Abstract
Prenatal cocaine exposure (PCE) in humans and animals has been shown to impair social development. Molecules that mediate synaptic plasticity and learning in the medial PFC (mPFC), specifically BDNF and its downstream signaling molecule, egr1 have been shown to affect the regulation of social interactions (SI). In this study we determined the effects of PCE on SI and the corresponding ultrasonic vocalizations (USVs) in developing mice. Furthermore, we studied the PCE-induced changes in constitutive expression of BDNF, egr1 and their transcriptional regulators in the mPFC as a possible molecular mechanism mediating the altered SI. In prenatal cocaine exposed (PCOC) mice we identified increased SI and USV production at P25, and increased SI but not USVs, at P35. By P45 the expression of both social behaviors normalized in PCOC mice. At the molecular level, we found increased BDNF exon IV and egr1 mRNA in the mPFC of PCOC mice at P30 that normalized by P45. This was concurrent with increased egr1 protein in the mPFC of PCOC mice at P30 suggesting a role of egr1 in the enhanced SI observed in juvenile PCOC mice. Additionally, by measuring the association of acH3K9,14, and MeCP2 at the promoters of BDNF exons I and IV, and egr1, our results provide evidence of promoter specific alterations in the mPFC of PCOC juvenile mice with increased association of acH3K9,14 only at the BDNF exon IV promoter. These results identify a potential PCE-induced molecular alteration as the underlying neurobiologic mechanism mediating the altered social development in juvenile mice.
Keywords: mPFC, prenatal cocaine, social interaction, ultrasonic vocalizations, egr1
Introduction
The development of limbic neurocircuits is sensitive to social environment and plays an important role in regulating social interactions (SI) [1, 2]. Thus, drugs that interfere with the development of limbic circuits would be expected to influence the ability to regulate SI. Prenatal cocaine exposure (PCE) has been associated with deficits in the development of social behaviors in humans [3] with increased passive-withdrawn negative engagement in infants [4], and increased aggression among juveniles [5]. While rodent studies show that PCE can lead to deficits in social behavior [6-9], the mechanisms that underlie the relationship between PCE and social regulation have not been clearly identified. In adult animals that were exposed to cocaine in utero several studies have reported depressed SI [6-8]. However, the effects of PCE on SI in juvenile animals show conflicting results. While Overstreet et al. 2000 [6] found that PCE depressed SI among juvenile rats tested at postnatal day 30 other similar studies of juveniles show no effect of treatment [9]. Furthermore, no study to date has evaluated how PCE contributes to molecular changes that might underlie SI dysregulation.
The medial prefrontal cortex (mPFC) plays an important role in SI regulation [10, 11]. Molecules that mediate synaptic plasticity and learning in the mPFC specifically the extracellular signal-regulated kinase 2 (ERK2) pathway, and its downstream signaling molecule early growth response protein 1 (egr1) have been shown to mediate SI [12, 13]. ERK2 expression is regulated by brain derived neurotrophic factor (BDNF) in the hippocampus [14]. Activation of BDNF in primary cortical cultures leads to the translocation of ERK2 into the nucleus where it activates the transcription of egr1 [15]. These results suggest that BDNF signaling pathways within the mPFC may be impacting SI via regulation of ERK2 and egr1.
Of the nine unique transcripts comprising the BDNF gene, those containing exons I and IV are the most abundantly transcribed in the mPFC of mice [16]. Transcription of BDNF from exons I and IV, as well as egr1 is dynamically regulated by changes in chromatin structure that is mediated in part by post-translational modifications of histone proteins. Acetylation of histone 3 at lysine residues 9 and 14 (acH3K9,14) act as a marker of transcriptional activation as it results in an open chromatin configuration that increases accessibility of transcription factors to specific DNA promoter regions. Methyl cytosine-binding protein 2 (MeCP2) is one such transcription factor that regulates the transcription of BDNF exons I and IV, and egr1 by altering its binding status at specific sites in their promoter regions [17-19]. In this study, we were interested in determining the effects of PCE on different aspects of the regulation of SI in juvenile (postnatal day P25-P35) and adolescent (postnatal day P45) mice and their production of ultrasonic vocalizations (USVs) during these interactions. Furthermore, we aimed to identify the effects of PCE on the constitutive expression of BDNF and egr-1 and their transcriptional regulators, specifically in the mPFC, as a possible molecular mechanism mediating the altered SI.
Materials and Methods
Animals and Prenatal Treatments
Wild-type male mice on the Swiss Webster background were used for all experiments. A transplacental cocaine treatment regimen as previously described [20] was used to expose mouse embryos to cocaine. Adult timed-pregnant Swiss Webster dams were purchased from Taconic (Germantown, New York) with each dam being assigned to one of two treatment groups and receiving twice-daily subcutaneous (SC) injections (at 7:00AM and 7:00PM) from E8 to E17, inclusive, of cocaine HCl (Sigma-Aldrich, St. Louis, Missouri; 20 mg/kg/injection, SC, dissolved in saline) totaling 40 mg/kg per day (prenatal cocaine exposed offspring (PCOC)) or 0.9% saline (prenatal saline exposed offspring (PSAL)). Though dams injected with cocaine gained less weight during gestation, there was no effect of prenatal cocaine exposure on the number of live born pups per litter (data not shown). Within 24 hours of birth, all pups were surrogate fostered to control dams (Swiss Webster; Taconic Labs), which had delivered within the preceding 24-72 hours.
For behavioral studies, surrogates and the newly born pups were placed on a reversed 12-hour dark/ light (11:00PM light-11:00AM dark) cycle. Pups were weaned and group housed on P21 with each cage containing two males and two females from the same litter. Only one male from each cage was tested for SI. For molecular studies, surrogates and their pups remained on a regular 12-hour (7:00AM light-7:00PM dark) cycle. Only male offspring were used for these studies. To avoid the problem of oversampling [21], no more than one animal per litter was used for any of the molecular experiments reported. For behavioral experiments a maximum of 2 male pups from each litter was used for all experiments. All experimental protocols were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee, and were in accordance with NIH directives for animal studies.
Social Interaction
Individual test mice were tested for SI during their dark cycle at three ages (P25, P35 and P45), timepoints that correspond to previous studies of social interaction in adolescent mice [22].Twenty-four hours prior to the test, one male animal from each cage was individually housed (test animal) leaving one male (stimulus animal) and two females group housed in their original home cage. After 5 min of habituation to the testing environment, the stimulus mouse was introduced into the cage containing the test animal and the interaction was video recorded for a total of 5 mins. Trained raters manually scored social behavior of the test mouse directed toward stimulus mouse during the entire 5 min test period. The SI score was derived by adding the duration that the test mouse engaged in the following social activities: investigation of head/neck, flank, and anogenital regions of the stimulus mouse, allogrooming and proximity [22]. Between each SI test, both the test and stimulus mice were returned to their original home cage and group housed with the females.
USVs were recorded during all SI tests as previously described [22]. Briefly, an ultrasonic microphone (UltraSoundGate Model CM16, Avisoft Bioacoustics, Berlin, Germany) was suspended just above the inside of the test cage. Recordings were processed through the recording interface UltraSoundGate 116 (Avisoft Bioacoustics, Berlin, Germany) and assessed with vocalization analysis software (SAS-Lab Pro, Version 5.2.06, Avisoft). To analyze recordings, auditory signals below 30 khz were excluded using a high pass filter followed by a whistle tracking function to label vocalizations longer than 3 msec in duration. A trained observer blind to the experimental conditions visually verified all identified vocalizations.
Chromatin Immunoprecipitation Assay (ChIP)
Bilateral dissections of the mPFC were performed on wet ice and the tissue was cross-linked in 1% formaldehyde. Tissue was lysed with a hand-held homogenizer in cell lysis buffer (100mM EDTA, 50mM Tris-HCl, 1% SDS) containing protease inhibitors and centrifuged at 5000rpm. The nuclear pellet was re-suspended in a 2:1 ratio of ChIP dilution buffer (0.01% SDS, 1.2mM EDTA, 16.7mM Tris-HCl, 167mM NaCl, 1.1% Triton X-100) and nuclear lysis buffer (10mM NaCl, 10mM Tris-HCl, 3mM MgCl2, 1% NP-40) and fragmented by sonication. Samples were centrifuged at maximum speed and the supernatant was collected and stored at -80°C. An aliquot of the fragmented chromatin was set aside as total input control.
ChIP assays were performed using the ChIP-IT Express kit (Active Motif, Carlsbad, CA) following the protocol supplied by the company. For the ChIP reaction, 100ul of the fragmented chromatin was mixed with protein G magnetic beads and 5ul of anti-MeCP2 antibody (Abcam, Cambridge, MA) or anti-acH3K9,14 antibody (Millipore, Billerica, MA). The reaction mixture was incubated at 4°C for 24 hrs on a rotator. The beads were washed with wash buffer and the DNA was immunoprecipitated.
Tissue collection for mRNA and protein analyses
To measure the constitutive levels of BDNF and egr1 mRNA and protein, mice were euthanized by rapid decapitation, whole brains were removed from the skull and bilateral dissections of the mPFC were performed on dry ice.
Real Time RT-PCR
To study the expression of BDNF and egr1 mRNA, total RNA was extracted and cDNA was synthesized as previously published [20]. The relative amount of each transcript of interest present in the sample was measured by quantitative real-time PCR on 24-26ng of the resulting cDNA using SYBR Green detection (Applied Biosystems, Foster City, CA) with parameters as previously published [20]. All samples were measured in duplicates and mRNA levels were normalized to beta-actin mRNA to adjust for small differences in input RNA. Primers used for BDNF exons I and IV were as published in [23], egr1 as published in [24] and beta-actin as published in [25].
For qPCR performed on samples that underwent ChIP, the qPCR parameters used were as in [26]. Primers used for BDNF exon IV were previously reported in [17] and for BDNF exon I in [27]. Primers for egr1 were designed using methprimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi). The primer sequences for egr1 were forward 5’ CCAGTTGGGAACCAAGGAG 3’ and reverse 5’ GCCCAAATAAGGTCTGTTCC 3’. All samples were run in triplicates and the levels of immunoprecipitated DNA were normalized to the total input control.
BDNF ELISA
Mature BDNF protein level was measured using the BDNF Emax ImmunoAssay (ELISA) system (Promega, Madison, WI) as previously described [20]. Standard and samples were performed in duplicates.
Western Blot Analyses
Tissue prepared for BDNF ELISA assays was additionally used for egr1 western blot analyses. 40ug of protein was separated on an 8% gel along with a Kaleidoscope-prestained standard (Bio-Rad, Hercules, CA). Blots were incubated in primary antibody (egr1 1:400, Cell Signaling, Danvers, MA; actin 1:20,000, Millipore, Billerica, MA) for 12-16 hours at 4°C. Secondary antibody incubations were performed at room temperature in blocking buffer for 1 hour (horseradish peroxidase-linked IgG conjugated goat anti-rabbit 1:2500 for egr1, or horse anti-mouse 1:30,000 for actin, Vector Laboratories, Burlingame, CA). Membranes were visualized with Western Lightning Chemiluminescence solution (Perkin Elmer Life Science, Boston, MA). Optical density from films was analyzed using NIH Image (NIH, Bethesda, MD).
Statistical analyses
When analyzing the SI and USV data, a two-way anova (prenatal treatment X age) was performed and when significant (p<0.05) a Student’s t-test was performed at each developmental age. The relationships between SI scores and USV counts were determined by fitting a linear regression individually for each treatment group at all developmental time points and evaluated using Pearson’s correlation coefficient. Similarly, the analysis of the molecular data was performed using a Student’s t-test comparing PSAL and PCOC groups. Before any analyses were done on the behavioral or molecular data, an outlier test was performed and outliers (two standard deviations from the mean) were removed.
Results
PCE increased SI in offspring at P25 and P35 but not P45
To understand the impact of PCE on SI during development, prenatal cocaine (PCOC) and saline (PSAL) treated animals were subjected to the SI test at three developmental ages. For SI, we found a main effect of prenatal treatment (F1,128=4.216, p<0.05) and an interaction between prenatal treatment and age (F2,128=3.781, p<0.05). Post-hoc analyses revealed a significant increase in total time that the PCOC test mouse spent interacting with the stimulus mouse when tested at P25 and P35 (*p<0.05; Fig. 1) compared to the PSAL mice. This increase in interaction was calculated as a combination of all the social measures and not attributable to any one specific measure. No such differences in time spent interacting with the stimulus mouse were observed in PCOC mice at P45 (Fig. 1).
Figure 1. Prenatal cocaine exposure increased social interaction in offspring at P25 and P35 but not P45.
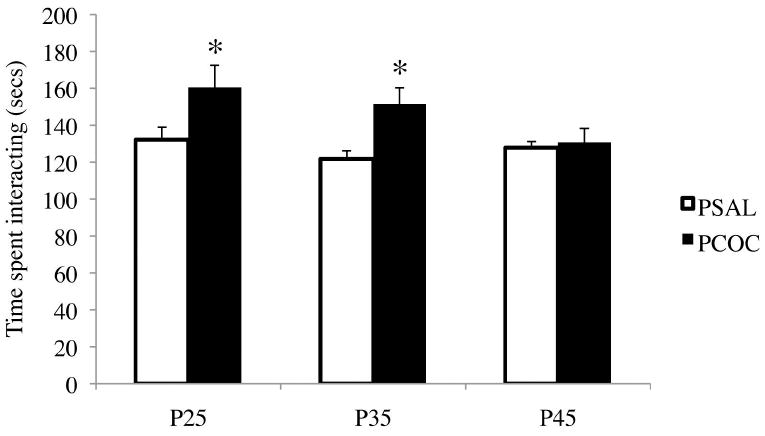
The total time that the test mouse interacted with the stimulus mouse at each of the developmental ages was measured. Total interaction time was a sum of head/ neck, flank, anogenital, allogrooming and proximity. There was a significant increase in the total interaction time in the PCOC mice at P25 and P35 compared to PSAL mice (*p<0.05 PCOC vs. PSAL; P25 PSAL n=26, PCOC n=21; P35 PSAL n=24, PCOC n=18; P45 PSAL n=24, PCOC n=21). Error bars represent the mean ± SEM.
PCE increased vocalization rates during SI at P25, but not P35 or P45
Using a 2x3 ANOVA, there was a significant effect of test age (F2, 126 = 11.62, p<0.0001), a marginal effect of treatment (F1,127 = 3.25, p=0.074) and no interaction between treatment and age (F2,126 = 2.36, p=0.098). However, using a 2x2 ANOVA and analyzing the USV data at P25 and P35 only we found a main effect of prenatal treatment (F1,85=14.76, p=0.0002) with no effect of age or an interaction between prenatal treatment and age. Post-hoc analyses revealed significantly elevated production of USVs in PCOC mice compared to PSAL mice at P25 (**p <0.01; Fig. 2a), but not when tested at P35.
Figure 2. Prenatal cocaine exposure increased vocalization rates during social interactions in offspring at P25, but not P35 or P45.
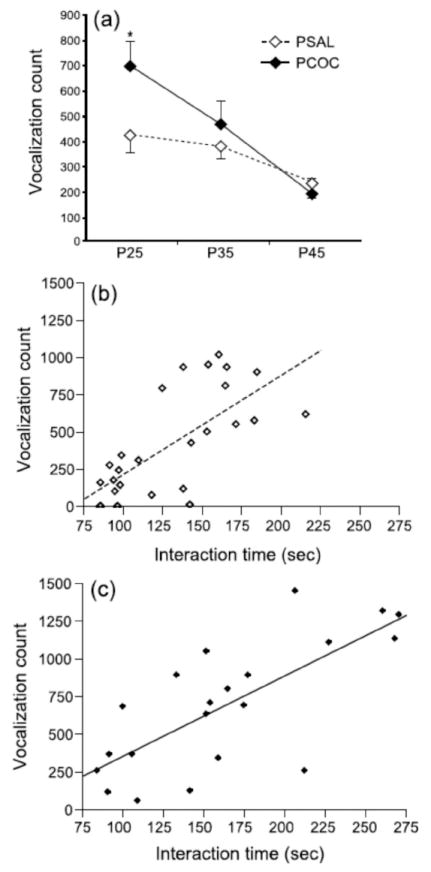
The total number of vocalizations produced by PCOC and PSAL social pairs was measured during social interaction at P25, P35, and P45. (a) PCOC mice emitted a greater number of USVs during social interaction relative to PSAL mice at P25, but not at P35 or P45. USV counts were strongly correlated with interaction time at P25 in both (b) PSAL mice and (c) PCOC mice.
When SI scores and USV counts were compared for individual mice, we found that these dependent variables were highly correlated at P25 for both PCOC (r=0.73, n=21, p<0.0005; Fig. 2b) and PSAL (r=0.7, n=26, p<0.0001, Fig. 2c) mice. However, these SI scores and USV counts were weakly correlated in PCOC mice at P35 (r=0.48, n=18, p<0.05; data not shown), were not correlated in PSAL mice at P35 (r=0.23, n=24, p=NS), and were not correlated in either treatment group at P45 (r<0.31, p=NS).
PCE selectively increased BDNF exon IV and egr1 mRNA expression in the mPFC of offspring at P30
To understand the molecular changes that might contribute to the increase in SI observed in PCOC mice at P25 and P35 but not P45, mRNA levels of BDNF and egr1 were measured in the mPFC at P30 and P45. At P30, there was a significant increase in BDNF exon IV (*p<0.05) and egr1 (**p<0.01) mRNA levels with no change in BDNF exon I mRNA levels in the mPFC of PCOC mice (Fig. 3a). At P45 there were no significant differences between prenatal treatment groups for mRNA levels of BDNF exons I and IV, and egr1 (Fig. 3b).
Figure 3. Prenatal cocaine exposure selectively increased BDNF exon IV and egr1 mRNA expression in the mPFC of offspring at P30 but not at P45.
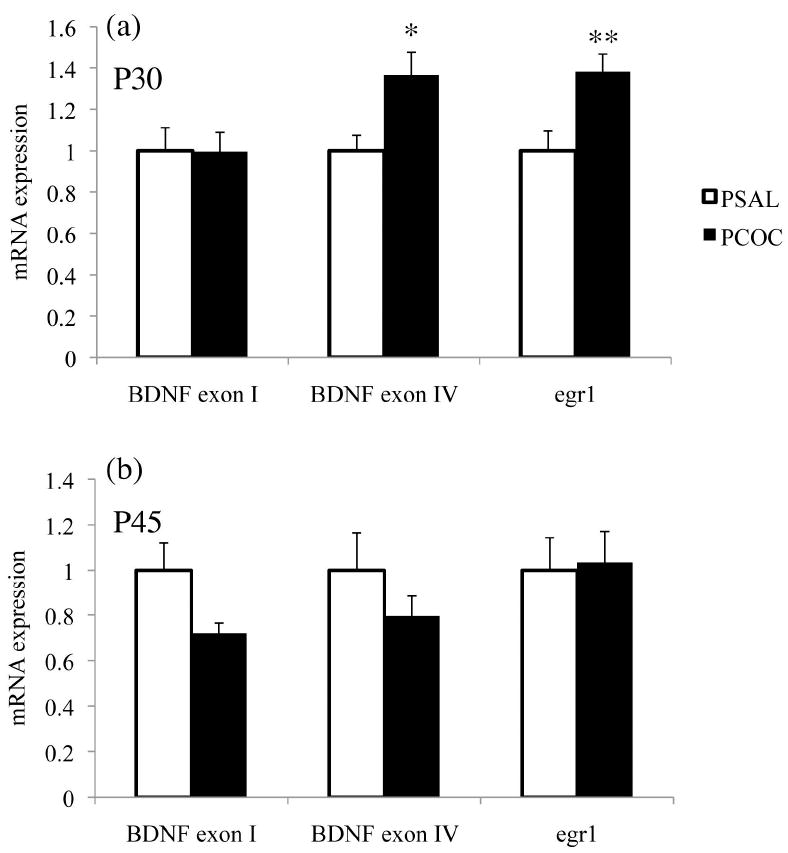
Constitutive mRNA levels of BDNF exons I and IV, and egr1 were measured in the mPFC of PSAL and PCOC mice at P30 and P45. (a) There was increased BDNF exon IV and egr1 mRNA levels in the mPFC of PCOC mice at P30 compared to PSAL mice. (b) There was no change in the mRNA levels of BDNF exons I and IV, nor of egr1 in the mPFC of PCOC mice at P45 compared to PSAL mice. (*p<0.05, **p<0.01 PCOC vs. PSAL; P30 PSAL n=14; PCOC n=13; P45 PSAL n=-6; PCOC n=6). Error bars represent the mean ± SEM.
PCE increased egr1 but not BDNF protein in the mPFC of offspring at P30
To determine whether the increased mRNA levels of BDNF exon IV and egr1 in the mPFC of PCOC mice corresponded to an alteration in their protein levels, we measured mature BDNF and egr1 protein in the mPFC. Because we observed changes in mRNA specifically at P30 but not P45, subsequent molecular experiments were performed only at this earlier age. ELISA was used to measure levels of the mature and functionally active isoform of the BDNF protein and Western blots were used to measure levels of the egr1 protein. In the mPFC of PCOC mice at P30, we observed no difference in BDNF protein levels (Fig. 4a). However, we did observe a significant increase in egr1 protein expression (*p<0.05; Fig. 4b) in the mPFC of PCOC mice at P30. These results suggest that PCE-induced enhancement in egr1 protein levels, but not BDNF protein in the mPFC may contribute to the increase in SI observed in these animals at P25 and P35.
Figure 4. Prenatal cocaine exposure increased egr1 protein but not BDNF protein in the mPFC of offspring at P30.
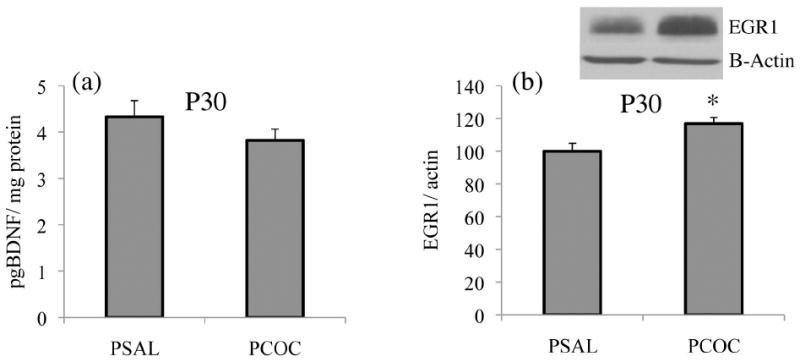
Constitutive protein levels of mature BDNF and egr1 were measured in the mPFC of PSAL and PCOC mice at P30. (a) There was no change in mature BDNF protein levels in the mPFC of PCOC mice compared to PSAL mice. (b) There was significantly increased egr1 protein in the mPFC of PCOC mice compared to PSAL mice (*p<0.05 PCOC vs. PSAL; PSAL n=14; PCOC n=13). Error bars represent the mean ± SEM.
PCE selectively increased acH3K9,14 at the BDNF exon IV promoter in the mPFC of offspring at P30
To understand the prenatal cocaine-induced transcriptional regulation of the BDNF and egr1 genes that showed increased mRNA levels in the mPFC at P30, the association of acH3K9,14 and MeCP2 to the promoters of these genes was studied. In the mPFC of PCOC mice at P30, there was increased acH3K9,14 (*p<0.05; Fig. 5a) and a trend towards decreased MeCP2 binding (p=0.06; Fig. 5b) specifically associated with the promoter of BDNF exon IV consistent with the increased BDNF exon IV mRNA that we observed in the mPFC of P30 PCOC mice. No changes in acH3K9,14 or MeCP2 binding were observed at the promoter of BDNF exon I (Fig. 5a, b).
Figure 5. Prenatal cocaine exposure selectively increased acH3K9,14 and trended towards decreased MeCP2 binding at the BDNF exon IV promoter in the mPFC of offspring at P30.
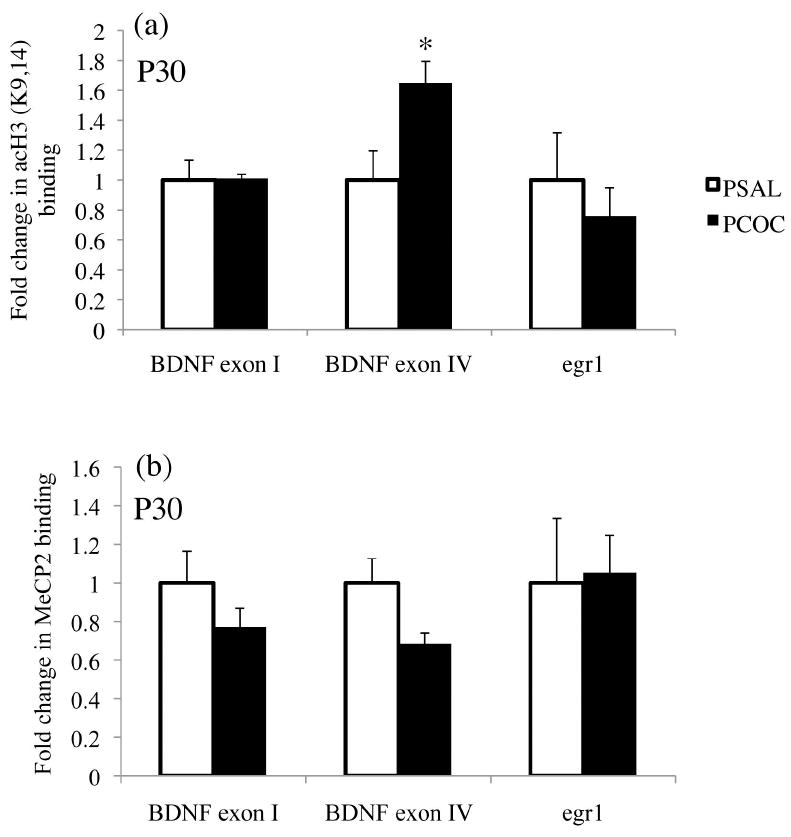
The association of acH3K9,14 and the binding of MeCP2 at the promoters of BDNF exons I and IV, and egr1 were identified in the mPFC of P30 mice. (a) There was a significant increase in the association of acH3K9,14 at the promoter of BDNF exon IV with no change in acH3K9,14 at the promoters of BDNF exon I and egr1. (b) There was a trend (p=0.06) towards a decrease in the binding of MeCP2 at the promoter of BDNF exon IV with no change in its binding at BDNF exon I and egr1. (*p<0.05 PCOC vs. PSAL; PSAL n=8, PCOC n=8). Error bars represent the mean ± SEM.
To identify changes in acH3K9,14 and the binding of MeCP2 at the promoter of egr1, we first screened the mouse egr1 promoter region for CpG islands. Using Methprimer software (http://www.urogene.org/methprimer/) [19, 28] we identified two CpG islands in the promoter of the egr1 gene immediately upstream to the transcription start site similar to what has been identified in the rat [19]. Based on our findings of increased egr1 expression in the mPFC of PCOC mice at P30, for our ChIP analyses we used primers designed to the more proximal CpG island in the promoter region of the mouse egr1 gene where MeCP2 binding has been thought to act as a transcriptional activator of egr1 expression [19]. We found no change in acH3K9,14 or MeCP2 binding within this CpG island in the promoter of egr1 in the mPFC of PCOC mice at P30 (Fig. 5a, b).
The findings indicate that PCE can lead to chromatin remodeling marks that persist through development in spite of a lack of acute exposure to the drug that can influence gene transcription in a promoter-specific fashion. However, the alterations we investigated do not appear to be the mechanism contributing to the increased egr1 mRNA expression we observed in the mPFC of PCOC mice at P30.
Discussion
In this study we identified increased SI in PCOC mice at P25 and P35 that normalized by P45, together with increased USV’s specifically at P25. At the molecular level, we observed increased egr1 mRNA and protein in the mPFC of P30 PCOC mice that also normalized by P45 suggesting that the elevated constitutive egr1 expression in the mPFC of PCOC mice may be mediating the increased SI in these animals. As such, we provide evidence of a novel developmental alteration in SI behavior of PCOC mice coordinate with a possible molecular mechanism underlying the behavior. Furthermore, this is the first study to identify epigenetic modifications in the mPFC of PCOC mice as early as P30. However, as we did not identify specific changes in the binding of MeCP2 or the association of acH3K9,14 at the egr1 promoter in the mPFC of PCOC mice, our findings suggest that other epigenetic marks on the egr1 promoter may be altered following prenatal exposure that impact the developmental expression of this gene, or that other mechanisms are operative.
The social behaviors of PCOC and PSAL mice differed markedly during early adolescence (P25) but not later (P45). While we find that prenatal cocaine exposure enhances social interaction in mice, other studies that assess rat social interactions show that these behaviors are either insensitive to prenatal cocaine exposure [9] or are reduced by exposure [6]. There are several possible explanations for the differences between our results and findings of these earlier reports, including differences in species tested, dose or timing of exposure, and behavioral test methodology. In the present study, test mice were socially isolated for twenty-four hours prior to the test, whereas in studies using rats [9] [6], test animals were not isolated prior to behavioral testing. The duration of social isolation prior to testing influences SI response in mice [29] and rats [30] and may be important for capturing the effects of prenatal cocaine exposure on social behavior. Furthermore, rats subjects were tested under low white light in a novel environment [6] and see [31], whereas our behavioral tests were conducted in the home cage during the dark cycle under red light conditions, which provides optimal conditions for social interactions among adolescent mice [22, 29, 32].
Although the SI test is widely used for assessing social deficits, abnormalities in the rodent responses are difficult to interpret. For instance, the fragile –X knockout mouse, one of the most extensively studied genetic models of a disability that features social deficits, can express greater levels of SI than their wild-type controls [33, 34]. While the direction of this difference is unexpected, it might be attributed to a loss in social regulation [35]. Likewise, the increased SI scores of early adolescence mice exposed in utero to cocaine might also indicate impaired social regulation. These results might also suggest less inhibition and an increased likelihood of risktaking behavior in adolescent mice following prenatal cocaine exposure. Furthermore, prenatal cocaine exposure increases frontal cortex reactivity to stress [36] and environmental changes [37] that likely accompany social isolation. From this perspective, one might consider the possibility that PCOC mice may be especially sensitive to the enhancing effect of short-term isolation on social approach, rendering them more likely to engage in longer bouts of social interaction. In this regard, elucidating of the interactions between prenatal cocaine exposure, egr1 expression, and frontal cortex reactivity might help explain why behavioral differences between experimental and control groups are lost later in maturation; declining levels of egr1 in late adolescent PCOC mice might normalize sensitivity to the short period of isolation and restore social interaction to normal levels. Other behavioral tests, such as social conditioned place preference [22, 35] and empathy testing [38, 39], might also elucidate the underlying psychological experiences that contribute to abnormal social interaction.
Our results at P25 are consistent with other studies that have shown increased vocalization rates associated with more robust SI responses [22, 40]. This association has been extensively characterized for B6 mice during early adolescence [22]. Interestingly, the vocalization rate of P25 PCOC mice is twice that of age-matched saline controls (PCOC=701.15; PSAL= 427.77). However, despite the large difference in vocalizations between treatment groups at P25, this difference disappears with age, SI of PCOC animals at P35 remains elevated above saline controls. Perhaps SI is more sensitive to prenatal injury from cocaine exposure than is vocalization rate. Alternatively, recovery of vocalization rates in PCOC adolescent maturation may simply precede recovery of social interactive behavior.
Social behavior is sensitive to changes in egr1 and ERK2 expression within the mPFC [12, 13], gene products that are downstream of BDNF [15]. We identified increased levels of egr1 protein but not BDNF protein in PCOC mPFC at P30 compared to PSAL mice suggesting that these egr1 effects may be BDNF independent. Since egr1 is an immediate early gene, its expression is downstream of several other signaling pathways. Further experiments will need to be conducted to identify the specific upstream signaling pathways mediating the increased egr1 levels observed in the mPFC of PCOC mice. Furthermore, our results suggest that the expression of egr1 and not BDNF in the mPFC of PCOC mice may contribute to the change in SI observed in these animals at this age. To establish a direct causal role of egr1 would require demonstration of a normalizing of behavior following infusion of shRNA directly into the mPFC of PCOC mice before the SI test, a technique that has been performed in adult rats [13].
We identified increased mRNA levels of the activity driven transcripts including BDNF exon IV and egr1 in the mPFC of PCOC mice at P30. However, we did not observe any difference in the mRNA expression of BDNF exon I suggesting a transcript specific effect of PCE in the mPFC. Of particular interest was the normalization of the expression of these transcripts between P30 and P45 in the mPFC of PCOC mice. This is in agreement with our recent findings of unaltered constitutive levels of BDNF exons I and IV in the mPFC of adult PCOC mice [41]. Both BDNF and egr1, which are dynamic markers of synaptic activity, have increased expression during critical periods of early brain development when these genes play a crucial role in synaptic maturation and the pruning of unnecessary connections. However, later in adolescence and adulthood, the expression of these genes decreases [42-44]. Thus, the increased expression of these transcripts in the mPFC of P30 PCOC mice may suggest a delay in the maturation of this region. A similar phenomenon was observed in the ventral tegmental area (VTA) of prenatal cocaine exposed animals in which there was a significant delay in the switch from the immature calcium permeable AMPA and NMDA receptor subunits to the mature calcium impermeable receptor subunits [45]. One possible explanation of our findings is that the delayed glutamatergic maturation in the VTA [45] could feed-forward via its strong dopaminergic projections to the mPFC and impair the development of this region via dysmaturation of dopaminergic signaling.
We were unable to establish whether the epigenetic modifications we identified in the promoter regions of BDNF exon IV regulate the transcription of these genes. In the mPFC of P30 PCOC mice we found increased acH3K9,14, and a trend towards decreased MeCP2 binding, specifically at the promoter of BDNF exon IV. Such findings are analogous to epigenetic changes evident as increased rates of DNA methylation of BDNF exon VI evident in adolescents born to human cigarette smokers [46]. However, we did not observe any change in MeCP2 binding or the association of acetylated histone 3 at the promoter of egr1, in spite of observing an increase in its expression. These results suggest that prenatal cocaine exposure alters the epigenetic marks at specific promoter regions within the mPFC that are evident as early as P30 and may impact other epigenetic marks including DNA methylation that mediate the dysregulated egr1 expression observed in these animals.
To conclude, this study suggests that prenatal cocaine exposure results in a transient increase in SI during early adolescence, potentially due to a coincident upregulation of egr1 expression in the mPFC that engenders greater sensitivity to isolation distress. These results may suggest that targeted molecular and behavioral treatments during critical periods of brain development could normalize function in a region that plays a crucial role in cognitive maturation. Furthermore, the gradual disappearance of differences in social behavior between cocaine-exposed and control groups warrant consideration. Critically, early social experience can shape brain development and influence adult social functioning [28, 47]. Thus, the possibility of impaired adolescent social functioning following prenatal cocaine exposure may be highly relevant to other adult social behaviors and might suggest possible use of social interventions to normalize child development and subsequent social maturation following such exposures.
Acknowledgments
Funding
This research was supported by NIH-NIDA Grants DA017905 and DA00354 to Dr. Barry Kosofsky, and NIH-NIDA Grant DA02254-05 to Dr. Garet Lahvis.
Footnotes
Disclosure
The authors report no financial interests or conflicts of interest.
References
- 1.Sobrian SK, Holson RR. Social behavior of offspring following prenatal cocaine exposure in rodents: a comparison with prenatal alcohol. Front Psychiatry. 2011;2:66. doi: 10.3389/fpsyt.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakemore SJ. The developing social brain: implications for education. Neuron. 2010;65(6):744–7. doi: 10.1016/j.neuron.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987;111(4):571–8. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- 4.Tronick EZ, et al. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005;41(5):711–22. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- 5.Delaney-Black V, et al. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106(4):782–91. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- 6.Overstreet DH, et al. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70(1-2):149–56. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estelles J, et al. Prenatal cocaine exposure alters spontaneous and cocaine-induced motor and social behaviors. Neurotoxicol Teratol. 2005;27(3):449–57. doi: 10.1016/j.ntt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Johns JM, Noonan LR. Prenatal cocaine exposure affects social behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1995;17(5):569–76. doi: 10.1016/0892-0362(95)00017-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magalhaes A, et al. Prenatal exposure to cocaine and enriched environment: effects on social interactions. Ann N Y Acad Sci. 2006;1074:620–31. doi: 10.1196/annals.1369.060. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez LE, et al. Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res. 2000;887(1):7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- 11.van Kerkhof LW, et al. Social Play Behavior in Adolescent Rats is Mediated by Functional Activity in Medial Prefrontal Cortex and Striatum. Neuropsychopharmacology. 38(10):1899–909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrier N, Kabbaj M. Sex differences in social interaction behaviors in rats are mediated by extracellular signal-regulated kinase 2 expression in the medial prefrontal cortex. Neuroscience. 2012;212:86–92. doi: 10.1016/j.neuroscience.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stack A, et al. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35(2):570–80. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–91. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblum K, et al. The role of extracellular regulated kinases I/II in late-phase longterm potentiation. J Neurosci. 2002;22(13):5432–41. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinowich K, et al. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain. 2011;4:11. doi: 10.1186/1756-6606-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302(5646):885–9. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 18.Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2010;38(4):1067–74. doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, et al. Histone methylation regulates memory formation. J Neurosci. 2010;30(10):3589–99. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabir ZD, et al. Brain-derived neurotrophic factor genotype impacts the prenatal cocaine-induced mouse phenotype. Dev Neurosci. 2012;34(2-3):184–97. doi: 10.1159/000337712. [DOI] [PubMed] [Google Scholar]
- 21.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 22.Panksepp JB, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2(4):e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60(4):610–24. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyan SW, et al. Serum- and glucocorticoid-inducible kinase 1 enhances zif268 expression through the mediation of SRF and CREB1 associated with spatial memory formation. J Neurochem. 2008;105(3):820–32. doi: 10.1111/j.1471-4159.2007.05186.x. [DOI] [PubMed] [Google Scholar]
- 25.Giordano TP, 3rd, et al. Up-regulation of dopamine D(2)L mRNA levels in the ventral tegmental area and dorsal striatum of amphetamine-sensitized C57BL/6 mice: role of Ca(v)1.3 L-type Ca(2+) channels. J Neurochem. 2006;99(4):1197–206. doi: 10.1111/j.1471-4159.2006.04186.x. [DOI] [PubMed] [Google Scholar]
- 26.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–9. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24(24):5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammock EAL, L P. The discipline of neurobehavioral development: The emerging interface of proceses that build circuits and skills. Human Development. 2006;49(5):15. [Google Scholar]
- 29.Panksepp JB, et al. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195(2):239–45. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niesink RJ, van Ree JM. Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol Behav. 1982;29(5):819–25. doi: 10.1016/0031-9384(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 31.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2(3):219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 32.McFarlane HG, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 33.Spencer CM, et al. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4(7):420–30. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 34.Spencer CM, et al. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122(3):710–5. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- 35.Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav. 2011;10(1):4–16. doi: 10.1111/j.1601-183X.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elsworth JD, Morrow BA, Roth RH. Prenatal cocaine exposure increases mesoprefrontal dopamine neuron responsivity to mild stress. Synapse. 2001;42(2):80–3. doi: 10.1002/syn.1102. [DOI] [PubMed] [Google Scholar]
- 37.Morrow BA, Elsworth JD, Roth RH. Male rats exposed to cocaine in utero demonstrate elevated expression of Fos in the prefrontal cortex in response to environment. Neuropsychopharmacology. 2002;26(3):275–85. doi: 10.1016/S0893-133X(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4(2):e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35(9):1864–75. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10(1):44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PLoS One. 2013;8(12):e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aid T, et al. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–35. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberg ME, et al. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–7. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74(4):183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Bellone C, Mameli M, Luscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14(11):1439–46. doi: 10.1038/nn.2930. [DOI] [PubMed] [Google Scholar]
- 46.Toledo-Rodriguez M, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1350–4. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- 47.Zahn-Waxler C, R-Y M. The origins of empathic concern. Motivation and Emotion. 1990;14(2):23. [Google Scholar]


