presented as a monomeric complex with MD-2. The very high specific radioactivity of LOS.MD-2[125I] also facilitated competition studies with unlabeled ligand.MD-2 complexes, expediting analyses of the molecular and structural requirements for TLR4 binding and activation. Comparison in this way of TLR4 binding to complexes that are either potent TLR4 agonists (e.g., hexaacylated meningococcal LOS.MD-2), weak or very weak TLR4 agonists (e.g., LOS complex with mutant MD-2 (Y102A or F126A, respectively)) or essentially pure TLR4 antagonists (a complex of eritoran, a tetraacylated lipid A mimetic, with wt MD-2) showed essentially identical TLR4 binding despite markedly different TLR4 agonist properties (Fig. 3). Thus, equal numbers (i.e., surface density) of ligand.MD-2.TLR4 complexes can result in markedly different levels of TLR4-dependent cell activation, depending on the structure of the ligand and/or of MD-2 (see also 34).
Fig. 3. Comparison of TLR4 activation (left) and cell surface TLR4 binding (right) by the monomeric complexes of hexaacylated meningococcal LOS with either wild-type (wt) or mutant (F126A or Y102A) human MD-2 or of tetraacylated eritoran.MD-2.
TLR4 activation was measured by induced extracellular accumulation of IL-8; TLR4 binding was measured by inhibition of TLR4-dependent binding of LOS.MD-2[125I] (60 pM) as described in (33).
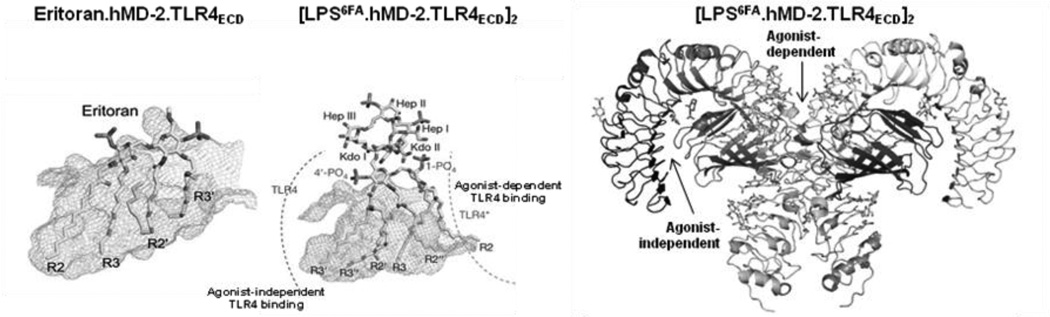
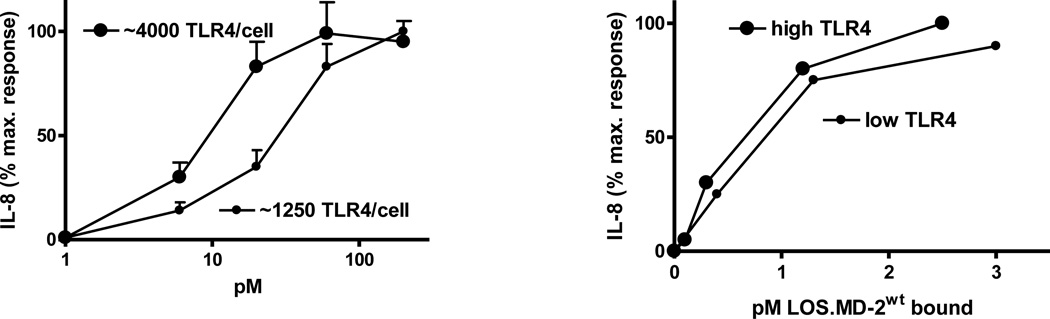
A likely explanation for these findings is suggested by the X-ray crystal structures of complexes of tetraacylated (eritoran or lipid IVA) and of hexaacylated (E. coli LPS) with MD-2 ± TLR4ECD. These structural analyses (36–38) revealed two topologically and functionally distinct interactions between MD-2 and TLR4 (Fig. 4): i) “agonistindependent” interactions that are manifest irrespective of the ligand bound to wt MD-2 or even in the absence of bound ligand, reflecting the likely role of these sites and interactions in the genesis of MD-2/TLR4 heterodimers by cells producing both TLR4 and MD-2; and ii) “agonist-dependent” interactions between neighboring ligand.MD-2.TLR4 ternary complexes in which the MD-2 ligand acts with MD-2 as a TLR4 agonist (e.g., hexaacylated endotoxin.MD-2). The very similar dose-dependent inhibition of LOS.MD-2[125I] binding by the various ligand.MD-2 complexes and by unlabeled monomeric MD-2 (39; Fig. 3), strongly suggests that the high affinity (pM) TLR4 binding of each of the ligand.MD-2 complexes corresponds to agonist-independent MD-2-TLR4 interactions within an individual ligand.MD-2/TLR4 ternary complex (i.e., intra-ternary complex interactions). Conversely, differences in TLR4 agonist potency of the various ligand.MD-2 complexes most likely reflect differences in agonist-dependent interactions: i.e., inter-ternary complex interactions induced by the presence of TLR4-activating ligand.MD-2 bound via agonist-independent interactions to TLR4. Viewed this way, the differences in TLR4 agonist potency of different ligand.MD-2 complexes may be explained by differences in the probability of the monomeric ternary complexes they form with TLR4 to interact (e.g., dimerize) and form an active receptor complex. If this interpretation is correct, the data shown in Fig. 3 and in ref. 33 indicate that LOS.MD-2Y102ALOS.MD-2F126Aand eritoran.MD-2 form ternary complexes with TLR4 that have, respectively, ca. 10%, 1%, and 0% the probability of dimerization and activation as that of LOS.MD-2wt.TLR4. Altering surface levels of TLR4 experimentally (Fig. 5) or as occurs naturally when a polymorphic variant of TLR4 (D299G.T399I) is expressed without MD-2 (e.g., airway epithelial cells; 30, 33, 40), changes the potency of TLR4 agonists but not the number of cell surface ternary complexes needed for TLR4-dependent cell activation (Fig. 5; 33). Ligands of MD-2 that act as TLR4 antagonists (e.g., eritoran) act in a similar way to reducing surface expression of TLR4 by reducing the pool of TLR4 available to TLR4-activating ligand.MD-2 complexes (or of those ligands to MD-2/TLR4). Thus, TLR4 activation, presumably triggered by agonist-induced dimerization, requires occupation of each TLR4 of the active receptor complex with activating ligand (i.e., TLR4-activating ligand.MD-2). Increasing cell surface expression of TLR4 does not change the probability of TLR4 activation by a given number of ligand.MD-2.ternary complexes but increases the TLR4 agonist potency of a ligand.MD-2 complex by resulting in increased numbers (surface density) of ligand.MD-2.TLR4 complexes formed when a particular dose of ligand.MD-2 complex is added. The ability of LOS.MD-2wt to induce appreciable TLR4-dependent cell activation at doses nearly 100-fold below the KD of its interaction with cell surface TLR4, at even relatively low surface levels of TLR4 expression, indicates a remarkable efficiency of ternary complex dimerization/receptor occupation when TLR4 is occupied by a ligand.MD-2 complex with strong TLR4-activating properties and also, quite likely, robust signaling by the active receptor complex.
Fig. 4. Ribbon and stick models of eritoran or LPS.MD-2 ± TLR4ECD derived from published X-ray crystal structures (36–38).
Note that the left two panels show only the ligand.MD-2 structures while the TLR4 structure is not displayed.
Fig. 5.
Effect of change in surface TLR4 expression on potency of LOS.MD-2wt, as expressed by concentration of LOS.MD-2wt added (left) or amount of LOS.MD-2wt bound (right
NMR studies of complexes of [13C]LOS.MD-2 (wt vs. F126A) ± TLR4ECD
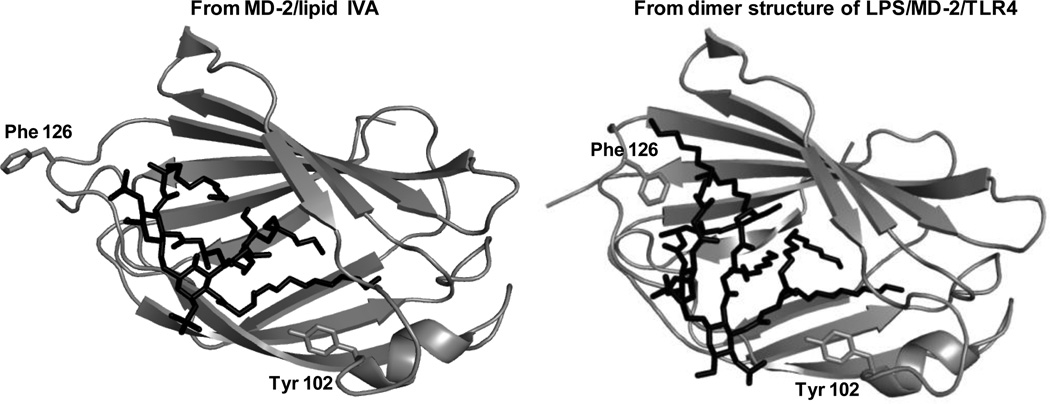
A major goal of high resolution structural studies of ligand.MD-2 binary complexes and ligand.MD-2.TLR4ECD ternary complexes (36–38, 41) and also of molecular modeling studies (42, 43) has been to identify the structural correlates of agonist-induced TLR4 activation. Comparison of the crystal structures of MD-2 bound to hexaacylated (TLR4-activating) LPS and TLR4ECD vs. those of MD-2 bound ± TLR4ECD to tetraacylated ligands (lipid IVA or eritoran) that act as TLR4 antagonists revealed several configurational differences in the ternary complexes that seemed potentially relevant to agonist-induced TLR4 activation, including the presence in the ternary complexes containing hexaacylated LPS of: i) ternary complex dimers; ii) a fatty acyl chain partially extruding from the hydrophobic pocket of MD-2; and iii) a local conformational change of MD-2 resulting from reorientation of the aromatic side chain of Phe126 from the surrounding aqueous solvent to contact with two of the six fatty acyl chains of the bound LPS (Fig. 6). This conformational change in MD-2 could promote agonist-dependent contacts between a TLR4-activating LPS·MD-2 complex of one ternary complex with TLR4 of a second complex by inducing: 1) hydrophilic MD-2/TLR4 interactions in the dimerization interface involving the backbone atoms of the shifted Phe126 loop; and 2) hydrophobic interactions between conserved and essential Phe 440 and 463 of TLR4 (42, 43) with the distal end of a fatty acyl chain (R2) of the bound hexaacylated E that protrudes from the hydrophobic pocket of MD-2 (see Fig. 4). However, what could not be judged from the comparative crystal structures was: 1) whetherprotrusion of a single fatty acyl chain and re-orientation of the aromatic side chain of Phe126 occurred before contact with TLR4 and, hence, were intrinsic structural properties of TLR4-activating hexaacylated E (ligand).MD-2 complexes; and 2) if Phe126 of MD-2 was instrumental in the positioning of the acyl chains of bound hexaacylated E.
Fig. 6. Ribbon models of MD-2 bound with lipid IVA (36) or LPS (37) based on X-ray crystal structure.
The structure of MD-2 bound to LPS is based on LPS.MD-2.TLR4ECD with TLR4 not displayed “. The MD-2 is shown in gray while the ligand is shown in black. Note the different orientation of Phe126 in the two complexes. Note also the position of the side chain of Tyr102 within the hydrophobic pocket of MD-2.
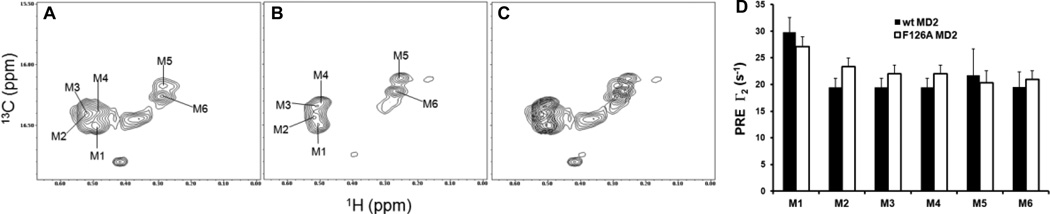
To address these questions, we produced and purified [13C]LOS.MD2wt and [13C]LOS.MD-2F126A making use of the same acetate auxotroph of Neisseria meningitidis To address these questions, we produced and purified [13C]LOS.MD-2wt and [13C]LOS.MD-2F126A making use of the same acetate auxotroph of Neisseria meningitidis to produce and purify [13C]LOS after bacterial growth in minimal medium supplemented with 1-[12C], 2-[13C]-acetate (32). This resulted in [13C] labeling of every other C atom in each fatty acyl chain starting with the terminal methyl group of each fatty acid. High resolution 13C/1H HSQC resolved six distinct signals in the methyl region of the spectrum (Fig. 7), indicative of the distinct location of each of the terminal 13CH3 groups of the six fatty acyl chains of LOS bound to wt (Fig. 7A) and F126A (Fig. 7B) MD-2. Overlay of these spectra (Fig. 7C) demonstrated differences in each of the 13CH3 group signals from LOS bound to wt or F126A MD-2, indicating an effect of Phe126 on the positioning (location) of the lipid A region of LOS as a whole. This effect of Phe126 is most compatible with re-orientation of the Phe126 side chain toward fatty acyl chains of the bound LOS, thus indicating that this local conformational change in MD-2 is TLR4-independent; i.e., an intrinsic property of the TLR4-activating LOS.MD-2wt complex. Differences in 13CH3 group signals from LOS bound to wt vs. F126A MD-2 were also seen after binding of purified [13C]LOS.MD2wt and [13C]LOS.MD-2F126A to TLR4ECD to form monomeric ternary complexes. In addition, 13CH2 methylene group signals were more markedly attenuated after TLR4ECD binding of [13C]LOS.MD-2F126A vs. [13C]LOS.MD-2wtfurther suggesting an important role of Phe126 in the positioning of the fatty acyl chains of bound TLR4-activating endotoxin. The relative surface exposure of individual 13CH3 groups was examined using a neutral chelated gadolinium compound (e.g., Gd(DPTA-BMA)) which by paramagnetic relaxation causes quenching of 13C/1H signals from methyl groups that are less sequestered and more readily affected by the Gd reagent present in solution. In both wt and mutant MD-2 binary complexes, five of the 13C/1H LOS methyl cross peaks (M2-M6) showed closely similar attenuation but one (M1) was more susceptible to paramagnetic attenuation (Fig. 7D) suggesting that this 13CH3 group (fatty acyl chain) is protruding out of the hydrophobic pocket of both wt and F126A MD-2. Experiments are planned to test if the difference in the overall positioning of the fatty acids bound to F126A vs. wt MD-2 affects FA-TLR4 contacts in the mutant complex and thereby contributes to reduced TLR4 agonist activity. Experiments are also in progress to determine more precisely the structural properties of MD-2 ligands required for induction of this conformational change in MD-2 and its relation to TLR4 activation. Of note, the side chain of Tyr102 also resides within the hydrophobic ligand binding pocket of MD-2 (Fig. 6). Thus, the intermediate TLR4 agonist potency of LOS.MD-2F102A (vs. LOS.MD-2wt and LOS.MD-2F126A; Fig. 3) could reflect perturbation of bound LOS-fatty acyl positioning while Phe126-induced MD-2-TLR4 contacts are retained.
Fig. 7. NMR data for [13C]LOS.MD-2wt and [13C]LOS.MD-2F126A complexes.
13C/1H high resolution HSQC spectra (A–C) of 13CH3 region of [13C]LOS.MD-2wt (A) and [13C]LOS.MD-2F126A (B) complexes; (C) represents the overlay of the two spectra. (D) Comparison of the paramagnetic relaxation effect (PRE) of Gd(DPTA-BMA) on the 13CH3 peaks of [13C]LOS.MD-2 (wt and F126A) complexes. Data were collected on Avance II 800 MHz NMR spectrometer.
Concluding remarks
The novel reagents and experimental approaches that we have developed have allowed detection and quantitative analysis of specific, pM E-protein and TLR4 interactions. The sensitivity of our assays has permitted testing of various host-E interactions at these very low, physiologically relevant endotoxin concentrations, facilitating testing of the mechanism of action of several natural and synthetic regulators of TLR4 activation. Our discovery and purification of stable, water-soluble monomeric complexes of E.MD-2 that, depending on the structure of bound E or MD-2, can act at pM concentrations as TLR4 agonists or antagonists have provided unique reagents to probe the molecular and structural requirements for endotoxin-triggered TLR4 activation and the structural limits of pattern recognition of endotoxin by MD-2 and TLR4. Increasing evidence suggests physiologic and pathophysiologic roles of TLR4 extending beyond host responses to invading GNB. Thus, knowledge of the molecular and cellular rules regulating TLR4 function should provide new insights into what determines the nature and strength of TLR4-dependent responses in settings including but not limited to GNB infection. Knowledge gained from these studies should also yield insights applicable to the design and testing of novel TLR4-directed immune modulators.
Acknowledgements
This review is dedicated to the memory of Dr. Theresa L. Gioannini, an exemplary partner at work and in all phases of life. The work reported herein was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development grant [1I01BX0000949-01A1 to T.L.G.) and grants to J.P.W. from the National Institute of Allergy and Infectious Disease, National Institutes of Health [grant numbers AI059372, AI088372, and AI044642].
Biography
![]()
References
- 1.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 2.Munford RS. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect. Immun. 2008;76:45–54. doi: 10.1128/IAI.00939-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 4.Weiss J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defense against Gram-negative bacteria. Biochem. Soc. Trans. 2003;31:785–790. doi: 10.1042/bst0310785. [DOI] [PubMed] [Google Scholar]
- 5.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014 doi: 10.1146/annurev-biochem-060713-035600. [Epub Ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 7.Caroff M, Karibian D, Cavaillon JM, Haeffner-Cavaillon N. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 2002;4:915–926. doi: 10.1016/s1286-4579(02)01612-x. [DOI] [PubMed] [Google Scholar]
- 8.Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schultze N, Beutler B, Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213:193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. Journal of Dental Research. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 10.Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- 11.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, Miller SI, Skerrett SJ, Ernst RK. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giardina PC, Gioannini T, Buscher BA, Zaleski A, Zhang DS, Stoll L, Teghanemt A, Apicella MA, Weiss J. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem. 2001;276:5883–5891. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 14.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem. 2005;280:38383–38394. doi: 10.1074/jbc.M508063200. [DOI] [PubMed] [Google Scholar]
- 15.Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodeling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013;11:167–181. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Needham BD, Carroll SM, Giles SK, Georgiou G, Whiteley M, Trent MS. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci USA. 2013;110:1464–1469. doi: 10.1073/pnas.1218080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol. 2003;3:119–128. doi: 10.1016/s1567-5769(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 19.Gioannini TL, Teghanemt A, Zhang DS, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–123. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 20.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin.protein complexes with Tolllike receptor 4 ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 22.Gioannini TL, Zhang D, Teghanemt A, Weiss JP. An essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J Biol Chem. 2002;277:47818–47825. doi: 10.1074/jbc.M206404200. [DOI] [PubMed] [Google Scholar]
- 23.Esparza GA, Teghanemt A, Zhang D, Gioannini TL, Weiss JP. Endotoxin.albumin complexes transfer endotoxin monomers to MD-2 resulting in activation of TLR4. Innate Immun. 2012;18:478–491. doi: 10.1177/1753425911422723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 25.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci U.S.A. 2001;98:12156–12161. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy MN, Mullen GE, Leifer CA, Lee C, Mazzoni A, Dileepan KN, Segal DM. A complex of soluble MD-2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J Biol Chem. 2004;279:34698–34704. doi: 10.1074/jbc.M405444200. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh S, Akashi S, Yamada T, Tanimura N, Matsumoto F, Fukase K, Kusumoto S, Kosugi A, Miyake K. Ligand-dependent Toll-like receptor 4 (TLR4)-oligomerization is directly linked with TLR4-signaling. J Endotoxin Res. 2004;10:257–260. doi: 10.1179/096805104225005904. [DOI] [PubMed] [Google Scholar]
- 28.Hyakushima N, Mitsuzama H, Nishitani C, Sano H, Kuronama K, Konishi M, Himi T, Miyake K, Kuroki Y. Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signaling. J Immunol. 2004;173:6949–6954. doi: 10.4049/jimmunol.173.11.6949. [DOI] [PubMed] [Google Scholar]
- 29.Visintin A, Halmen KA, Latz E, Monks BG, Golenbock DT. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J Immunol. 2005;175:6465–6472. doi: 10.4049/jimmunol.175.10.6465. [DOI] [PubMed] [Google Scholar]
- 30.Prohinar P, Rallabhandi P, Weiss J, Gioannini T. Expression of functional D299G.T399I polymorphic variant of TLR4 depends more on coexpression of MD-2 than does wild-type TLR4. J Immunol. 2010;184:4362–4367. doi: 10.4049/jimmunol.0903142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teghanemt A, Re F, Prohinar P, Widstrom R, Gioannini TL, Weiss JP. Novel roles in human MD-2 of phenylalanines 121 and 126 and tyrosine 131 in activation of Toll-like receptor 4 by endotoxin. J Biol Chem. 2008;283:1257–1266. doi: 10.1074/jbc.M705994200. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Phillips R, Zhang D, Teghanemt A, Weiss J, Gioannini T. NMR studies of hexaacylated endotoxin bound to wild-type and F126A mutant MD-2 and MD-2·TLR4 ectodomain complexes. J Biol Chem. 2012;287:16346–16355. doi: 10.1074/jbc.M112.343467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teghanemt A, Weiss JP, Gioannini TL. Radioiodination of endotoxin.MD-2 complex generates a novel sensitive, high-affinity ligand for TLR4. Innate Immun. 2013;19:545–560. doi: 10.1177/1753425913475688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 35.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 37.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 39.Teghanemt A, Widstrom R, Gioannini T, Weiss J. Isolation of monomeric and dimeric secreted MD-2.Endotoxin.sCD14 and Toll-like receptor 4 ectodomain selectively react with the monomeric form of secreted MD-2. J Biol Chem. 2008;283:21881–21889. doi: 10.1074/jbc.M800672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia HP, Kline JK, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB., Jr Endotoxin Responsiveness of Human Airway Epithelia is Limited by Low Expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 41.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci USA. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resman N, Vasl J, Oblak A, Pristovsek P, Gioannini T, Weiss J, Jerala R. Essential roles of hydrophobic residues in both MD-2 and toll-like receptor 4 in activation by endotoxin. J Biol Chem. 2009;284:15052–15060. doi: 10.1074/jbc.M901429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resman N, Oblak A, Gioannini TL, Weiss JP, Jerala R. Tetraacylated lipid A and paclitaxel-selective activation of TLR4/MD-2 conferred through hydrophobic interactions. J Immunol. 2014;192:1887–1895. doi: 10.4049/jimmunol.1302119. [DOI] [PubMed] [Google Scholar]