Abstract
Isorhamnetin is an O-methylated flavonol present in fruit and vegetables. We recently reported the identification of isorhamnetin as an activator of the human pregnane X receptor (PXR), a known target for abrogating inflammation in inflammatory bowel disease (IBD). The current study investigated the role of isorhamnetin as a putative mouse PXR activator in ameliorating chemically induced IBD. Using two different models (Ulcerative colitis-like and Crohn’s disease-like) of experimental IBD in mice, we demonstrated that isorhamnetin abrogated inflammation through inhibiting the activity of myeloperoxidase (MPO), the levels of TNF-α and IL-6, the mRNA expression of pro-inflammatory mediators (iNOS, ICAM-1, COX2, TNF-α, IL-2 and IL-6), and the phosphorylation of IκBα and NF-κB p65. PXR gene overexpression inhibited NF-κB luciferase activity, and the inhibition was potentiated by isorhamnetin treatment. PXR knockdown by siRNA demonstrated the necessity for PXR in isorhamnetin-mediated upregulation of xenobiotic metabolism genes. Ligand pocket-filling mutants (S247W/C284W and S247W/C284W/S208W) of human PXR weakened the effect of isorhamnetin on PXR activation. Molecular docking studies and time-resolved fluorescence resonance energy transfer (TR-FRET) competitive binding assays confirmed the ligand (isorhamnetin) binding affinity. These results clearly demonstrated the ameliorating effect of isorhamnetin on experimental IBD via PXR-mediated upregulation of xenobiotic metabolism and downregulation of NF-κB signaling. The novel findings may contribute to the effective utilization of isorhamnetin or its derivatives as a PXR ligand in the treatment of human IBD.
Keywords: Inflammatory bowel disease, pregnane X receptor, xenobiotic metabolism, NF-κB, isorhamnetin
1. Introduction
Inflammatory bowel disease (IBD), which primarily manifests as ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic and relapsing inflammatory condition of the gastrointestinal (GI) tract. It is usually accompanied by severe GI symptoms such as diarrhea, bleeding, abdominal pain, weight loss, and anemia. The etiology of IBD is still not completely understood; however, it is widely accepted that the development of IBD is associated with the interplay of genetic, bacterial and environmental factors and dysregulation of the intestinal immune system [1,2].
The pregnane X receptor (PXR) belongs to the nuclear receptor superfamily, members of which are transcription factors characterized by a ligand-binding domain (LBD) and a DNA-binding domain. The primary function of PXR is to sense the presence of foreign toxic substances and upregulate the expression of metabolism enzymes (e.g., CYP3A4, CYP2C9 and CYP1A2) and xenobiotic transporters (e.g., MDR1, MRP2 and OATP2) involved in the detoxification and clearance of these substances from the body [3,4]. As a master regulator of xenobiotic metabolism and detoxification, PXR is abundantly expressed in the mammalian intestine and liver. It is hypothesized that the detoxification properties of PXR and its target genes are necessary to maintain the integrity of the intestinal epithelial barrier [5]. Loss of PXR function has been associated with intestinal inflammation in animal studies [6,7], and low levels of PXR expression have been found in the intestine of UC patients [8]. We and others have shown that activation of PXR leads to transcriptional regulation of cellular detoxification genes and inhibition of NF-κB activity, which results in a decrease in intestinal inflammation [9-11]. Pregnenolone 16α-carbonitrile (PCN), a mouse PXR ligand, decreases the susceptibility of mice to DSS-induced colitis via PXR-mediated repression of inflammatory NF-κB signaling in the colon, suggesting the potential value of PXR agonists as therapeutic agents for IBD [12].
Isorhamnetin (3′-methoxy-3,4′,5,7-tetrahydroxyflavone) is an O-methylated flavonol present in plants of the Polygonaceae family. It is also an intermediate metabolite of quercetin in mammals and plants [13,14]. Isorhamnetin has anti-inflammatory, anti-oxidant and anti-cancer activities [15-17]. Several studies indicated that isorhamnetin downregulates the key molecules such as COX2, PGE2, TNF-α and NF-κB involved in inflammation. A recent investigation suggested an ameliorating effect of isorhamnetin on clinical symptoms of DSS-induced colitis in mice, but failed to provide the mechanism [17]. We recently reported the identification of isorhamnetin as an activator of the human PXR, upregulating the expression of CYP3A4 in HepG2 cells [18]. The present study details the role of isorhamnetin in ameliorating chemically induced IBD (DSS- or TNBS-induced) in association with its effects as a PXR ligand.
2. Materials and Methods
2.1. Cell lines and reagents
The human colon adenocarcinoma cell lines HT-29 and LS174T and mouse macrophage cell line RAW264.7 were from the American Type Culture Collection (ATCC) and cultured according to ATCC recommendations. HT-29 cells have low expression of PXR and LS174T cells have the abundant expression of PXR. Isorhamnetin (purity ≥ 98%, HPLC) was kindly provided by the Shanghai R&D Center for Standardization of Traditional Chinese Medicine, Shanghai, China. DSS (MW 36-50 KDa) was acquired from MP Biochemical LLC, Solon, OH. Donkey serum, paraformaldehyde, methylcellulose, TNBS, LPS, rifampicin, hyperforin, formalin, Tween-20, ethanol and DMSO were obtained from Sigma-Aldrich, St.Louis, MO. The NF-κB reporter vector pGL4.32[luc2P/NF-κB-RE/Hygro] and the Dual-Luciferase reporter assay system were from Promega, Madison, WI. SYBR Premix ExTaq Mix was from Takara Bio Inc., Otsu, Japan. The LanthaScreen ™ TR-FRET PXR competitive binding assay system, SuperScript III first-strand synthesis system, Fluor 488-conjugated anti-rabbit IgG (A-21206), Triton X-100, Trizol, and DAPI reagent were from Invitrogen, Carlsbad, CA. BSA and protease inhibitor cocktail tablets were from Roche Diagnostics, Mannheim, Germany. PXR siRNA duplex (sc-44057) and control siRNA duplex (sc-37007) were from Santa Cruz Biotechnology, CA. Horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology. The human PXR antibody (ab85451) was from Abcam, Cambridge, MA. TNF-α reagent and mouse antibodies to the following proteins: NF-κB p65 (#8242), phospho-p65 (#3033), phospho-IκBα (#2859), IκBα (#4812), and beta-actin (#4970) were from Cell Signaling Technology, Danvers, MA. Mouse TNF-α and IL-6 ELISA kits were from R&D systems, Minneapolis, MN. The MPO activity assay kit was from CytoStore, Calgary, AB, Canada. Agarose, ethidium bromide and enhanced chemiluminescence (ECL) western blotting detection reagent were from Thermo Scientific, Waltham, MA.
2.2. Mice
Healthy 8-week-old female C57BL/6 mice (20 ± 2 g) were obtained from Shanghai Laboratory Animal Center, and studies performed in accordance with the guidelines approved by the Animal Ethics Committee of Shanghai University of TCM (SHUTCM). Standard mouse chow pellets and water were supplied ad libitum. All mice were housed under a specific pathogen-free facility at SHUTCM and kept under the same laboratory conditions of temperature (25 ± 2°C) and lighting (12-h light-dark cycle).
2.3. DSS-induced colitis
DSS colitis was induced in mice as described previously [10,11]. Eight-week-old female C57BL/6 mice were placed into four groups (n = 10 per group) in the DSS-induced IBD study as follows: Group 1, vehicle controls were administered 100 μl 0.5% methylcellulose by oral gavage once per day; Group 2 received isorhamnetin at 20 mg/kg of body weight via oral gavage once per day; Group 3 received 100 μl 0.5% methylcellulose by oral gavage once per day and 4% DSS (MW 36000-50000, MP Biomedicals, Solon, OH) in the drinking water for 7 days; Group 4 received isorhamnetin by oral gavage (20 mg/kg of body weight) for 7 days, beginning in coordination with the start of DSS exposure. Isorhamnetin dosing (20 mg/kg per body weight) was based on previous reports in which oral administration of 10-30 mg/kg of isorhamnetin showed a potent anti-inflammatory effect in carrageenan-induced paw swelling and an anti-oxidant effect in streptozotocin-induced diabetes [19].
2.4. Clinical and histological scores of colitis
Mice were monitored daily for body weight, diarrhea and bloody stool. Four hours after receiving the last gavage, the mice were sacrificed under anesthesia. The entire colon was removed, and the total length of the colon was measured. The distal colons were taken, fixed in 10% buffered formalin for 24 h at room temperature, embedded in paraffin and stained with hematoxylin-eosin (H&E) for histological evaluation. Histological scoring was performed in a blinded fashion by two pathologists to obtain a combined score of inflammatory cell infiltration (score 0–3) and tissue damage (score 0–3) as described previously [11].
2.5. Immunoblot analysis
When LS174T cells reached 70% confluence, they were incubated with isorhamnetin (25 μM) for 24 h and then subjected to cell lysis. Colon tissues were disrupted by homogenization on ice and centrifuged at 4°C (12,000 ×g, 15 min), and the supernatants were collected. Equal amounts of protein (40 μg) were separated on 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked in 5% (w/v) skim milk and incubated with antibodies against PXR (1:1000), phospho-p65 (1:1000), phospho-IκBα (1:1000) and IκBα (1:1000), respectively. Blots were incubated with horseradish peroxidase-conjugated secondary antibodies and developed by ECL western blot detection reagents. Quantification of protein expression was performed by densitometric analysis of the blots.
2.6. Semi-quantitative and real-time quantitative (q) PCR
LS174T cells were incubated with isorhamnetin (0, 1, 10, 25 μM) for 24 h. RNA was extracted from cultured cells or colon tissues using TRIzol reagent. Semi-quantitative and real-time qPCR was performed using cDNA generated from 3 μg total RNA with the SuperScript III Reverse Transcriptase kit according to the manufacturer’s recommendations. The primer sequences used in semi-quantitative PCR amplification are as follows: 5′-TGGCTTCCAGCAACTTCTAC-3′/5′-GCATCAGCACATACTCCTCC-3′ for hPXR, 5′-GACTTAGTTGCGTTACACCCTTTCT-3′/5′-ACTGCTGTCACCTTCACCGTTC-3′ for hbeta-actin. The DNA thermal cycler conditions used were 94°C for 5 min (pre-denature), and 35 cycles of 94°C for 1 min, annealing at 58°C for 30 s and extension at 72°C for 45 s, followed by a final extension of 72°C for 2 min. PCR products were separated by electrophoresis on 2% agarose gels and stained with ethidium bromide. The amount of target gene was normalized with beta-actin as the internal control gene. The primer sequences used in qPCR amplification are as follows: 5′-AATAGTTGTCCTAGCACCTGAC-3′/5′-TCTTTCACATCCTCCCTTTG-3′ for hUGT1A1, 5′-AGCCCATCCTGTTTGACTGC-3′/5′-TGTATGTTGGCCTCCTTTGC-3′ for hMDR1a, 5′-GGAAATCGTGCGTGACATTA-3′/5′-TCAGGCAGCTCGTAGCTCTT-3′ for hbeta-actin, 5′-GCACGAAGTTGTGGTCATAG-3′/5′-AATCTTGATTAAAGGCAGTCC-3′ for mUGT1A1, 5′-TGTGATTGCGTTTGGAGGAC-3′/5′-CCATACCAGAATGCCAGAGC-3′ for mMDR1a, 5′-GGGAATCTTGGAGCGAGTTG-3′/5′-GTGAGGGCTTGGCTGAGTGA-3′ for miNOS, 5′-CGCTGTGCTTTGAGAACTGT-3′/5′-AGGTCCTTGCCTACTTGCTG-3′ for mICAM-1, 5′-GAAGTCTTTGGTCTGGTGCCT-3′/5′-GCTCCTGCTTGAGTATGTCG-3′ for mCOX2, 5′-CGTGGAACTGGCAGAAGAGG-3′/5′-AGACAGAAGAGCGTGGTGGC-3′ for mTNF-α, 5′-TCAGCAACTGTGGTGGACTT-3′/5′-AGTGATTAGCAAGGGTGAGA-3′ for mIL-2, 5′-ACCACGGCCTTCCCTACTTC-3′/5′-CATTTCCACGATTTCCCAGA-3′ for mIL-6, and 5′-CAGCCTTCCTTCTTGGGTAT-3′/5′-TGGCATAGAGGTCTTTACGG-3′ for mbeta-actin. PCR reactions were carried out using SYBR Premix ExTaq Mix in an ABI Prism 7900 real-time PCR System (Life Technologies, Carlsbad, CA). The thermal cycler parameters were as follows: 1 cycle of 95°C for 30 s and 40 cycles of denaturation (95°C, 5 s) and combined annealing/extension (60°C, 30 s). Gene expression changes were calculated by the comparative Ct method, and the values were normalized to the internal beta-actin control.
2.7. Wild-type h/mPXR and hPXR mutant transactivation reporter assays
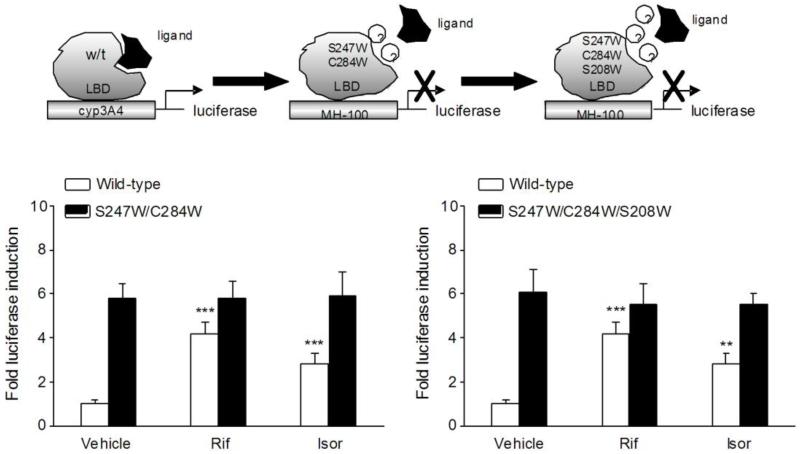
HT-29 cells (1×106) were electroporated using a Lonza Nucleofector II instrument (Amaxa Biosystems, Germany) as described previously [10,11]. In brief, HT29 cells were cotransfected with plasmids expressing hPXR, mPXR, hPXR double mutants (S247W/C284W), and hPXR triple mutants (S247W/C284W/S208W) with appropriate reporters, as shown in schematic diagrams (Fig. 5). Plasmid information refers to our previously published studies [20,21]. For human and mouse wild-type PXR transactivation assays, cells were incubated with isorhamnetin (0, 1, 10, 25 μM). For hPXR double and triple mutant transactivation assays, cells were incubated with rifampicin (10 μM) or isorhamnetin (25 μM). Cell extracts were assayed for luciferase activity after 24 h of treatment. The results are expressed as the fold induction of control cells.
Figure 5.
The effects of isorhamnetin on PXR mutants activation. (A) Transient transcription assays were performed in HT-29 cells cotransfected with plasmids expressing wild-type, double mutants (S247W/C284W) or triple mutants (S247W/C284W/S208W) of hPXR with their appropriate reporters. After 8 h of transfection, cells were treated with rifampicin (10 μM) or isorhamnetin (25 μM) for 24 h. Cell extracts were assayed for luciferase activity, and results are expressed as fold induction of control cells. Data are presented as the mean ± SD of three independent experiments. ** P < 0.01, *** P < 0.001 vs. vehicle-treated cells transfected with wild-type hPXR..
2.8. PXR-mediated NF-κB repression reporter assay
HT-29 cells (1×106) were electroporated with pGL4.32[luc2P/NF-κB-RE/Hygro] reporter alone or co-electroporated with pSG5-hPXR and pRL-TK as described previously [10]. The pGL4.32 reporter is a NF-κB reporter vector containing NF-κB response elements and firefly luciferase gene. Cells were then seeded into 48-well plate following transfection. After overnight incubation, cells were treated either with TNF-α (20 ng/ml) alone or cotreated with isorhamnetin (1, 10 and 25 μM) for 24 h. A standard dual luciferase assay was performed with cell lysates, and results are expressed as fold induction of control cells.
2.9. Gene silencing
Human PXR gene knockdown in LS174T cells was performed using a Lonza Nucleofector II instrument (Amaxa Biosystems, Germany). In brief, 1×106 LS174T cells were electroporated with PXR siRNA targeting the human PXR mRNA. Control siRNA, a non-targeting siRNA, was used as a negative control. Cells were then seeded into 6-well plate following transfection. After overnight incubation, cells were treated with or without isorhamnetin (25 μM) for 24 h. At the end of the incubation, cells were rinsed, scraped and used in qRT-PCR or western blot studies as described above.
2.10. Determination of TNF-α and IL-6 levels
Colon segments were homogenized in ice-cold PBS. The homogenates were centrifuged at 3,000 ×g for 10 min, and the cytokine levels in the supernatants were determined as described previously [22]. The level of each cytokine was evaluated using ELISA kits according to the manufacturer’s protocols (R&D systems, Minneapolis, MN), and the results are expressed as pg/mg of protein in each sample.
2.11. Myeloperoxidase (MPO) assay
Tissue MPO activity, which is linearly related to neutrophil infiltration in inflamed tissue, was assayed to monitor the degree of inflammation. MPO activity was measured in pieces of the colon according to the manufacturer’s instructions (CytoStore, Alberta, Canada). MPO activity is expressed as units/mg of protein.
2.12. Molecular modeling
The molecular modeling analysis of the hPXR ligand affinity was performed as described previously with some modifications [11]. The hPXR ligand hyperforin served as template molecule to evaluate the ligand affinity for hPXR. The three-dimensional structure of hPXR co-crystalized with hyperforin was obtained from the Protein Data Bank (PDB code: 1M13). The co-crystalized structure was prepared using MOE (Molecular Operating Environment Program, version 2012.10, Chemical Computing Group, Montreal, Canada) to correct structural errors, such as broken bonds, missing loops, etc. The binding site for hPXR has been well characterized based on structural information derived from variety of co-crystals (PDB Code: 1ILH/1M13/1SKX/2QNV/3R8D). PLIF (Protein Ligand Interaction Fingerprints) in MOE was used to analyze the co-crystals and identify the conserved pocket residues. The prepared structure was submitted to FlexX (BioSolveIT, Germany) for molecular docking. The residues within 7 Å around hyperforin (template ligand) were selected as the binding pocket, which includes all the critical residues. Then, hyperforin was removed, and hydrogen atoms were added to prepare the structure for docking experiments. The docked complex was built by Molecule Builder in MOE. The prepared structure of isorhamnetin was submitted as a ligand to FlexX. Classical Triangle Matching was chosen as the placement method, and the docking poses were evaluated by the FlexX Score. The best-ranked complex was then energy minimized using the Amber12:EHT force field in MOE. Before minimization, the residues around the ligand within 7 Å were tethered (the strength is 10), and those farther than 7 Å from the ligand were fixed. The binding mode was analyzed in MOE after minimization.
2.13. TR-FRET assay
The PXR binding assay was performed using the LanthaScreen™ TR-FRET PXR competitive binding assay system as described previously [21], in which a test compound competes with and displaces a reference fluorescently labeled ligand from the recombinant terbium-labeled PXR-LBD. Briefly, 10 μl of isorhamnetin (final concentration, 25 μM) was placed in quadruplicate into the wells of a black, round-bottomed 384-well assay plate. Next, 5 μl 4× Fluormone PXR (SXR) Green was added into each well, followed by 5 μl 4× PXR-LBD (GST)/DTT/4×Tb anti-GST antibody. The plate was gently mixed and then incubated in the dark at room temperature for 1 h. TR-FRET was measured using the EnVision® Multilabel Plate Reader (PerkinElmer, CA) with an excitation wavelength of 340 nm and emission wavelengths of 520 and 495 nm. The TR-FRET ratio was calculated by dividing the emission signal at 520 nm by that at 495 nm. The curve was fit to the data (TR-FRET ratio versus log test compound) using a sigmoidal dose-response (variable slope) equation in GraphPad Prism ™ software (Version 3.0). Rifampicin (final concentration, 10 μM) was included as a positive control PXR ligand.
2.14. TNBS-induced colitis
Colitis was induced in mice with TNBS as described previously with some modifications [10]. Eight-week-old female C57BL/6 mice were placed into four groups (n = 10 per group) in the TNBS-induced IBD study. Group 1 and group 2 refer to the DSS-induced IBD model; Group 3 received 100 μl 0.5% methylcellulose by oral gavage once per day, and 2.5 mg TNBS in 100 μl 50% ethanol was administered intrarectally to fasted and anesthetized mice via a catheter inserted 3 cm proximally to the anus on d1; Group 4 received a daily gavage of isorhamnetin (20 mg/kg of body weight) for 7 days, beginning in coordination with the start of TNBS exposure.
2.15. Statistics
All data are expressed as the mean ± SD. The differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post-hoc test. Statistical analysis was performed by the SPSS 16.0 software package. A value of p<0.05 was considered statistically significant.
3. Results
3.1. Isorhamnetin treatment attenuates DSS-induced colitis
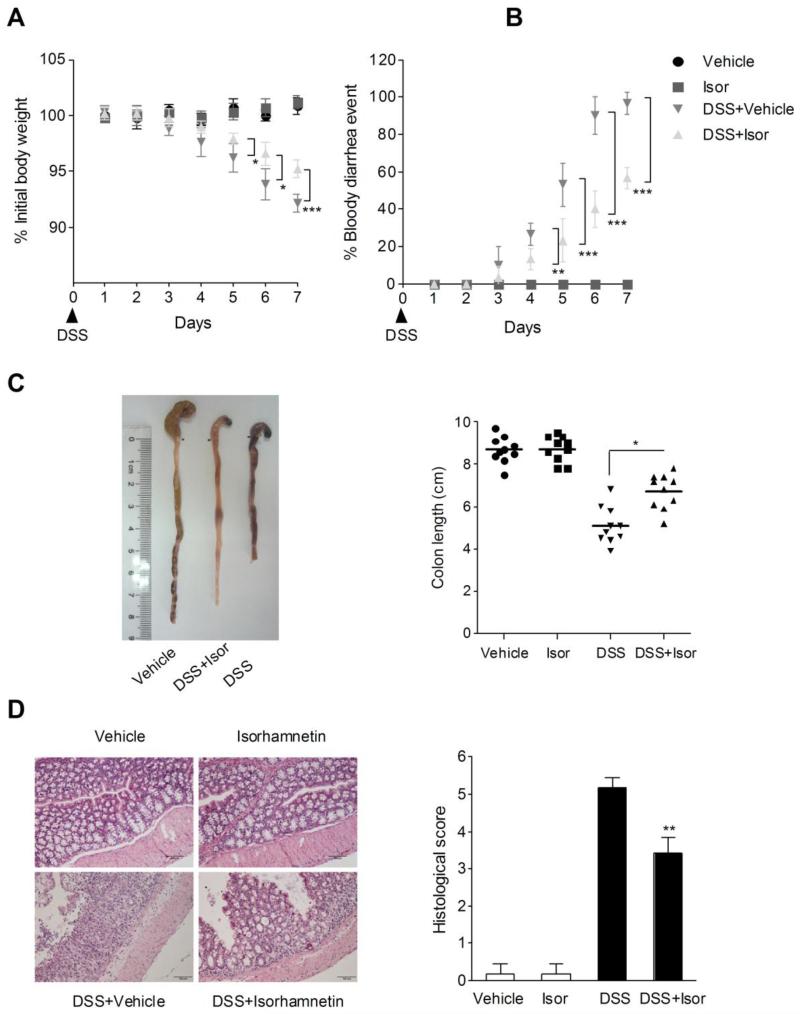
Oral administration of DSS in mice induces colitis resembling human UC, and the inflammation was mainly localized to the colon [23]. In mice receiving DSS treatment alone, we observed a significant body weight loss, bloody diarrhea, colon shortening and neutrophil infiltration. (Fig. 1). Moreover, the mucosal content of TNF-α and IL-6 and the activity of MPO were markedly increased in mice exposed to DSS (Table 1). These parameters were significantly attenuated in mice receiving isorhamnetin treatment. In addition, none of the mice receiving isorhamnetin alone exhibited loss of body weight, diarrhea, colon shortening or mucosal disruption at any point during the study (Fig. 1).
Figure 1.
Isorhamnetin attenuated DSS-induced colitis in mice. (A) Body weight changes following DSS induction of colitis. Data plotted as a percentage of basal body weight. (B) The occurrence of bloody diarrhea. Data plotted as percentage of total mice that had bloody diarrhea at different time points of DSS treatment. (C) Macroscopic observation and assessment of colon shortening. (D) Representative H&E-stained colon sections (Magnification 200 ×) and histology score. Values were expressed as the mean ± SD of n = 10 mice in each group. *p<0.05, **P< 0.01, ***p<0.001 vs. DSS-treated group.
Table 1.
Effects of isorhamnetin on the activity of MPO and the levels of TNF-α and IL-6 in DSS- or TNBS-induced colitis mice.
| DSS |
TNBS |
|||||
|---|---|---|---|---|---|---|
| Group | Vehicle | Isor | Vehicle | Isor | Vehicle | Isor |
| TNF-α (pg/mg pr.) |
26.5 ± 1.6 | 28.6 ±14.7 | 257.1 ± 23.6 | 84.2 ± 16.8 *** | 226.5 ± 38.1 | 92.9 ± 27.5 ** |
|
| ||||||
| IL-6 (pg/mg pr.) |
14.9 ± 3.9 | 18.7 ±1.5 | 148.0 ± 23.4 | 82.9 ± 6.5 ** | 186.1 ± 27.0 | 64.9 ± 17.4 ** |
|
| ||||||
| MPO (U/mg pr.) |
3.7 ± 0.6 | 4.4 ±0.3 | 26.8 ± 2.2 | 14.7 ± 0.4 ** | 22.7 ± 0.9 | 10.5 ± 2.8 ** |
Colon segments from mice (n = 6 per group) were excised and homogenized. The supernatants were assayed for the determination of the activity of MPO and the levels of TNF-α and IL-6 as described in the Methods. Values are expressed as the mean ± SD.
p < 0.01
P < 0.001 vs. DSS- or TNBS-treated group.
3.2. Isorhamnetin blocks the activation of NF-κB in the colon and downregulates NF-κB target genes in the colon
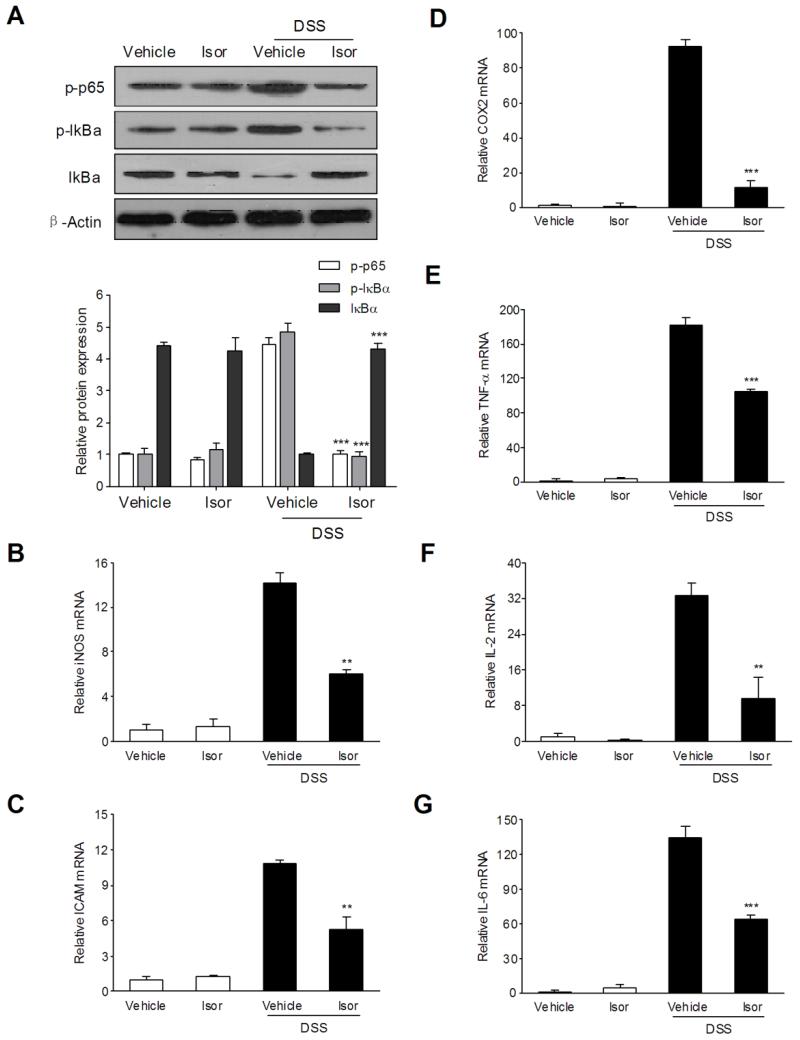
NF-κB is a major transcriptional factor of pro-inflammatory signaling pathways involved in IBD [24,25]. We reasoned that the anti-inflammatory effect of isorhamnetin in response to chemically induced IBD is associated with the blockade of NF-κB activation. Immunoblot analysis showed that DSS-induced phosphorylation/degradation of IκBα and phosphorylation of NF-κB p65 were blocked by isorhamnetin administration (Fig. 2A). To further elucidate the potential mechanism of isorhamnetin in abrogating chemical colitis, qPCR analyses of several NF-κB target genes in the colon were performed. The results showed that mRNA expression of iNOS, ICAM-1, COX2, TNF-α, IL-2 and IL-6 was remarkably induced in the inflamed colons of mice that were exposed to DSS (Fig. 2B-G). In contrast, the increase in these inflammatory mediators following DSS treatment was significantly decreased in mice receiving isorhamnetin. The results suggest that isorhamnetin ameliorates experimental colitis through repression of NF-κB signaling.
Figure 2.
The effects of isorhamnetin on NF-κB activation and NF-κB target genes expression in the colon of DSS-induced colitis mice. (A) Total protein from colon samples (n = 6 per group) was extracted and analyzed by western blot. mRNA expression of iNOS (B), ICAM-1 (C), COX2 (D), TNF-α (E), IL-2 (F) and IL-6 (G) was determined by qRT-PCR in colon samples isolated from mice (n = 6 per group). Expression was normalized to beta-actin, and each bar represents the mean ± SD of two independent experiments with samples in triplicate. ** p < 0.01, ***p < 0.001 vs. DSS-treated group.
3.3. Isorhamnetin inhibits NF-κB activity in a PXR dependent manner
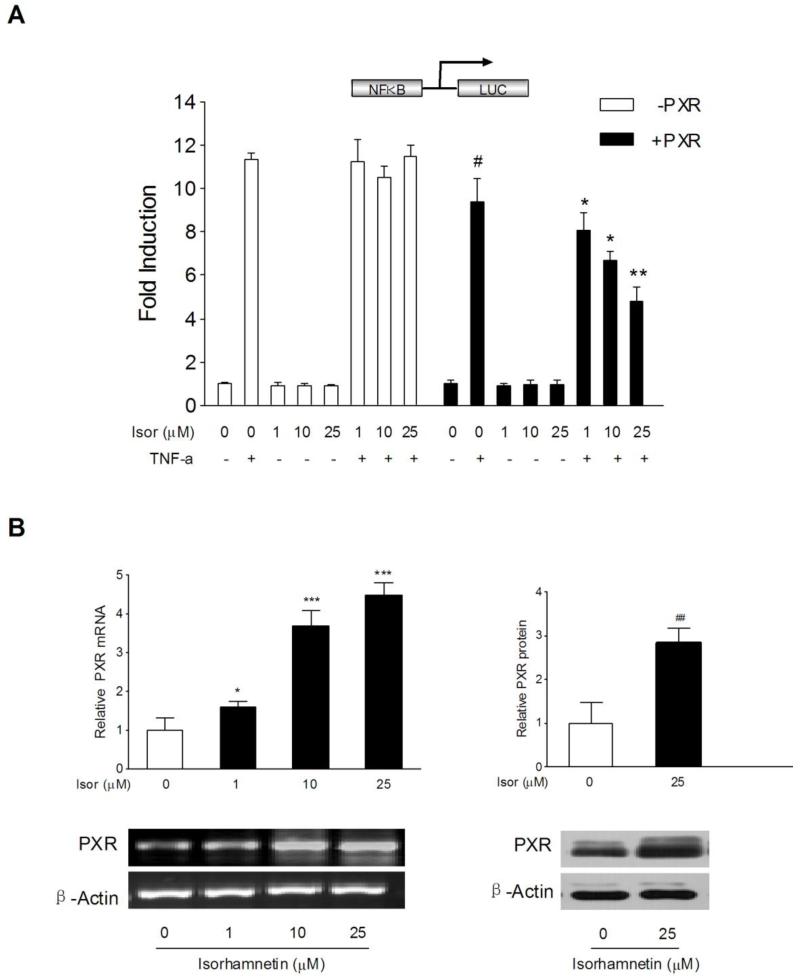
Recent studies have demonstrated that PXR activation decreases the susceptibility of mice to DSS-induced colitis via suppression of NF-κB [5,9]. We infer that the immunosuppressive effect of isorhamnetin in chemical colitis might be linked to PXR activation. The effect of isorhamnetin on the regulation of PXR was determined in HT-29 colon cancer cells transfected with an NF-κB reporter in the presence or absence of an human PXR expression plasmid. HT-29 cells have low or undetectable human PXR expression [26,27]. Treatment with the known NF-κB pathway activator TNF-α led to a marked increase in NF-κB reporter activity, and interestingly, overexpression of human PXR inhibited NF-κB reporter activity (Fig. 3A). In addition, we observed that NF-κB activity was dose-dependently inhibited by isorhamnetin in the presence of human PXR expression, but isorhamnetin had no significant effect on NF-κB activity in the absence of human PXR expression (Fig. 3A). These results suggest that PXR is required for the isorhamnetin-mediated inhibition of NF-κB activity.
Figure 3.
Role of PXR on isorhamnetin-mediated NF-κB luciferase inhibition and the effects of isorhamnetin on PXR expression. (A) HT-29 cells were electroporated with the pGL4.32[luc2P/NF-κB-RE/Hygro] reporter alone or coelectroporated with pSG5-hPXR and pRL-TK. After transfection, cells were treated either with TNF-α (20 ng/ml) alone or cotreated with isorhamnetin (0, 1, 10, 25 μM) for 24 h. Luciferase activity was determined with cell lysates, and the results are expressed as fold induction of control cells (designated as 1). Data are presented as the mean ± SD of three independent experiments. # P < 0.05 vs. TNF-α alone-treated samples without hPXR transfection; *p < 0.05, **P < 0.01 vs. TNF-α alone-treated samples with hPXR transfection. (B) The mRNA expression of PXR (n=3) was assessed by semi-quantitative RT-PCR in LS174T cells treated with isorhamnetin (0, 1, 10, 25 μM); the protein expression of PXR (n=3) was determined by western blot in LS174T cells treated with isorhamnetin (25 μM). *p < 0.05, ** p < 0.01, ***P < 0.001 vs. vehicle-treated group.
3.4. Isorhamnetin upregulates the expression of PXR and xenobiotic detoxification genes
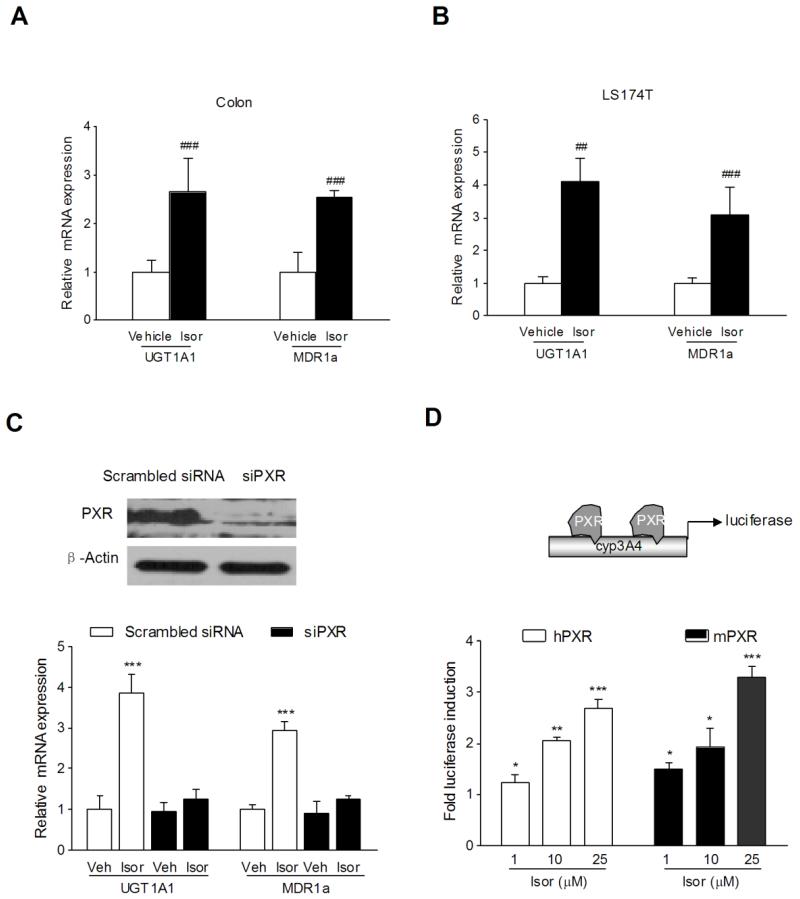
In addition to the activation of PXR, isorhamnetin also upregulated the mRNA and protein expression of PXR in LS174T colon cancer cells (Fig. 3B). As a xenobiotic sensor nuclear receptor, PXR activation upregulates the expression of xenobiotic oxidation and conjugation enzymes and transporters involved in elimination of potentially harmful chemicals from the body. It has been hypothesized that the detoxification properties of PXR and its target genes (e.g., CYP3A4, UGT1A1, MDR1, MRP2, etc.) are necessary to maintain the integrity of the intestinal epithelial barrier and, therefore, contribute to the anti-inflammatory effect in intestines [28,29]. Accordingly, we observed a significant upregulation of the expression of UGT1A1 and MDR1a in the colon of mice receiving 7 days of isorhamnetin administration (Fig. 4A). However, we did not observe the upregulation of CYP3A11 in the colon of the same mice exposed to isorhamnetin (data not shown). Consistent with the in vivo results, we observed a robust increase in the expression of UGT1A1 and MDR1a in LS174T colon cancer cells exposed to 25 μM isorhamnetin (Fig. 4B). These results suggest that the upregulation of xenobiotic detoxification genes might contribute to the effects of isorhamnetin in abrogating experimental IBD.
Figure 4.
Role of PXR on isorhamnetin-mediated upregulation of xenobiotic detoxification genes and the effects of isorhamnetin on PXR activation. The mRNA expression of UGT1A1 and MDR1a was assessed by qRT-PCR in colon samples isolated from mice (n = 6 per group) orally fed with 20 mg/kg isorhamnetin (A) or in LS174T cells (n = 3) treated with 25 μM isorhamnetin (B). ## p<0.01, ### p<0.001 vs. vehicle-treated samples. (C) Depletion of PXR by specific siRNA in LS174T cells was verified by western blot (top panel). The mRNA expression of UGT1A1 and MDR1a was assessed by qRT-PCR in isorhamnetin-treated LS174T cells transfected with hPXR siRNA or control siRNA (bottom panel). Data are expressed as the mean ± SD of triplicate of two independent experiments. ***P< 0.001 vs. wild-type vehicle-treated wells. (D) Wild-type h/mPXR transactivation reporter assay. Transient transcription assays were performed in HT-29 cells cotransfected with plasmids expressing hPXR or mPXR with appropriate reporters. After 8 h of transfection, cells were incubated with isorhamnetin (0, 1, 10, 25 μM) for 24 h. A standard dual luciferase assay was performed with cell extracts, and results are expressed as fold induction of control cells. * p < 0.05, ** P < 0.01, *** P < 0.001 vs. vehicle-treated cells.
3.5. Isorhamnetin upregulates xenobiotic detoxification genes in a PXR dependent manner
To explore the mechanistic involvement of PXR in the upregulation of xenobiotic detoxification genes induced by isorhamnetin, the human PXR gene was silenced by PXR siRNA transfection. As illustrated in Figure 4C, treatment of LS174T cells with a PXR siRNA almost completely blocked the expression of PXR (Fig. 4C, top panel). Further, we observed that the effect of isorhamnetin on the upregulation of xenobiotic detoxification genes (UGT1A1 and MDR1a) was lost in LS174T cells transfected with a PXR siRNA (Fig. 4C, bottom panel). These results indicate that PXR is required for the isorhamnetin-mediated upregulation of cellular detoxification genes.
3.6. Isorhamnetin activates both mouse and human PXR in vitro
Using the HepG2 liver cancer cell model, we previously demonstrated that isorhamnetin activates human PXR in a dose-dependent manner [18]. To determine whether isorhamnetin can also activate mouse PXR, human and mouse PXR transactivation reporter assays were performed in HT-29 cells exposed to isorhamnetin. The results indicate that isorhamnetin can activate both human and mouse PXR in a dose-dependent manner (Fig. 4D).
3.7. Ligand pocket-filling mutants of human PXR attenuates isorhamnetin-mediated PXR activation
To assess whether isorhamnetin binds to the ligand-binding pocket of human PXR and subsequently actives PXR, we performed a transient transactivation reporter assay using the double-mutant construct (S247W/C284W) and triple-mutant construct (S247W/C284W/S208W) of the human PXR ligand-binding pocket. The S247W mutant was originally described as a pocket-filling mutant, with the serine replaced by a larger tryptophan [20,30]. Because PXR has a promiscuous binding pocket, it is conceivable that even the S247W mutant can accommodate smaller ligands such as isorhamnetin (MW316.3) yet exclude larger ones such as rifampicin (MW 822.9). To address this problem, we used double and triple mutants of the PXR ligand-binding pocket by combining the S247W mutant with either S208W and/or C284W, which effectively fill the ligand-binding pocket of PXR, leaving insufficient room for even the smallest established ligand (like SR12813) to bind to the receptor, resulting in ligand-binding occlusion and ligand-independent constitutive activation [20,21]. We showed that both rifampicin and isorhamnetin can activate wild-type human PXR, whereas none of them can activate either double or triple mutants (Fig. 5). The results imply that isorhamnetin indeed binds to the ligand-binding pocket of human PXR.
3.8. Docking of isorhamnetin to the PXR ligand-binding pocket reveals a high-confidence ligand interaction
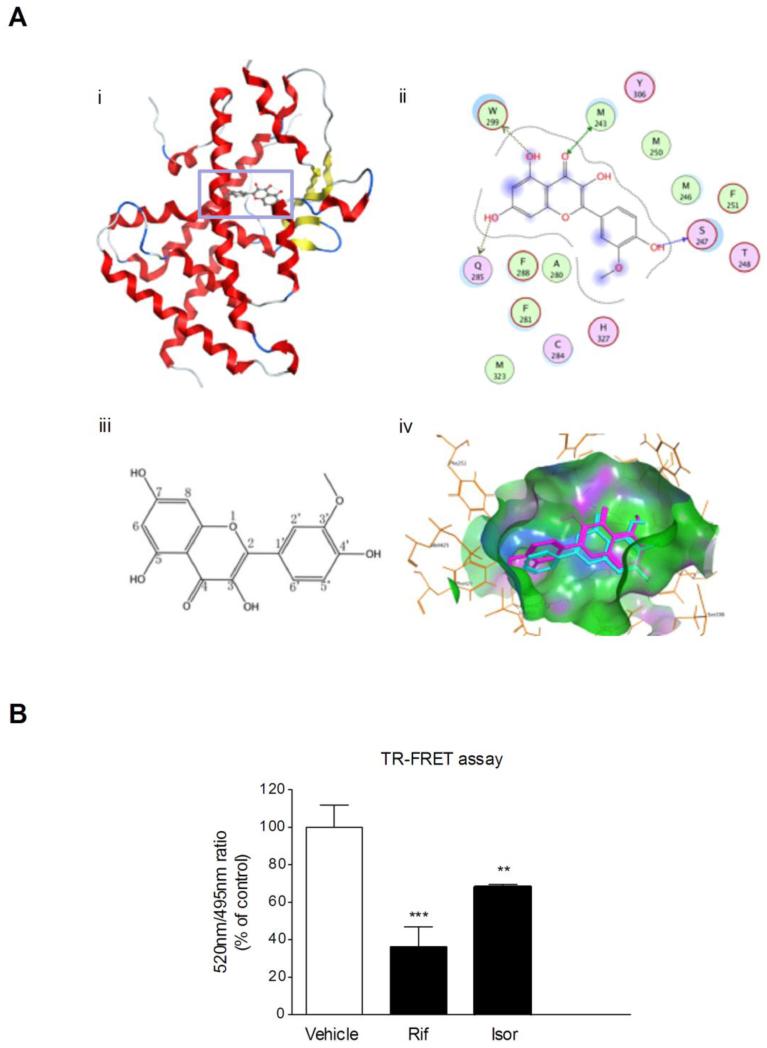
To confirm the results of the study from human PXR mutants transactivation, computational molecular modeling studies were carried out. The docking mode of isorhamnetin in the binding site of human PXR is illustrated in Fig. 6A. Docking studies showed that isorhamnetin had hydrogen-bond interactions with residues Gln285, Leu209, Glu321 and His407 in different directions. The carbonyl group at C-4 and the hydroxyl group at C-3 share the same hydrogen-bond interaction with residue Gln285, which may leads to more stable ligand binding. Furthermore, the hydrophobic interactions with residue His407, Leu209, Met323, Phe281, Leu411, Phe429 and Phe251 may contribute to the high binding affinity, and the methoxyl group at C-3’ position may enhance the hydrophobic interactions. These data confirm the human PXR ligand (isorhamnetin)-binding affinity.
Figure 6.
Molecular docking analyses and TR-FRET assay. (A) Molecular docking of isorhamnetin to hPXR ligand-binding domain. i) Docking mode of isorhamnetin in the binding site of hPXR (shown in ribbon representation and colored by Terminus). ii) 2D-interaction schematic diagram of isorhamnetin docking to the hPXR ligand-binding domain was generated by the Ligand Interactions module of MOE. The binding-site residues are colored according to different types, with hydrophobic residues in green, polar residues in purple and charged residues highlighted with bold contours. Blue spheres and contours indicate matching regions between ligand and receptor. Hydrogen-bond interactions are shown by green dotted lines with arrows for side chain and main chain interactions, respectively. iii) Molecular structure of isorhamnetin. iv) The binding poses of isorhamnetin (purple) in the ligand-binding pocket of hPXR. (B) The interaction between isorhamnetin and the ligand-binding pocket of hPXR was further characterized using a LanthaScreen TR-FRET PXR competitive binding assay system. The TR-FRET ratio was calculated by dividing the emission signal at 520nm by that at 495nm. Data are expressed at means ± SD of quadruplicate of a representative experiment. ** P < 0.01, *** P < 0.001 vs. vehicle-treated wells.
3.9. Verification of ligand binding by TR-FRET assay
The LanthaScreen TR-FRET PXR competitive binding assay was performed to confirm the direct binding of isorhamnetin to the ligand-binding pocket of human PXR. The known human PXR agonist rifampicin (10 μM) decreased the TR-FRET emission ratio by 64%, which is in accord with the previous report [21]. Isorhamnetin (25 μM) decreased the TR-FRET ratio by 32% (Fig. 6B). This observation confirmed the ability of isorhamnetin to bind directly to the ligand-binding pocket of human PXR.
3.10. Isorhamnetin treatment attenuates TNBS-induced colitis
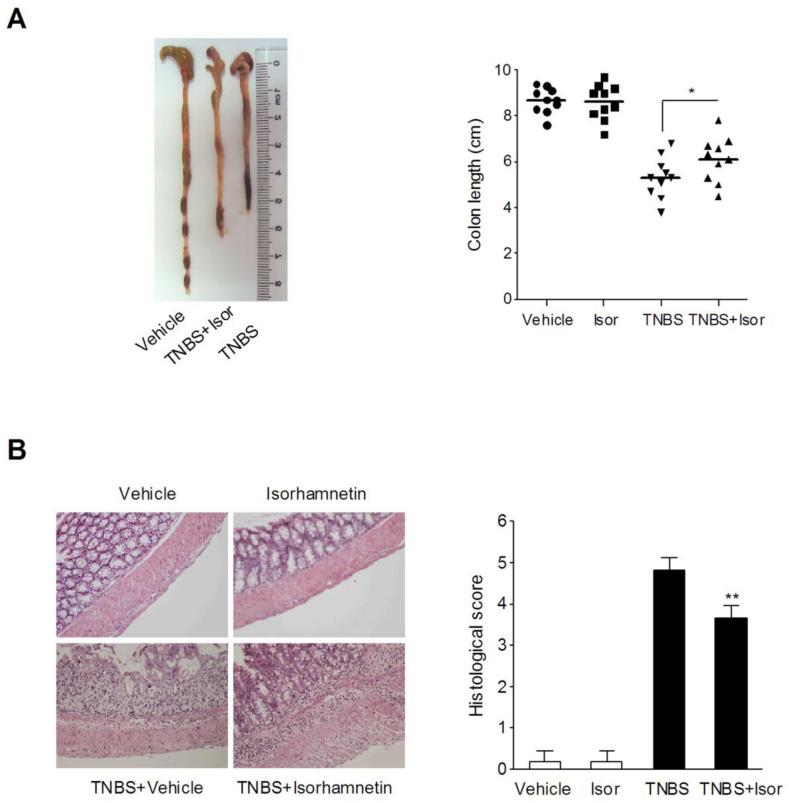
To confirm the results of isorhamnetin on DSS-induced colitis model, we tested the influences of isorhamnetin on some parameters of colitis induced by TNBS, which constitutes a Crohn’s disease model [31]. Rectal administration of TNBS dissolved in ethanol induced severe colitis in mice that was characterized by colon shortening, ulcers in the rectum, enhanced rectal MPO activity, and increased TNF-a and IL-6 levels (Fig. 7 and Table 1). Oral administration of isorhamnetin markedly improved these parameters. In addition, there was no colon shortening or histological damage observed in vehicle or isorhamnetin alone-treated mice (Fig. 7).
Figure 7.
Isorhamnetin attenuated TNBS-induced colitis in mice. (A) Macroscopic observation and assessment of colon shortening (n=10). (B) Representative H&E-stained colon sections (Magnification 200×) and histology score. Values are expressed as the mean ± SD of triplicates of two independent experiments. **p < 0.05, ***P < 0.01 vs. TNBS-treated group.
4. Discussion
Intestinal inflammation induced by DSS and TNBS are well established chemical IBD models with clinical features resembling human UC and CD, respectively [32,33]. In the current study, we have shown that exposure of DSS-induced colitic mice to isorhamnetin ameliorated disease hallmarks such as body weight loss, bloody diarrhea, colon shortening and histological damage. We reasoned that the beneficial effect of isorhamnetin in response to chemical injury is associated with the blockade of NF-κB activity. The results showed that phosphorylation/degradation of IκBα and phosphorylation of NF-κB p65 in the inflamed colon were significantly inhibited by isorhamnetin treatment. Administration of isorhamnetin not only decreased NF-κB activity but also downregulated downstream genes (iNOS, ICAM-1, COX2, TNF-α, IL-2 and IL-6), reduced the activity of MPO, and reduced the accumulation of TNF-α and IL-6 in inflamed colon. To confirm a direct role for PXR in the suppression of NF-κB signaling following isorhamnetin treatment, an in vitro model using LS174T colon cancer cells was assessed. Interestingly, overexpression of human PXR significantly inhibited NF-κB luciferase activity, and the inhibition was further potentiated in the presence of isorhamnetin. Collectively, these findings suggest that isorhamnetin appears to exert its amelioration of DSS-induced colitis through inhibition of PXR-mediated NF-κB signaling.
Previous studies have shown that PXR can be activated by a wide range of structurally diverse chemicals that bind to the LBD of PXR, which triggers the detoxification process by enhancing downstream gene expression and provides protection against chemically induced IBD [29,34,35]. Although we previously demonstrated the effect of isorhamnetin on human PXR activation, it is insufficient to explain the direct ligand binding and predict the in vivo effect of PXR-mediated attenuation of intestinal inflammation. To address this problem, we performed a transient transfection functional assay for wild-type PXR and double mutants (S247W/C284W) and triple mutants (S247W/C284W/S208W) of the human PXR ligand-binding pocket. These mutants are shown to effectively fill the ligand-binding pocket of PXR, leaving insufficient room to allow even the smallest established ligand (like SR12813) to bind to the receptor [20,36]. As expected, we observed activation of wild-type PXR and loss of PXR activation in mutants in this system after isorhamnetin treatment. Furthermore, molecular docking studies and the TR-FRET-based in vitro competitive binding assay confirmed the PXR ligand (isorhamnetin)-binding affinity.
It has long been known that activated PXR functions as a xenobiotic sensor overseeing the detoxification of a wide range of xenobiotics, which is critical for the maintenance of mucosal integrity [5]. In the DSS-induced mouse IBD model, administration of PCN, a mouse PXR agonist, attenuates the severity of intestinal inflammation and also upregulates the expression of phase II detoxification enzymes (GSTa1, GSTm1 and GSTt1) and xenobiotic transporters (MDR1a and MRP2) in the colon [12]. Hence, PXR target genes are thought to play a critical role in intestinal barrier function against xenobiotics and bacteria [6]. Support for this hypothesis comes from the observation that the expression of xenobiotic detoxification genes (UGT1A1 and MDR1) in the colon is upregulated after isorhamnetin treatment. Furthermore, the effect of isorhamnetin on the upregulation of xenobiotic detoxification genes was lost in LS174T colon cancer cells transfected with human PXR siRNA. Recently, dietary curcumin was reported to alleviate colonic inflammation in mice via a PXR-mediated upregulation of xenobiotic metabolism and a downregulation of inflammatory signaling [37]. Our results confirmed the concept that PXR-mediated upregulation of intestinal detoxification genes may also contribute to the beneficial effect of isorhamnetin in experimental IBD in addition to above described PXR-mediated NF-κB pathway inhibition.
IBD is associated with a considerable reduction in quality of life of the patients and an increased risk of colorectal cancer, and currently there is no curative treatment available [38,39]. Current medical treatments for IBD generally include 5-aminosalicylates or sulfasalazine, antibiotics, corticosteroids, immunomodulators and biological therapies (e.g., monoclonal antibodies). However, despite their efficacy, some patients are unresponsive to these therapies and often suffer from numerous side effects [40-42]. Thus, it is still necessary to develop new and specific therapies for IBD. To confirm the data from the DSS-induced colitis model, we next determined the attenuation of mucosal inflammation in the TNBS-induced colitis mouse model after exposure to isorhamnetin. Taken together, our findings provide the first evidence that oral isorhamnetin administration decreases chemically induced IBD (UC-like and CD-like) probably through PXR-mediated upregulation of xenobiotic metabolism and downregulation of the NF-κB signaling pathway. The present study may provide insight into the potential use of isorhamnetin in human IBD treatment.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81273572, U1032604); Natural Science Foundation of Shanghai (12ZR1431400); National Institutes of Health Grant RO1CA127231; Damon Runyon Foundation Clinical Investigator Award (CI 1502). W.D., Z.W. And S.M. designed the study and prepared the manuscript; W.D., J.Z, H.L., S.K., K.S., L.D. and G.R. performed the research and analyzed the data. All authors read and approved the final content of the manuscript.
Abbreviations
- CD
Crohn’s disease
- COX2
cyclooxygenase 2
- CYPs
cytochromes P450
- DSS
dextran sodium sulfate
- ELISA
enzyme linked immunosorbent assay
- GSTs
glutathione S-transferases
- IBD
inflammatory bowel disease
- ICAM-1
Intercellular adhesion molecule-1
- IL-2/6
interleukin 2/6
- iNOS
inducible NO synthase
- Isor
isorhamnetin
- LBD
ligand banding domain
- LPS
lipopolysaccharide
- MDR1
multidrug resistance 1
- MPO
myeloperoxidase
- MRP2
multidrug resistance protein 2
- NF-κB
nuclear factor-kappa B
- OATP2
organic anion transporting polypeptide 2
- PCN
pregnenolone-16α-carbonitrile
- mPGES-1
microsomal prostaglandin E synthase-1
- PXR
pregnane X receptor
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- RXR
retinoid X receptor
- TNBS
trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor-alpha
- TR-FRET
time-resolved fluorescence resonance energy transfer
- UC
ulcerative colitis
- UGTs
UDP-glucuronosyltransferases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest.
References
- 1.Abraham C, Cho JH. Infammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Infammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 4.Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45:60–72. doi: 10.3109/03602532.2012.746363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mencarelli A, Migliorati M, Barbanti M, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80:1700–7. doi: 10.1016/j.bcp.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Langmann T, Moehle C, Mauerer R, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto J, Saito Y, Honda A, et al. Bile acid malabsorption deactivates pregnane X receptor in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19:1278–84. doi: 10.1097/MIB.0b013e318281f423. [DOI] [PubMed] [Google Scholar]
- 8.Martínez A, Márquez A, Mendoza J, et al. Role of the PXR gene locus in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1484–7. doi: 10.1002/ibd.20252. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Shah YM, Gonzalez FJ. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci. 2012;33:323–30. doi: 10.1016/j.tips.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dou W, Zhang J, Zhang E, et al. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther. 2013;345:473–82. doi: 10.1124/jpet.112.201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dou W, Mukherjee S, Li H, et al. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One. 2012;7:e36075. doi: 10.1371/journal.pone.0036075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah YM, Ma X, Morimura K, et al. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–22. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Lee HJ, Lee EO, et al. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008;270:342–53. doi: 10.1016/j.canlet.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Boesch-Saadatmandi C, Egert S, Schrader C, et al. Effect of quercetin on paraoxonase 1 activity--studies in cultured cells, mice and humans. J Physiol Pharmacol. 2010;61:99–105. [PubMed] [Google Scholar]
- 15.Jiang JS, Shih CM, Wang SH, et al. Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells. J Ethnopharmacol. 2006;103:281–7. doi: 10.1016/j.jep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Yang JH, Kim SC, Shin BY, et al. O-methylated flavonol isorhamnetin prevents acute inflammation through blocking of NF-κB activation. Food Chem Toxicol. 2013;59C:362–72. doi: 10.1016/j.fct.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Saud SM1, Young MR, Jones-Hall YL, et al. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013;73:5473–84. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding LL, Zhang JJ, Dou W. Effects of isorhamnetin on CYP3A4 and herb-drug interaction. Yao Xue Xue Bao. 2012;47:1006–10. In Chinese. [PubMed] [Google Scholar]
- 19.Yokozawa T, Kim HY, Cho EJ, et al. Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J Agric Food Chem. 2002;50:5490–5. doi: 10.1021/jf0202133. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Li H, Moore LB, et al. The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Mol Endocrinol. 2008;22:838–57. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh M, Wang H, Cayer J, et al. In vivo and in vitro characterization of a first-in-class novel azole analog that targets pregnane X receptor activation. Mol Pharmacol. 2011;80:124–35. doi: 10.1124/mol.111.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dou W, Zhang J, Sun A, Zhang E, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalmasso G, Nguyen HT, Ingersoll SA, et al. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology. 2011;141:1334–45. doi: 10.1053/j.gastro.2011.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 25.Biasi F, Astegiano M, Maina M, et al. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr Med Chem. 2011;18:4851–65. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 26.Habano W, Gamo T, Terashima J, et al. Involvement of promoter methylation in the regulation of Pregnane X receptor in colon cancer cells. BMC Cancer. 2011;11:81. doi: 10.1186/1471-2407-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Ke S, Ouyang N, et al. Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J Biol Chem. 2009;284:9199–205. doi: 10.1074/jbc.M806193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu X, Ke S, Liu D, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 29.Ma X, Shah YM, Guo GL, et al. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther. 2007;322:391–8. doi: 10.1124/jpet.107.121913. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y, Moore LB, Orans J, et al. Crystal structure of the pregnane X receptor-estradiol complex provides insights into endobiotic recognition. Mol Endocrinol. 2007;21:1028–38. doi: 10.1210/me.2006-0323. [DOI] [PubMed] [Google Scholar]
- 31.Santucci L, Agostini M, Bruscoli S, et al. GITR modulates innate and adaptive mucosal immunity during the development of experimental colitis in mice. Gut. 2007;56:52–60. doi: 10.1136/gut.2006.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coccia EM, Remoli ME, Di Giacinto C, et al. Cholera toxin subunit B inhibits IL-12 and IFN-{gamma} production and signaling in experimental colitis and Crohn’s disease. Gut. 2005;54:1558–64. doi: 10.1136/gut.2004.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–35. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 34.Xie W, Tian Y. Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab. 2006;4:177–8. doi: 10.1016/j.cmet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Wahli W. A gut feeling of the PXR, PPAR and NF-kappaB connection. J Intern Med. 2008;263:613–9. doi: 10.1111/j.1365-2796.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 36.Watkins RE, Davis-Searles PR, Lambert MH, et al. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331:815–28. doi: 10.1016/s0022-2836(03)00795-2. [DOI] [PubMed] [Google Scholar]
- 37.Nones K, Dommels YE, Martell S, et al. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a−/−) mice, a model of inflammatory bowel diseases. Br J Nutr. 2009;101:169–81. doi: 10.1017/S0007114508009847. [DOI] [PubMed] [Google Scholar]
- 38.Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–175.e8. doi: 10.1053/j.gastro.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 39.van der Marel S, Majowicz A, van Deventer S, et al. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2:114–22. doi: 10.4291/wjgp.v2.i6.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fok KC, Ng WW, Henderson CJ, et al. Cutaneous sarcoidosis in a patient with ulcerative colitis on infliximab. J Crohns Colitis. 2012;6:708–12. doi: 10.1016/j.crohns.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Milia AF, Manetti M, Generini S, et al. TNFalpha blockade prevents the development of inflammatory bowel disease in HLA-B27 transgenic rats. J Cell Mol Med. 2009;13:164–76. doi: 10.1111/j.1582-4934.2008.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jobin C. Probiotics and ileitis: could augmentation of TNF/NF-κB activity be the answer? Gut Microbes. 2010;1:196–199. doi: 10.4161/gmic.1.3.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]