Abstract
In an otherwise eligible patient, inadequate mobilization of PBSCs is a limiting factor to proceeding with an auto-ASCT. In such situations, plerixafor is commonly added to improve PBSC collection yields along with cytokine (G-CSF alone) or chemomobilization (chemotherapy+G-CSF). Individually, both strategies are proven to be safe and effective. Here we report six patients who underwent successful mobilization with combination chemomobilization plus plerixafor after upfront failure of cytokine mobilization plus plerixafor. The median CD34+ cell yield after chemomobilization was 2.48 × 106/kg (range 0.99–8.49) after receiving one to two doses of plerixafor. All patients subsequently underwent ASCT without major unforeseen toxicities and engrafted successfully. No significant delays in time to neutrophil recovery were observed. Our experience highlights the safety and effectiveness of chemomobilization with plerixafor after G-CSF plus plerixafor (G+P) failure and suggests this is a viable salvage strategy after initial failed G+P mobilization.
INTRODUCTION
Auto-SCT is an important and potentially curative therapy for patients with relapsed lymphoma; however, 5–40% of lymphoma patients fail to mobilize adequate numbers of PBSCs, and thus cannot undergo a therapy that is known to improve long-term survival.1 Over the past decade, different strategies have been implemented to achieve adequate apheresis yields for successful engraftment. These strategies include cytokine growth factors, either alone or in combination with chemotherapy and, more recently, the partial CXC chemokine receptor-4 antagonist, plerixafor.2,3
Plerixafor disrupts the stromal cell-derived factor-1/CXCR4 interaction and reduces the binding and chemotaxis of hematopoietic stem cells to the BM stroma.4,5 Mobilization with G-CSF plus plerixafor (G+P) is an Food and Drug Administration-approved strategy for PBSC mobilization before ASCT in patients with non-Hodgkin lymphoma or multiple myeloma.6–8 This indication is based on two phase III, double-blind, randomized clinical studies in which combination G+P mobilized more hematopoietic stem cells in fewer apheresis sessions compared with G-CSF alone in MM and non-Hodgkin lymphoma patients.4,9–11 The combination of G+P has been shown to improve PBSC collection yields and potentially reduce mobilization failure rates.12–15 Of patients undergoing upfront utilization of G+P, 14% failed to achieve more than 2 × 106 CD34+ cells/kg.4 Despite utilization of upfront G+P, there remains a subset of patients unable to collect adequate stem cells.
In addition, G+P after chemotherapy as a front-line mobilization strategy safely and effectively allows the collection of an adequate number of PBSCs in order to perform ASCT in MM and lymphoma.16–18 Previous reports have outlined mobilization algorithms including a strategy to include plerixafor for poor mobilizers.19 However, there has not been a report outlining a successful collection strategy after failed G+P mobilization. We report on six patients with relapsed or refractory lymphoma who were deemed eligible for ASCT and subsequently underwent chemomobilization with the addition of plerixafor following failure of upfront mobilization with G+P between January 2012 and April 2013. Patients were eligible for inclusion if they failed to yield 2 × 106 stem cells/kg following initial mobilization attempt with G+P. Patients who failed initial mobilization following chemotherapy plus G+P were not included. All patient data were collected prospectively with informed consent and approval from the institutional review board at the Ohio State University. Here we describe our institution’s experiences and propose this option as a viable strategy in poor mobilizers who fail initial cytokine and plerixafor mobilization. The feasibility and efficacy of such a strategy has not been reported to our knowledge.
MATERIALS AND METHODS
This study is an institutional review board-approved descriptive case series of six consecutive patients who underwent chemomobilization with the addition of plerixafor following failure of mobilization with upfront G+P. Given the small sample size, descriptive statistics with a median and a range were used to summarize the time to neutrophil and platelet engraftment. Collection outcomes were described on an individual patient basis.
The target optimal CD34+ cell yield at our institution is at least 5 × 106/kg recipient body weight, whereas a minimum dose of at least 2 × 106/kg is recommended to proceed with ASCT. Our institutional standard is to add plerixafor on day 4 of G-CSF mobilization for patients who received radiation, 10 or more cycles of chemotherapy, are aged 60 or older or on day 5 for patients who had a CD34+ cell count of <10/μL that morning. After an unsuccessful attempt at mobilization with G+P (1–2 doses), these six individuals underwent chemomobilization with either cyclophosphamide (CY; 3 gm/m2) or etoposide 2000 mg/m2 (VP-16) at the discretion of the treating physician. G-CSF (10 μg/kg) was started on day 5 after chemotherapy. Plerixafor (0.24 mg/kg) was administered to patients who were in danger of failing to mobilize, with WBC recovery but persistently low levels of circulating CD34+ cells after chemotherapy. In both approaches, plerixafor was administered the evening before planned apheresis. Once the peripheral blood CD34+ count was ≥10/μL, apheresis was done using a Caridian Cobe Spectra machine (Terumo, Lakewood, CO, USA) and four blood volumes were processed according to institutional guidelines.
All patients were initially treated in a HEPA-filtered inpatient BMT unit and received fungal and herpes zoster prophylaxis as appropriate. Thawed autologous stem cells were infused through a central venous catheter on day 0 following conditioning chemotherapy. All patients received BEAM conditioning (carmustine, 300 mg/m2 on day − 6; etoposide, 100 mg/m2 and cytarabine, 100 mg/m2 twice daily on days − 5 through − 2; and melphalan, 140 mg/m2 on day − 1). All patients received irradiated and leukoreduced blood products. Post-transplant G-CSF was administered at 5 μg/kg per day starting on day +1 for patients who underwent transplant before November 2012 and starting on day +7 for patients who underwent transplant afterward. Neutrophil engraftment was defined as the first of three successive days with an absolute neutrophil count ≥0.5 × 109/L after post-transplant nadir, and platelet engraftment was defined as the first of three successive days with a platelet count ≥20 × 109/L without platelet transfusion.
RESULTS
Baseline characteristics of the six patients are shown in Table 1. For five of the six patients, chemomobilization+plerixafor was the second mobilization attempt. For patient #1, this was the third attempt after failed attempts at mobilization of chemotherapy with rituximab, ifosfamide, carboplatin, and etoposide (RICE) and subsequently G+P. The median age was 55 (range, 38–68). Five of the six patients were female. One had Hodgkin’s lymphoma, four had diffuse large B-cell lymphoma, and one had grade 3A follicular lymphoma. The patient with follicular lymphoma had stable disease going into collection. All of the other patients had chemosensitive disease, with a partial response20 or better following their most recent salvage. Karnofsky performance status ranged from 70 to 90, and the median comorbidity index was 3.5 (range, 0–9). The median number of prior therapies was 3, the median number of cycles of chemotherapy received was 10 (range, 8–14) and two of the six patients had received radiation. There were no patients with BM involvement by their lymphoma.
Table 1.
Demographics of six patients who underwent chemomobilization with G-CSF plus plerixafor
| Patient | Sex | Age at mobilization (year) | Diagnosis | Stage | CMI | Disease status | Total mob attempts | Number of prior therapies | Prior radiation | Prior therapies |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | DLBCL | 3A | 9 | PR2 | 3 | 3 | No | RITx5; RCEPPx6; RICEx3 |
| 2 | F | 40 | DLBCL | 2A | 5 | CR1r | 2 | 2 | No | RCHOPx8; RICEx2 |
| 3 | M | 38 | FL (G3) | 3A | 4 | SD | 2 | 3 | Yes | RCHOPx6; XRT; RESHAPx4; REPOCHx4 |
| 4 | F | 65 | cHL | 2A | 0 | CR2 | 2 | 4 | Yes (M) | ABVDx1; MOPP; XRT; ICE |
| 5 | F | 48 | DLBCL | 3B | 3 | CR2 | 2 | 2 | No | RCHOPx6; RICEx2 |
| 6 | F | 68 | DLBCL | 4A | 2 | CR2 | 2 | 3 | No | RCHOPx6; RICEx1; RGemOx x3 |
Abbreviations: CMI = comorbidity index; DLBCL =diffuse large B-cell lymphoma. CR1r indicates that patient initially had refractory disease to RCHOP but responded to salvage RICE, was in CR before SCT (M) mediastinal involvement. Prior therapies include RIT (rituximab); RCEPP (cyclophosphamide, etoposide, procarbazine, prednisone); RICE (rituximab, ifosfamide, carboplatin, etoposide); RCHOP (rituximab, cyclophosphamide, doxorubicin, prednisone); XRT (radiation); RESHAP (rituximab, etoposide, methylprednisolone, cisplatin, cytarabine); REPOCH (rituximab, etoposide, prednisone, vincristine, doxorubicin); ABVD (doxorubicin, bleomycin, vinblastine, decarbazine); MOPP (nitrogen mustard, vincristine, procarbazine, prednisone); RGemOx (rituximab, gemcitabine, oxaliplatin).
Table 2 describes the results of PBSC mobilization with G+P and salvage mobilization in all six patients. The median peak peripheral blood CD34+ cell count obtained during G+P mobilization was 7/μL (range, 6–20). The median CD34+ cell count after salvage mobilization was 15.5/μL (range, 14–105). The median CD34+ cell yield after salvage mobilization was 2.48 × 106/kg (range, 0.99–8.49 × 106) receiving one to two doses of plerixafor. One patient (#3) had already yielded 1.42 × 106/kg during his previous G+P mobilization, so an additional 0.99 × 106/kg provided an adequate number to move forward with ASCT. All six patients received high dose therapy with BEAM. One patient (#1) received 100 mg/m2 of melphalan. The median time to neutrophil engraftment was 12 days (range, 9–13). The median time to platelet recovery was 13.5 days (range, 10–24). All six patients are alive and without disease progression at a median follow-up of 231 days (range, 29–622).
Table 2.
Results of PBSC mobilization with G-CSF+plerixafor and salvage mobilization in all six patients
| Patient | No of prior doses of plerix during G+P mobilization |
Peak PB CD34 during G+P mobilization |
Chemo- mobilization |
No of plerix after chemo- mobilization |
Day of first plerix after chemo- mobilization |
Day 1st collection after chemo- mobilization +plerix |
Peak PB CD34 after chemo- mobilization +plerix |
No of apheresis sessions |
TNC ×108/kg |
CD34+ ×106/kg yield |
Total CD34 infused |
Days to neutrophil, platelet recovery |
PFS since SCT (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 7 | VP | 2 | 20 | 21 | 16 | 2 | 5.73 | 2.34 | 2.34 | 10, 18 | 622 |
| 2 | 2 | 6 | VP | 1 | 16 | 17 | 105 | 1 | 7.58 | 8.49 | 8.49 | 9, 13 | 302 |
| 3 | 2 | 20a | CY | 2 | 14 | 15 | 14 | 2 | 4.71b | 0.99 | 2.41 | 12, 10 | 224 |
| 4 | 1 | 8 | VP | 2 | 15 | 16 | 15 | 2 | 6.81 | 2.02 | 2.02 | 13, 24 | 238 |
| 5 | 2 | 8 | CY | 2 | 17 | 18 | 18 | 2 | 6.25 | 3.61 | 3.61 | 12, 12 | 33 |
| 6 | 2 | 7 | CY | 2 | 19 | 20 | 14 | 3 | 6.69 | 2.62 | 2.62 | 12, 14 | 29 |
Abbreviation: CY =cyclophosphamide 3 gm/m2; VP = VP-16 or etoposide 2000 mg/m2; G+P =G-CSF plus plerixafor.
Yielded 0.71 × 106/kg PBSCs on two apheresis sessions.
Yielded an additional 2.96 ± 3.96 × 108/kg TNCs previously.
DISCUSSION
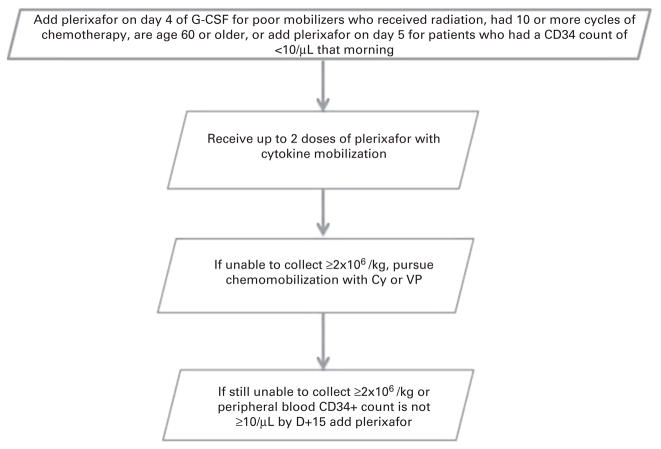
Although not inexpensive, plerixafor has provided many patients with the opportunity to collect an adequate number of stem cells for ASCT who otherwise would be deprived of a potentially life saving treatment. Plerixafor combined with G-CSF or plerixafor combined with chemomobilization have each shown to be effective mobilization strategies.21 A debate continues regarding when each strategy should be used. Our approach at Ohio State, highlights a safe and effective approach applicable to all individuals (Figure 1).22 However, it more importantly highlights an effective strategy to mobilize patients who have failed mobilization, as well as those with risk factors for collection failure. Thanks to employment of this strategy, mobilization failure is not a significant barrier to proceeding with ASCT at our institution.
Figure 1.
Flow chart on an effective mobilization strategy based on our single institution experience.
In this case series, we examined the utility of adding plerixafor to chemomobilization in patients who failed mobilization with upfront G+P. We found that all six patients were successfully mobilized following either CY or VP-16 with the addition of G+P. Interestingly, one patient (#1) failed collection with initial chemomobilization, and then failed G+P, but was ultimately successfully mobilized after VP-16 and G+P. This patient highlights the superiority of adding plerixafor to chemomobilization.
Although our patients did not have overwhelming risk factors for poor mobilization rates, they did have notable risk factors including increasing age, prior radiotherapy and multiple prior lines of intensive therapy as listed in Table 1.5 None of our patients had BM involvement or directly BM toxic therapy. Fortunately, in this cohort, all of our patients successfully underwent ASCT with only slight delay in engraftment, which is not unexpected, given the lower infused cell doses. At last follow-up, all of our patients were without progression of their disease. Other groups have reported inferior ASCT outcomes in patients who mobilized poorly.23–25 This has not been our experience with this small number of patients, but our follow-up is short.
We acknowledge the small sample size, the need for further validation, the inherent bias of our retrospective analysis and the heterogeneity among patients as limitations of this study. Despite these limitations, it appears that chemomobilization with G+P is an effective option in patients who have previously failed to mobilize with G+P. In addition, there is a void of published patients treated with this approach and as the age of patient eligibility continues to be pushed higher, this strategy seems to be a viable option in lymphoid malignancies and certainly warrants further exploration in the elderly with multiple myeloma.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18:1191–1203. doi: 10.1016/j.bbmt.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 3.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 4.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 5.To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118:4530–4540. doi: 10.1182/blood-2011-06-318220. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- 7.Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41:331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 8.Shaughnessy P, Uberti J, Devine S, Maziarz RT, Vose J, Micallef I, et al. Plerixafor and G-CSF for autologous stem cell mobilization in patients with NHL, Hodgkin’s lymphoma and multiple myeloma: results from the expanded access program. Bone Marrow Transplant. 2013;48:777–781. doi: 10.1038/bmt.2012.219. [DOI] [PubMed] [Google Scholar]
- 9.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 10.Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-Hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15:1578–1586. doi: 10.1016/j.bbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Maziarz RT, Nademanee AP, Micallef IN, Stiff PJ, Calandra G, Angell J, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19:670–675. doi: 10.1016/j.bbmt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Attolico I, Pavone V, Ostuni A, Rossini B, Musso M, Crescimanno A, et al. Plerixafor added to chemotherapy plus G-CSF is safe and allows adequate PBSC collection in predicted poor mobilizer patients with multiple myeloma or lymphoma. Biol Blood Marrow Transplant. 2012;18:241–249. doi: 10.1016/j.bbmt.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Wood WA, Whitley J, Moore D, Sharf A, Irons R, Rao K, et al. Chemomobilization with etoposide is highly effective in patients with multiple myeloma and overcomes the effects of age and prior therapy. Biol Blood Marrow Transplant. 2011;17:141–146. doi: 10.1016/j.bbmt.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K, et al. Safety and preliminary ef3cacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin’s lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45:39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- 15.Wood WA, Whitley J, Goyal R, Brown PM, Sharf A, Irons R, et al. Effectiveness of etoposide chemomobilization in lymphoma patients undergoing auto-SCT. Bone Marrow Transplant. 2013;48:771–776. doi: 10.1038/bmt.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basak GW, Mikala G, Koristek Z, Jaksic O, Basic-Kinda S, Cegledi A, et al. Plerixafor to rescue failing chemotherapy-based stem cell mobilization: it’s not too late. Leuk Lymphoma. 2011;52:1711–1719. doi: 10.3109/10428194.2011.578312. [DOI] [PubMed] [Google Scholar]
- 17.D’Addio A, Curti A, Worel N, Douglas K, Motta MR, Rizzi S, et al. The addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSF. Bone Marrow Transplant. 2011;46:356–363. doi: 10.1038/bmt.2010.128. [DOI] [PubMed] [Google Scholar]
- 18.Hubel K, Fresen MM, Salwender H, Basara N, Beier R, Theurich S, et al. Plerixafor with and without chemotherapy in poor mobilizers: results from the German compassionate use program. Bone Marrow Transplant. 2011;46:1045–1052. doi: 10.1038/bmt.2010.249. [DOI] [PubMed] [Google Scholar]
- 19.Faison E, Chow E, Khan T, Covington D, Shea T, Rao K. Effectiveness of an algorithm-based approach to filgrastim-based mobilization using predetermined decision points for the inclusion of plerixafor. Biol Blood Marrow Transplant. 2013;19:S175. [Google Scholar]
- 20.Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29:541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Jantunen E, Kuittinen T, Mahlamaki E, Pyorala M, Mantymaa P, Nousiainen T. Ef3cacy of pre-emptively used plerixafor in patients mobilizing poorly after chemomobilization: a single centre experience. Eur J Haematol. 2011;86:299–304. doi: 10.1111/j.1600-0609.2010.01573.x. [DOI] [PubMed] [Google Scholar]
- 22.Haverkos B, Geyer S, McBride A, Penza S, Devine S, Andritsos L, et al. Mobilization for autologous stem cell transplantation in Hodgkin’s lymphoma (HL) and non-Hodgkin lymphoma (NHL): a single institution experience. Biol Blood Marrow Transplant. 2014;20:S111–S112. [Google Scholar]
- 23.Tomblyn M, Burns LJ, Blazar B, Wagner J, Lee C, Rogers T, et al. Difficult stem cell mobilization despite adequate CD34+ cell dose predicts shortened progression free and overall survival after autologous HSCT for lymphoma. Bone Marrow Transplant. 2007;40:111–118. doi: 10.1038/sj.bmt.1705708. [DOI] [PubMed] [Google Scholar]
- 24.Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44:815–820. doi: 10.1080/1042819031000067585. [DOI] [PubMed] [Google Scholar]
- 25.Kanate AS, Watkins K, Cumpston A, Craig M, Hamadani M. Salvage bone marrow harvest in patients failing plerixafor-based stem cell mobilization attempt: feasibility and autologous transplantation outcomes. Biol Blood Marrow Transplant. 19:1133–1135. doi: 10.1016/j.bbmt.2013.04.019. [DOI] [PubMed] [Google Scholar]