Abstract
Background
In 2009, we reported a novel form of delayed anaphylaxis to red meat related to serum IgE antibodies to the oligosaccharide galactose-alpha-1,3-galactose (alpha-gal). Although patients were remarkably consistent in their description of a 3- to 6-hour delay between eating mammalian meat and the appearance of symptoms, this delay has not been demonstrated under observed studies.
Objectives
We sought to formally document the time course of clinical symptoms after the ingestion of mammalian meat in subjects with IgE to alpha-gal and to monitor ex vivo for the appearance of markers of an allergic reaction.
Methods
Open food challenges were performed with mammalian meat in 12 subjects with a history of severe urticarial reactions 3 to 6 hours after eating beef, pork, or lamb, as well as in 13 control subjects. Blood samples were taken hourly during each challenge.
Results
Ten of 12 subjects with IgE to alpha-gal had clinical evidence of a reaction during the food challenge (vs none of the control subjects, P < .001). The reactions occurred 3 to 7 hours after the initial ingestion of mammalian meat and ranged from urticaria to anaphylaxis. Tryptase levels were positive in 3 challenges. Basophil activation, as measured by increased expression of CD63, correlated with the appearance of clinical symptoms.
Conclusion
The results presented provide clear evidence of an IgE-mediated food allergy that occurs several hours after ingestion of the inciting allergen. Moreover, here we report that in vivo basophil activation during a food challenge occurs in the same time frame as clinical symptoms and likely reflects the appearance of the antigen in the bloodstream. (J Allergy Clin Immunol 2014;)
Keywords: Anaphylaxis, alpha-gal, basophil, mammalian meat, food allergy
Shortly after the approval of cetuximab in 2005, it became clear that a significant number of patients were experiencing severe hypersensitivity reactions during their first infusion of this mAb.1 Detailed investigation of serum antibodies by our group established that these reactions were occurring in patients who had pre-existing IgE antibodies specific for glycosylation products on the Fab fragment of the mAb.2 The relevant oligosaccharide is galactose-alpha-1,3-galactose (alpha-gal), which is a blood group substance of nonprimate mammals.3,4 Since that time, IgE antibodies to alpha-gal have been associated with a novel form of food allergy that appears to occur in some patients after tick bites.2,5-8 Specifically, patients with IgE to alpha-gal reported that they had generalized urticaria, angioedema, or anaphylaxis and that this occurred 3 to 6 hours after eating beef, pork, or lamb.6,7 Although we are now aware of more than 2000 persons on at least 4 continents who report delayed allergic reactions to mammalian meat or cow's milk–containing products (most of whom have documented IgE to alpha-gal), direct observation of the delayed reactions has not been confirmed.9-16
Here we report detailed observations on the appearance of symptoms after food challenges with mammalian meat in subjects with IgE to alpha-gal and present results showing that clinical symptoms do not appear until at least 3 hours after the consumption of beef or pork. These studies also included 13 control subjects with negative results for IgE to alpha-gal who underwent mammalian meat food challenges and did not have symptoms.
Although tryptase levels (presumed to be from mast cells) largely do not increase in patients with food-induced anaphylaxis, basophils are postulated to be involved in allergic reactions to food, and specific markers of activation have been identified.17-21 Dr Shreffler and colleagues22 have shown that regulation of the basophil activation markers CD63 and CD203c corresponds to peanut sensitization and that activation is decreased with oral immunotherapy. Using subjects allergic to insect venom, Dr Saini's group23 showed that in an intentional sting challenge model there was an increase in these same activation markers.
We report here a detailed clinical investigation of delayed allergic symptoms after consumption of mammalian meat in subjects with IgE to alpha-gal. The results provide direct evidence for a delayed, IgE-mediated food allergy. We also sought to assess basophil CD63 levels ex vivo during the food challenges, and these results imply there is a delay in the entrance of the relevant form of antigen into the circulation.
METHODS
Basophil activation assay
The various stimulation conditions included RPMI medium (ThermoFisher Scientific, Waltham, Mass) containing 10 μg/mL beef thyroglobulin (Sigma-Aldrich, St Louis, Mo), 100 μg/mL cetuximab (ImClone, Bridgewater, NJ), 1 μg/mL cetuximab, 1 μg/mL anti-IgE antibody (Invitrogen Life Technologies, Grand Island, NY), or 2 μmol/L fMLP (Sigma-Aldrich). RPMI medium alone was used for all unstimulated control subjects. Blood was collected into Vacutainer tubes containing acid citrate dextrose buffer (BD, Franklin Lakes, NJ) before oral food challenge and at hourly intervals for up to 6 hours after food consumption. Additionally, before the activation assay itself, all solutions and whole peripheral blood collected before ingestion of mammalian meat were separately incubated for 15 minutes at 37°C to allow for temperature equilibration.
For the basophil activation assay, 1 mL of warmed whole peripheral blood was mixed with 1 mL of warmed stimulus medium and incubated for 30 minutes, 1 hour, 2 hours, and 4 hours at 37°C. Afterward, 350 μL of PBS plus 20 mmol/L EDTAwas added to each sample to stop the activation process. For hourly interval time points collected during the food challenge, 3 mL of whole peripheral blood was mixed directly with 350 μL of PBS plus 20 mmol/L EDTA. (Note: no ex vivo stimulation was performed on samples collected during a meat challenge.) All samples were spun at 1400 rpm for 10 minutes, with the resulting supernatant manually removed and the remaining cell pellet immediately stained for flow cytometric analysis.
Flow cytometric analysis
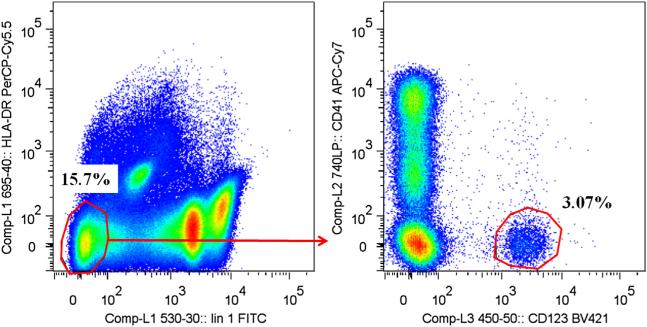
Multiple gating strategies were used over the initial mammalian meat food challenges to establish optimal fluorochromes for flow cytometric analysis of whole blood basophils. Although we did perform Ficoll purification of basophils, we did not find that this additional purification step led to appreciable differences in results. Included in the optimization process was assessment of whether differences emerged when collecting peripheral blood through intravenous needle draw, as well as the conditions, protocols, and reagents for measuring mediators. We report activation as the percentage of CD63 cells over baseline. Our analysis includes CD203c as well; however, we found this marker most reliable for in vitro assays in which a controlled stimulation occurred for 15 to 30 minutes. CD203c was not a consistent marker of activation across subjects during meat challenges (where samples were taken hourly), and this is likely because of the more rapid nature of CD203c as an activation marker.E1 For multicolor FACS analysis, specific mAbs were directly added to the stimulated whole peripheral blood samples and incubated for 30 minutes in the dark at 4°C. Antibodies used were at a final concentration of 1 μg/100 μL and allophycocyanin-conjugated anti-CD63 (MEM-259; BioLegend, San Diego, Calif), Brilliant Violet 421–conjugated anti-CD123 (9F5; BD Biosciences, San Jose, Calif), PerCP-Cy5.5–conjugated anti-HLA-DR (LN3; eBioscience, San Diego, Calif), fluorescein isothiocya-nate–conjugated lineage cocktail 1 (anti-CD3, anti-CD14, anti-CD16, anti-CD19, anti-CD20, and anti-CD56; BD Biosciences), allophycocyanin-Cy7–conjugated anti-CD41 (HIP8; BioLegend, San Diego, Calif), and phycoerythrin-conjugated anti-CD203c (97A6; Beckman Coulter, Indianapolis, Ind). Single color compensation controls were created by using anti-mouse immunoglobulin beads (BD Biosciences). Stained cells were washed with FACS buffer (PBS supplemented with 0.5% BSA and 2 mmol/L EDTA). Red blood cells were then lysed by adding FACS Lysing Solution (BD Biosciences) to each sample for 15 minutes. Stained cell suspensions were analyzed with a FACScalibur flow cytometer (BD Biosciences) with a Cytek DxP10 upgrade (Cytek, Fremont, Calif) and FlowJo software, version 7.6.5 (Tree Star). For all analyses, compensation and gating controls (cells stained with all reagents minus one) were included. A minimum of 1000 Lin 1–HLA-DR–CD41–CD123+ events (ie, basophils) were recorded for each condition or the sample was excluded.
Allergen stimulation generates a distinct subpopulation of basophils that express CD63 with a high density and consequently can be expressed as a percentage of activated CD63+ basophils for each condition or time point.E2 This percentage was corrected for nonspecific activation by gating the percentage of activated (CD63+) basophils in the appropriate negative control or baseline condition at 1% (Figs 2 and E2). Although some authorities have suggested that a percentage of CD63+ basophils of 8% to 10% might reflect activation,E3 we found that a percentage of at least 15% CD63+ cells was more consistently correlated with clinical symptoms. Therefore “basophil activation” was set at greater than 15% CD63+.
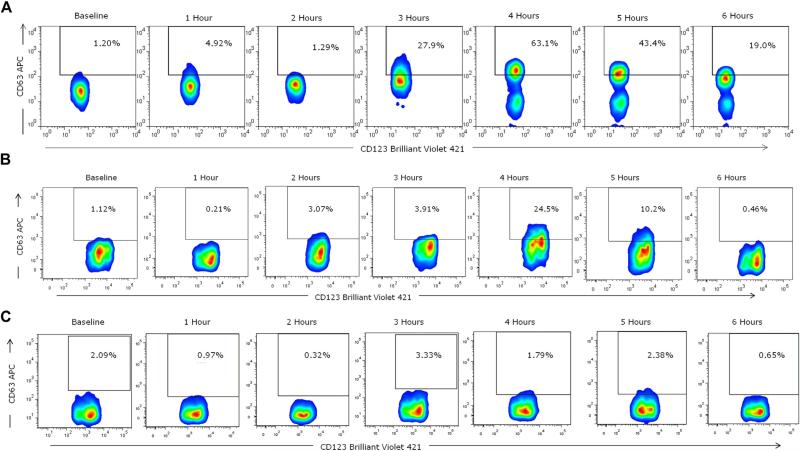
FIG 2.
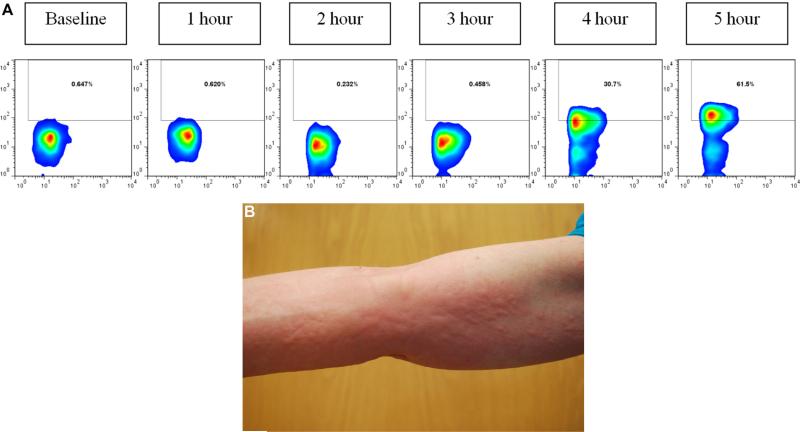
Basophil cell-surface expression of CD63 during mammalian meat challenge of patient FC-09 with IgE to alpha-gal (A) and control subjects (B and C). Whole blood was collected at baseline and hourly during a mammalian meat challenge, as described in the Methods section. CD63 expression peaked at hour 4 (63.1%) in the subject (Fig 2, A) and at hour 4 (24.5%) in a control subject (Fig 2, B). The control subject in Fig 2, C, did not show evidence of basophil activation.
FIG E2.
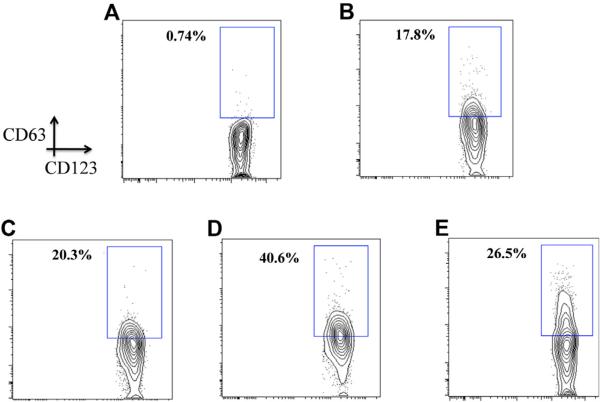
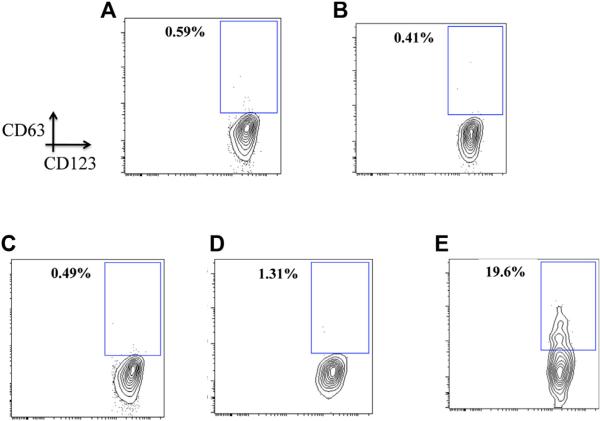
Representative FACS contour plots from patient FC-02 using whole blood basophils stimulated in vitro for 30 minutes with medium (A), 50 μg of beef thyroglobulin (B), 5 μg of cetuximab (C), 5 μg of anti-IgE (D), and 10 μmol/L fMLP (E).
Key messages.
The delay in reactions to red meat reported by patients with IgE to alpha-gal has been confirmed in observed challenges.
Basophil activation during the food challenge corresponds to the timing of clinical symptoms.
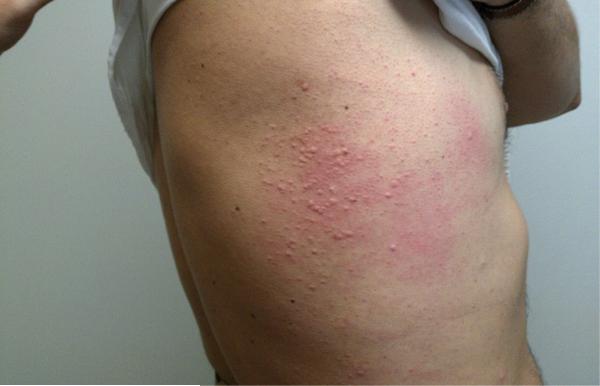
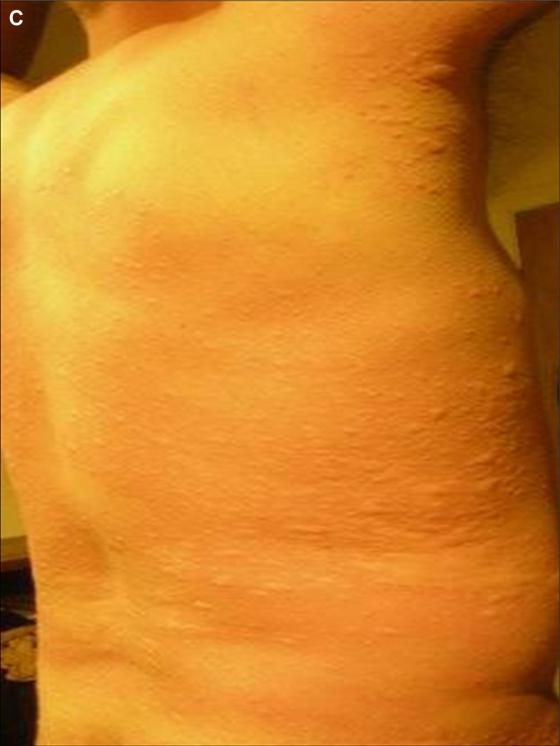
FIG 1.
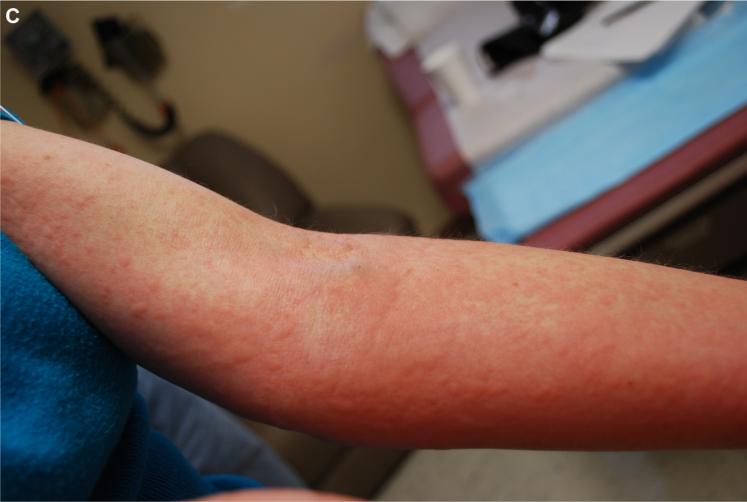
Representative urticaria appearing in patient FC-09 at 257 minutes after eating 150 g of pork sausage. The right flank is shown.
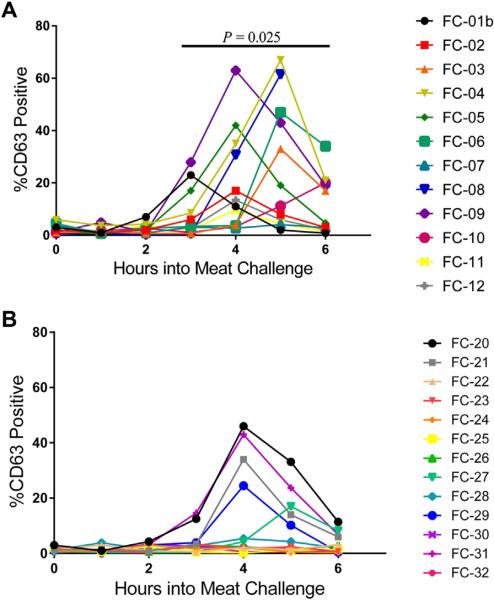
FIG 3.
Basophil CD63 expression at hourly time points in individual patients with positive results for IgE antibody (A) and control subjects (B) over the course of a mammalian meat challenge. P = .025 for mean maximal activation of patients (33.4 ± 6.4) versus control subjects (14.7 ± 4.6).
FIG E1.
Gating strategy for the basophil activation assay. Basophils were identified by means of flow cytometry as HLA-DR–lineage 1– and then CD41–CD123+ cells. Basophil activation was assessed based on expression of CD63 and reported as a percentage of CD63+ cells, with baseline set to 1%. APC, Allophycocyanin; FITC, fluorescein isothiocyanate.
FIG E3.
Representative FACS contour plots from patient FC-02 with IgE to alpha-gal using whole blood basophils stimulated in vitro for 4 hours with medium (A), 50 μg of beef thyroglobulin (B), 5 μg of cetuximab (C), 5 μg of anti-IgE (D), and 10 μmol/L fMLP (E).
FIG E4.
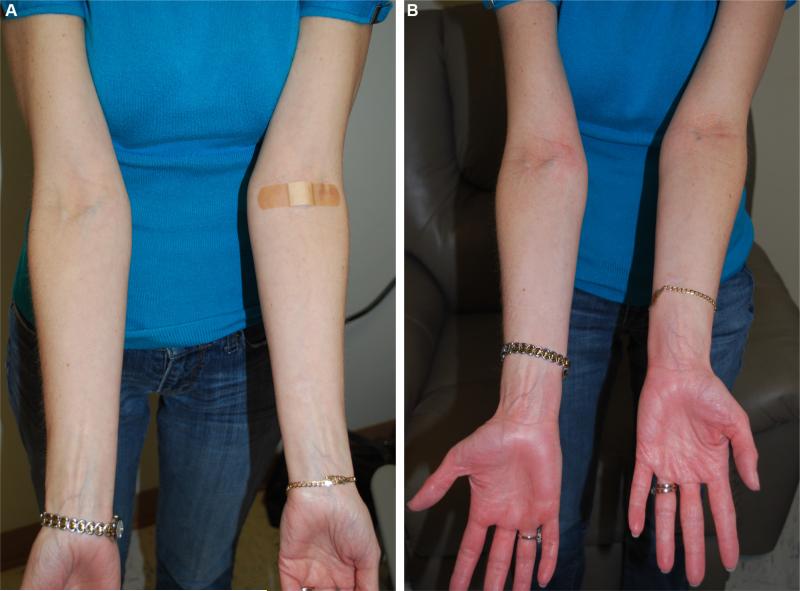
Patient with IgE to alpha-gal (FC-08) demonstrating baseline forearms and palms (A) compared with distinct palmar erythema (B) that appeared at 4 hours and 38 minutes after eating 150 g of pork sausage.
FIG E5.
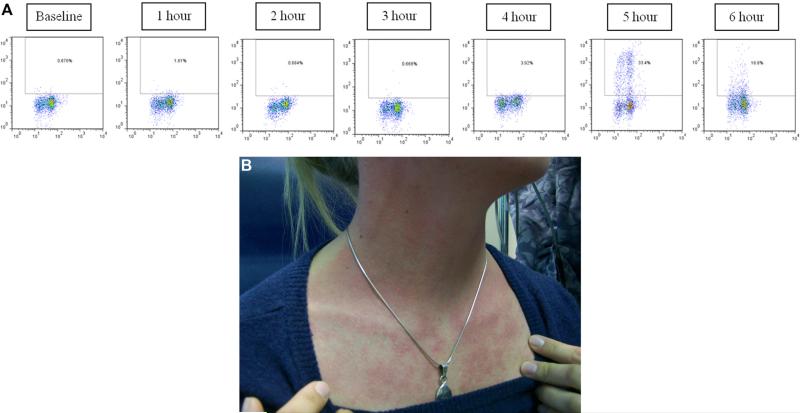
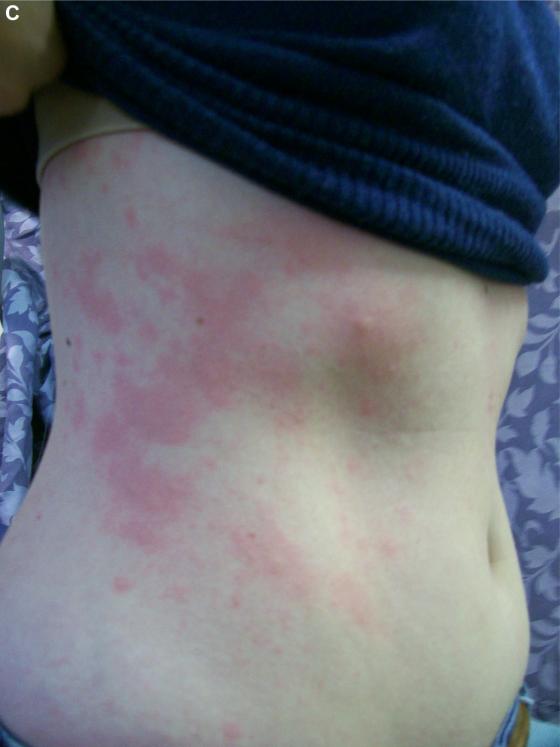
A, Basophil cell-surface expression of CD63 during mammalian meat challenge of patient FC-08 shown in Fig E4. No sample was obtained at 6 hours because of clinical care of the subject. B and C, Additional photos of hives from patient FC-08.
FIG E6.
Basophil cell-surface expression of CD63 (A) and supporting photos (B and C) during mammalian meat challenge of patient FC-03.
FIG E7.
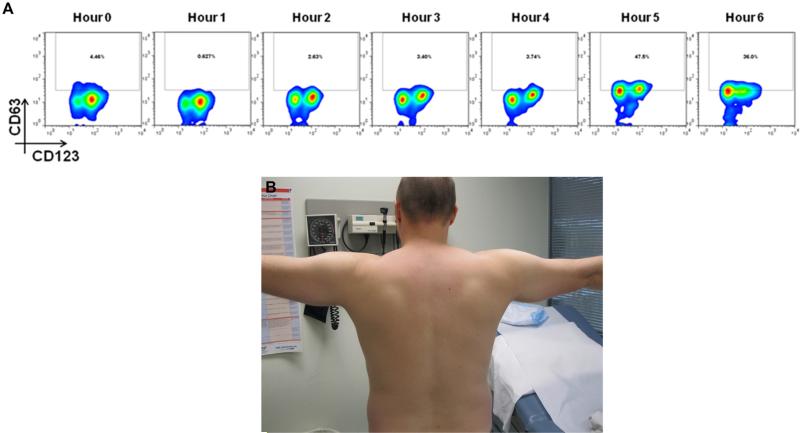
A, Basophil cell-surface expression of CD63 during mammalian meat challenge of patient FC-06. B, Patient FC-06 before eating mammalian meat. C, Patient FC-06 at 6 hours and 45 minutes after consuming 150 g of mammalian meat.
TABLE I.
Patients’ characteristics and mammalian meat food challenge results
| Subject ID |
Age (y)/sex |
History of tick bites |
IgE to alpha-gal |
Total IgE (IU/mL) |
Alpha- gal/total IgE |
Cow's milk IgE (IU/mL) |
IgE to casein (IU/mL) |
Beef IgE (IU/mL) |
Pork IgE (IU/mL) |
Symptoms | Time to symptoms |
Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC-01b | 54/M | Yes | 26.7 | 757 | 3.5% | 3.9 | <0.35 | 6.8 | 6.5 | Pruritus, urticaria | 4:15 | AH |
| FC-02 | 28/M | Yes | 22.4 | 109 | 21% | 2.3 | <0.35 | 5.5 | 4.9 | Pruritus, urticaria, heartburn | 4:22 | AH |
| FC-03* | 25/F | Yes | 17.6 | 184 | 9.5% | 3.4 | 0.6 | 4.8 | 4.5 | Cough, urticaria, chest tightness, abdominal cramping, hypotension | 3:54 | AH, prednisone, albuterol, epinephrine |
| FC-04 | 55/M | Yes | 43.6 | 78.1 | 56% | 0.6 | <0.35 | 8.3 | 7.8 | None | NA | None |
| FC-05 | 32/M | Yes | 10.1 | 27.0 | 37% | 1.1 | <0.35 | 3.0 | 2.7 | “Grumbling stomach” | 4:18 | None |
| FC-06 | 26/M | Yes | 9 | 204 | 4.4% | 0.8 | <0.35 | 2.2 | 2.1 | Pruritus, urticaria | 4:52 | AH |
| FC-07* | 52/M | Yes | 22.6 | 77.9 | 29% | 1.5 | <0.35 | 3.3 | 2.9 | Pruritus, urticaria | 4:40 | AH |
| FC-08 | 46/F | Yes | 30.3 | 146 | 21% | 1.2 | <0.35 | 3.7 | 3.0 | Palmar erythema, pruritus, urticaria, chills, hypotension | 4:38 | AH, prednisone, epinephrine |
| FC-09 | 48/M | Yes | 31.7 | 143 | 22% | 4.4 | <0.35 | 11.6 | 11.2 | Palmar itching, pruritus, urticaria | 3:23 | AH, prednisone |
| FC-10 | 19/M | Yes | 25.7 | 324 | 7.9% | 1.5 | <0.35 | 9.5 | 9.8 | Heart burn, abdominal cramping | 5:04 | AH |
| FC-11* | 37/M | Yes | 10.3 | 163 | 6.3% | 2.9 | <0.35 | 5.0 | 5.1 | Palmar and plantar itching | 4:09 | None |
| FC-12 | 51/F | Yes | 13.2 | 143 | 9.2% | 0.6 | <0.35 | 2.9 | 2.5 | Palmar itching, flushing, urticaria | 2:45 | AH, prednisone |
AH, Antihistamine; F, female; M, male; NA, not applicable.
Subjects who were avoiding cow's milk, limiting dairy intake, or both before food challenge because of associated symptoms.
TABLE II.
Control subjects’ characteristics and mammalian meat food challenge results
| Subject ID | Age (y)/sex | History of tick bites | IgE to alpha-gal (IU/mL) | Total IgE (IU/mL) | Cow's milk IgE (IU/mL)* | Beef IgE (IU/mL) | Pork IgE (IU/mL) | Symptoms (IU/mL)† |
|---|---|---|---|---|---|---|---|---|
| FC-20 | 34/F | No | <0.35 | 83.8 | <0.35 | <0.35 | <0.35 | None |
| FC-21 | 29/M | Yes | <0.35 | 252 | <0.35 | <0.35 | <0.35 | None |
| FC-22 | 28/F | No | <0.35 | 22.9 | <0.35 | <0.35 | <0.35 | None |
| FC-23 | 31/F | No | <0.35 | 42.3 | <0.35 | <0.35 | <0.35 | None |
| FC-24 | 37/M | Yes | <0.35 | <2 | <0.35 | <0.35 | <0.35 | None |
| FC-25 | 25/F | No | <0.35 | 107 | <0.35 | <0.35 | <0.35 | None |
| FC-26 | 44/M | Yes | <0.35 | 2.9 | <0.35 | <0.35 | <0.35 | None |
| FC-27 | 27/M | No | <0.35 | 7.7 | <0.35 | <0.35 | <0.35 | None |
| FC-28 | 46/F | Yes | <0.35 | 53.8 | <0.35 | <0.35 | <0.35 | None |
| FC-29 | 36/M | No | <0.35 | 11.1 | <0.35 | <0.35 | <0.35 | None |
| FC-30 | 23/F | Yes | <0.35 | 127 | <0.35 | <0.35 | <0.35 | None |
| FC-31 | 39/M | Yes | <0.35 | 96.1 | <0.35 | <0.35 | <0.35 | None |
| FC-32 | 22/F | No | <0.35 | 32.4 | <0.35 | <0.35 | <0.35 | None |
F, Female; M, male.
Measurement of IgE levels to casein (milk component) was not performed in subjects with a negative immunoassay result for IgE to cow's milk.
None of the control subjects reported symptoms, and therefore no treatment was administered.
TABLE III.
Basophil activation and tryptase measurements during mammalian meat challenge of subjects with and without IgE to alpha-gal
| Patient | CD63 activation* | Serial tryptase† |
|---|---|---|
| Subjects with positive results for IgE antibody to alpha-gal | ||
| FC-01b | 23% at 3 h | No change |
| FC-02 | 17% at 4 h | No change |
| FC-03 | 33% at 5 h | 4.9/4.7/9.9/10.7/9.6 |
| FC-04∥ | 67% at 5 h | No change |
| FC-05∥ | 42% at 4 h | No change |
| FC-06 | 47% at 5 h | ND |
| FC-07 | None | No change |
| FC-08 | 30% at 4 h | 4.2/3.8/5.1/18.2/20.1 |
| FC-09 | 27% at 3 h | 6.2/5.9/14.8/18.3‡ |
| FC-10 | 20% at 6 h | No change |
| FC-11 | None | No change |
| FC-12 | None§ | ND |
| Control subject | CD63 activation | Serial tryptase |
|---|---|---|
| Subjects with negative results for IgE antibody to alpha-gal | ||
| FC-20 | 46% at 4 h* | No change |
| FC-21 | 34% at 4 h | No change |
| FC-22 | None | No change |
| FC-23 | None | No change |
| FC-24 | None | No change |
| FC-25 | None | No change |
| FC-26 | None | No change |
| FC-27 | 17% at 5 h | No change |
| FC-28 | None | No change |
| FC-29 | 24% at 4 h | No change |
| FC-30 | None | No change |
| FC-31 | 43% at 4 h | No change |
| FC-32 | None | No change |
ND, Not done.
Basophil activation was defined as the first time point when the percentage of CD63+ basophils was greater than 15%. The actual percentage of activation was noted with the corresponding hourly time point.
Tryptase was expressed as nanograms per milliliter and listed in order of collection (baseline/2 hours/3 hours/4 hours/5 hours); serial mature tryptase levels were considered positive if the level increased greater than 11.4 mg/mL or 2+1.2 times baseline.26
Patient FC-09 did not have a 5-hour tryptase sample.
The basophil CD63 percentage reached 13% at hour 4.
Subject considered to have a negative meat challenge result.
Acknowledgments
We thank the Organic Butcher of Charlottesville for continuing to be a purveyor of fine meats.
S. P. Commins has received research support from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Disease and has received royalties from UpToDate. H. R. James, W. Stevens, S. L. Pochan, C. King, and S. Mozzicato have received research support from the NIH. T. A. E. Platts-Mills has received research support from the NIH, has received royalties from UpToDate, is a patent holder for technology used to biotinylate antigens for detection of IgE, has been on the scientific advisory board for ViaCor-IBT laboratories, and has received select reagents & supplies used in his laboratory from Phadia/ThermoFisher.
Abbreviations used
- Alpha-gal
Galactose-alpha-1,3-galactose
- FACS
Fluorescence-activated cell sorting
- fMLP
Formyl-methionyl-leucyl-phenylalanine
Footnotes
Disclosure of potential conflict of interest: M. H. Land declares that he has no relevant conflicts of interest.
REFERENCES
- 1.O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 2.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 4.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, et al. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104:559–64. doi: 10.1073/pnas.0610012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009;124:652–7. doi: 10.1016/j.jaci.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-a-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caponelto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-alpha-1,3-galactose. J Allergy Clin Immunol: In Practice. 2013;1:302–3. doi: 10.1016/j.jaip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meat-induced anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol. 2009;124:603–5. doi: 10.1016/j.jaci.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Jappe U. [Update on meat allergy : α-Gal: a new epitope, a new entity?]. Hautarzt. 2012;63:299–306. doi: 10.1007/s00105-011-2266-y. [DOI] [PubMed] [Google Scholar]
- 12.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya K, Fukutomi Y, Nakazawa T, Taniguchi M, Akiyama K. Delayed anaphylactic reaction to mammalian meat. J Investig Allergol Clin Immunol. 2012;22:446–7. [PubMed] [Google Scholar]
- 14.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlén G, et al. Identification of galactose-α-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68:549–52. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- 15.Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–1. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 16.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012;129:1334–42. e1. doi: 10.1016/j.jaci.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–6. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 18.Lemon-Mulé H, Nowak-Wegrzyn A, Berin C, Knight AK. Pathophysiology of food-induced anaphylaxis. Curr Allergy Asthma Rep. 2008;8:201–8. doi: 10.1007/s11882-008-0034-6. [DOI] [PubMed] [Google Scholar]
- 19.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–4. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 20.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 21.MacGlashan D. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012;42:1197–205. doi: 10.1111/j.1365-2222.2012.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gober LM, Eckman JA, Sterba PM, Vasagar K, Schroeder JT, Golden DB, et al. Expression of activation markers on basophils in a controlled model of anaphylaxis. J Allergy Clin Immunol. 2007;119:1181–8. doi: 10.1016/j.jaci.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandené L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40–8. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 26.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiro RG, Bhoyroo VD. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984;259:9858–66. [PubMed] [Google Scholar]
- 28.Cianferoni A, Khullar K, Saltzman R, Fiedler J, Garrett JP, Naimi DR, et al. Oral food challenge to wheat: a near-fatal anaphylaxis and review of 93 food challenges in children. World Allergy Organ J. 2013;6:14. doi: 10.1186/1939-4551-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Erp FC, Knulst AC, Kentie PA, Pasmans SG, van der Ent CK, Meijer Y. Can we predict severe reactions during peanut challenges in children? Pediatr Allergy Immunol. 2013;24:596–602. doi: 10.1111/pai.12107. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy J, Stallings A, Platts-Mills T, Oliveira W, Workman L, James H, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131:1–8. doi: 10.1542/peds.2012-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oole-Groen CJ, Brand PL. Double-blind food challenges in children in general paediatric practice: useful and safe, but not without pitfalls. Allergol Immunopathol (Madr) 2013 doi: 10.1016/j.aller.2013.06.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rydberg L, Bjorck S, Hallberg E, Magnusson S, Strokan V, Svalander C, et al. Extracorporeal (“ex vivo”) connection of pig kidneys to humans. II. The anti-pig antibody response. Xenotransplantation. 1996;3:340–53. doi: 10.1111/j.1399-3089.1996.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 34.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–42. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 35.Strokan V, Mölne J, Svalander CT, Breimer ME. Heterogeneous expression of Gal alpha1-3Gal xenoantigen in pig kidney: a lectin and immunogold electron microscopic study. Transplantation. 1998;66:1495–503. doi: 10.1097/00007890-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 36.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 37.Jönsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–96. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonen B, O'Donnell P, Post TJ, Quinn TJ, Schulman ES. Very low density lipoproteins (VLDL) trigger the release of histamine from human basophils. Biochim Biophys Acta. 1987;917:418–24. doi: 10.1016/0005-2760(87)90121-4. [DOI] [PubMed] [Google Scholar]
- 39.Virgolini I, Li SR, Yang Q, Koller E, Sperr WR, Leimer M, et al. Characterization of LDL and VLDL binding sites on human basophils and mast cells. Arterioscler Thromb Vasc Biol. 1995;15:17–26. doi: 10.1161/01.atv.15.1.17. [DOI] [PubMed] [Google Scholar]
- 40.Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662–9. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Platz IJ, Binder M, Marxer A, Lischka G, Valent P, Bühring HJ. Hymenoptera-venom-induced upregulation of the basophil activation marker ecto-nucleotide pyrophosphatase/phosphodiesterase 3 in sensitized individuals. Int Arch Allergy Immunol. 2001;126:335–42. doi: 10.1159/000049531. [DOI] [PubMed] [Google Scholar]
- 42.de Weck AL, Sanz ML. Cellular allergen stimulation test (CAST) 2003, a review. J Investig Allergol Clin Immunol. 2004;14:253–73. [PubMed] [Google Scholar]
- E1.Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662–9. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- E2.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- E3.Platz IJ, Binder M, Marxer A, Lischka G, Valent P, Bühring HJ. Hymenoptera-venom-induced upregulation of the basophil activation marker ecto-nucleotide pyrophosphatase/phosphodiesterase 3 in sensitized individuals. Int Arch Allergy Immunol. 2001;126:335–42. doi: 10.1159/000049531. [DOI] [PubMed] [Google Scholar]