Abstract
Broccoli sprouts are a convenient and rich source of the glucosinolate, glucoraphanin, which can generate the chemopreventive agent, sulforaphane, an inducer of glutathione S-transferases (GSTs) and other cytoprotective enzymes. A broccoli sprout-derived beverage providing daily doses of 600 μmol glucoraphanin and 40 μmol sulforaphane was evaluated for magnitude and duration of pharmacodynamic action in a 12-week randomized clinical trial. Two hundred and ninety-one study participants were recruited from the rural He-He Township, Qidong, in the Yangtze River delta region of China, an area characterized by exposures to substantial levels of airborne pollutants. Exposure to air pollution has been associated with lung cancer and cardiopulmonary diseases. Urinary excretion of the mercapturic acids of the pollutants, benzene, acrolein, and crotonaldehyde, were measured before and during the intervention using liquid chromatography tandem mass spectrometry. Rapid and sustained, statistically significant (p ≤ 0.01) increases in the levels of excretion of the glutathione-derived conjugates of benzene (61%), acrolein (23%), but not crotonaldehyde were found in those receiving broccoli sprout beverage compared with placebo. Excretion of the benzene-derived mercapturic acid was higher in participants who were GSTT1-positive compared to the null genotype, irrespective of study arm assignment. Measures of sulforaphane metabolites in urine indicated that bioavailability did not decline over the 12-week daily dosing period. Thus, intervention with broccoli sprouts enhances the detoxication of some airborne pollutants and may provide a frugal means to attenuate their associated long-term health risks.
Keywords: Air pollution, broccoli, sulforaphane, benzene, chemoprevention
The International Agency for Research on Cancer (IARC) has recently classified air pollution and particulate matter (PM) from air pollution as carcinogenic to humans (1). China is now the world’s largest emitter of anthropogenic air pollution and levels of outdoor air pollution in China are among the highest in the world (2,3). The Yangtze River delta region of China, which includes our study site of Qidong, is the fastest growing economic development area in China. Air pollution from expanding industrialization in this region masks the horizon on many days, especially during the winter months. Increases in fossil fuel use in China’s industry, transport and residential sectors have resulted in a steep rise in emissions. The Yangtze River delta region, which constitutes only 2% of the area of China, contributes upwards of 15% of countrywide emissions of greenhouse gases (4). These emissions include PM. There is substantial evidence that the most harmful components of PM are in the fine fraction of PM (particles with an aerodynamic diameter < 2.5 μm; PM2.5) which can be inhaled into the deep lungs (5,6). In Chinese cities, until recently, only the larger PM, PM10, were routinely monitored and reported. A large, recent study in Europe indicated that PM, irrespective of particle size, contributes to lung cancer incidence (7). Adsorbed onto these inhaled particles are heavy metals, as well as carcinogenic polycyclic aromatic hydrocarbons and volatile organic chemicals such as benzene and aldehydes, which, following desorption from PM, may contribute to lung cancer risk (8,9).
We have previously reported 5-fold higher levels of the polycyclic aromatic hydrocarbon biomarker, phenanthrene tetraol, in the urines of non-smoking Qidongese compared to non-smoking residents of the Twin Cities region of Minnesota (10), perhaps reflective of different ambient air quality in these two regions. Qidong is located on the northeastern tip of the mouth of the Yangtze River delta and is undergoing a rapid transition from isolated rural farm communities to an industrialized manufacturing center. Levels of mercapturic acids formed in the metabolism of benzene [S-phenylmercapturic acid (SPMA)], acrolein [3-hydroxypropylmercapturic acid (3-HPMA),] and crotonaldehyde [3-hydroxy-1-methylpropylmercapturic acid (HMPMA)] are also substantially higher in these Qidongese compared to non-smoker residents of Singapore (11). In the US, the predominant exposure to benzene arises from on-road mobile-source emission, although other sources including emissions from coal and oil combustion, evaporation from industrial sites and gasoline service stations are noted (12). Smoking is also an important source of exposure to benzene, acrolein, and crotonaldehyde, based on analyses of their mercapturic acids before and after cessation, as well as other data (13). We have also observed 2- to 3-fold higher rates of excretion of crotonaldehyde and acrolein mercapturic acids in the urine of Qidong smokers compared to non-smokers (10).
Mercapturic acids are detoxication products resulting from glutathione conjugation of the parent aldehydes, or in the case of benzene, a primary metabolite, benzene oxide, is conjugated with glutathione followed by dehydration giving SPMA. They can be formed non-enzymatically or by glutathione S-transferase (GST)-catalyzed reactions (14-16). These biomarkers can play multiple, seemingly paradoxical roles in studies on human health. Commonly, they are used as indices of internal dose, and as such are physiologically integrated measures of either ambient or occupational exposures that have been applied across study populations amid a range of exposures linked to adverse health effects. Dose-response relationships between workplace air measures of benzene and urinary excretion of SPMA have been reported (17). These biomarkers may also serve as measures of pharmacodynamic action in randomized clinical trials to assess the impact of interventions to enhance carcinogen detoxication (10).
To determine possible enhancement of detoxication of airborne pollutants by a broccoli sprout beverage, we conducted a placebo-controlled, randomized intervention trial in China. A bioactive component derived from broccoli, sulforaphane (SF) (18), is an effective anti-carcinogen in animal models (19), and acts in part through inducing detoxication enzymes including GSTs. The safety of broccoli sprout beverage has been well established in several Phase I clinical trials (10, 20, 21). Unlike the previous clinical studies, this trial used a beverage with a blended, well-defined content of SF (40 μmol) and its biogenic precursor glucoraphanin (GR) (600 μmol). Therefore, the primary goals of this study were to determine i) to what extent daily consumption of a broccoli sprout beverage could elevate the initial rate of detoxication of pervasive toxic air pollutants among individuals exposed to excessive ambient levels and ii) whether such a protective response would be sustainable with daily doses across a 12-week time-frame.
Materials and Methods
Study design and participants
Adults in good general health without a history of major chronic illnesses were randomized into a placebo-controlled trial for assessing the pharmacokinetics and pharmacodynamics of a beverage enriched with GR and SF from broccoli sprouts. Study participants were recruited from the villages of Qing Jia, Ji Zi, and Jiang Luo in the rural farming community of He-He Township, Qidong, Jiangsu Province, China. One-thousand two-hundred and five individuals were screened at local clinics over 6 days in September 2011. Written informed consents were obtained from all participants. The protocol was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health, the University of Pittsburgh, the University of Minnesota, and the Qidong Liver Cancer Institute and registered with ClinicalTrials.gov (NCT 01437501). A medical history, physical examination and routine hepatic and renal function tests were used to screen the individuals, aged 21 to 65 years, by methods identical to those described for our previous interventions in this region (10, 22). Five-hundred and thirty-nine individuals from the screened group were eligible, of which the initial 300 were randomized using a fixed randomization scheme with a block size of 10. Two-hundred and ninety-one of these selected participants returned to the clinics on the first day of the study where they provided informed consents for the intervention study and were given their identification code. Overall, there were 62 men (21%) and 229 women (79%) with a median age of 53 (range 21-65) years. Although this was a tightly controlled dietary intervention, participants were under no dietary restrictions throughout the trial.
The trial was conducted from mid October 2011 to early January, 2012. Participants consumed a placebo beverage or a broccoli sprout beverage for 84 consecutive days (12 weeks). Participants met local doctors and study investigators at one of ten designated local sites between 16:30 and 18:00 each evening for distribution of the intervention beverages. Compliance was determined by visual observation of consumption and measures of urinary excretion of SF metabolites (see below). Placebo and broccoli sprout beverages were prepared fresh each afternoon from bulk powders and brought to He-He daily for distribution. To control pH, ascorbic acid was added to urine collection containers shortly before distribution to participants, and complete overnight and daytime (about 12 h each) urine samples were collected following consumption of the beverage on days 1, 7, 14, 28, 42, 56, 70 and 84. In addition, a 12-hour overnight urine was collected on the day prior to consuming the first beverage (day 0). Once collected, urine volumes were measured, and aliquots prepared and transported to the Qidong Liver Cancer Institute for immediate storage at −20°C. Blood samples were collected on days 0, 28, 56, and 84 of the study. Serum alanine aminotransferase activities were determined on all collected samples. Aliquots of urine and serum from each sample were shipped frozen to Baltimore at the end of the study, and serum samples were transferred immediately to a clinical laboratory (Hagerstown Medical Laboratory, Hagerstown, MD) for comprehensive blood chemistry analyses.
Preparation of the broccoli sprouts beverages
The study was conducted using re-hydrated, previously lyophilized broccoli sprout powders rich in either GR or SF that were produced by the Cullman Chemoprotection Center at Johns Hopkins University, School of Medicine, Department of Pharmacology, for clinical study use as an Investigational New Drug. Broccoli sprouts were grown from specially selected BroccoSprouts™ seeds (cv. DM1999B) with technology licensed from Johns Hopkins University. Briefly, seeds were surface-disinfected, and grown in a commercial sprouting facility under controlled light and moisture conditions. After 3 days of sprout growth, an aqueous extract was prepared in a steam jacketed kettle at a GMP food processing facility (Oregon Freeze Dry, Albany, OR). Sprouts were plunged into boiling deionized water and allowed to boil for 30 minutes. The resulting aqueous extract contained about 5 mM GR, the biogenic precursor of SF.
A GR-rich powder was prepared by filtering and lyophilizing this aqueous extract at Oregon Freeze Dry. Total GR titer was determined in the resulting powder by HPLC (23) to be 329 μmol/g powder when assayed just prior to use in the clinical study. To prepare our SF-rich powder, the aqueous extract was filtered, cooled to 37°C, and treated with myrosinase, an enzyme released from a small amount of daikon (Raphanus sativus) sprouts, for 4 hours in order to hydrolyze the glucosinolates to isothiocyanates. Total isothiocyanate and SF levels were then quantified by cyclocondensation analysis (24) and by direct HPLC (25), respectively. This hydrolyzed aqueous extract was also lyophilized at Oregon Freeze Dry. SF content at time of use was 202 μmol/g powder and represented 91% of the total isothiocyanate content in the powder.
The bulk powders were tested for microbial contaminants prior to release by Oregon Freeze Dry and again upon receipt in Baltimore (IEH-JL Analytical Services, Modesto, CA and Eurofins Strasburger & Siegel, Hanover, MD), heavy metals (Elemental Analysis, Inc., Lexington, KY) and benzene (TestAmerica, Pittsburgh, PA). Following air shipment to China, both powder preparations were stored in sealed bags in a locked, dedicated −20°C freezer until reconstitution of the study beverages.
To prepare 150 daily doses, allotments of each powder (360 g GR-rich and 24.8 g SF-rich powders) were dissolved in sterile water. An equal volume of pineapple juice (Dole, Manila, Philippines) was added along with lime juice (Safeway, USA) in a final ratio of 47:47:6 water:pineapple juice:lime juice (by volume) with vigorous mixing prior to transfer of 100-mL individual doses into sterile 330-mL commercial bottled water bottles for daily distribution to study participants. The individual daily dose was 600 μmol of GR and 40 μmol of SF. The placebo beverage contained the same liquid components, to which 1% molasses v/v was added to provide color masking.
Quality control of beverages
The juices served to mask odor and taste, but had no effect on the stability of the phytochemicals; and contributed minimal enzyme inducer activity to the beverage. Extra beverages prepared at early, middle, and late time-points during the trial were stored at −20°C and returned to Baltimore for analyses of GR and SF content as well as enzyme inducer activity. NAD(P)H: quinone acceptor oxidoreductase inducer activity in the beverages, measured by the Prochaska assay (26) confirmed 40 ± 1.4 μmol of SF equivalents (mean ± SD) in the broccoli beverage in the absence of treatment with myrosinase and 635 ± 100 μmol of equivalents following incubation with myrosinase, per 100 mL. Direct analyses of GR and SF content in the broccoli beverage (23, 25) indicated striking concordance with the bioassay results: 614 ± 15 μmol of GR and 40.5 ± 0.8 μmol of SF. Equivalent measures were seen across the frozen samples saved from the early, middle, and late time-point preparations. No GR or SF was detected in the placebo beverage. Negligible basal inducer activity was detected (0.87 ± 0.24 μmol of SF equivalents) in the placebo beverage.
Air pollution biomarkers
Data for the PM10 levels in Qidong during the study period were provided by the Qidong Environmental Monitoring Station. Values for PM10 in Shanghai were obtained from the Shanghai Environmental Monitoring Center, Shanghai Environmental Protection Bureau. All mercapturic acids were quantified by isotope-dilution mass spectrometry as described previously (13, 27). Urinary creatinine was assayed by the Hagerstown Medical Laboratory, Hagerstown, MD.
Glucoraphanin and sulforaphane in urine
Measurement of GR and SF metabolites in urine was performed by isotope-dilution mass spectrometric assay as previously reported by Egner et al. (28). Positive ESI-MS/MS was carried out using a Thermo-Finnigan TSQ Advantage triple quadrupole mass spectrometer coupled to a Thermo-Finnigan Accela UPLC and HTC Pal autoinjector (ThermoElectron Corporation, San Jose, CA). Chromatographic separation of analytes was achieved using a 1.9 μm 100 × 1 mm Thermo Hypersil Gold column maintained at 40°C.
Genotyping and Single Nucleotide Polymorphism (SNP) analyses
Genomic DNA was isolated from serum with a Qiagen QIAamp Mini Blood isolation kit. GSTM1 and GSTT1 genotypes were identified by real-time PCR as described previously (21). The primer and probe sequences for the NRF2 rSNP-617 were as follows: forward primer sequence CAGTGGGCCCTGCCTAG; reverse primer sequence TCAGGGTGACTGCGAACAC; reporter 1 dye_VIC TGGACAGCGCCGGCAG; reporter 2 dye_FAM TGTGGACAGCTCCGGCAG (Applied Biosystems, Grand Island, NY).
Statistical analyses
The analyses comprised four components: (i) a comparison of levels of air pollutant biomarkers by treatment arm at baseline, prior to the administration of the broccoli sprout beverage (i.e., day 0), (ii) a comparison of the persistent effects (days 1 through 84) of the beverage on air pollutant excretion in urine, (iii) a comparison of air pollutant excretion by genotype, and (iv) a description of SF metabolites excreted in urine at the individual level.
For the baseline comparison of the treatment and placebo arms, a two-sample t-test of geometric means for each biomarker was conducted. To describe the acute and persistent effects of treatment, separate log-linear mixed effects (random intercepts and slopes) models for each biomarker were fit. In this setting, the independent variables were treatment assignment (placebo as reference), time in weeks (from day 1 to day 84), and the interaction between treatment assignment and time. Specifically, the model was of the form:
where and corresponding to the between a and b follow a bivariate normal distribution with means equal to 0, variance components corresponding to the between-individuals differences in intercept (level at day 1) and slopes (change per week), and are statistically independent of the residuals (e) which also follow a normal distribution, whose variance corresponds to the within-individual variability of the biomarkers across visits. The parameter α0 is interpreted as the average biomarker level for the placebo group at day 1 and α1 describes the difference in biomarker level due to treatment at day 1 (acute effect). The parameter β0 is the average change in biomarker level per each week for the placebo group and β1 is the effect of treatment on this slope (persistent effect).
For benzene, a portion (14%) of urine samples were below the limit of detection (i.e., <0.125 pmol/mL). Since these observations were standardized to heterogeneous urine creatinine concentrations (mg/mL), the resulting left-censored values were also heterogeneous. To appropriately incorporate the left-censored observations to the mixed effects models, we programmed the maximum likelihood method using the flexible procedure NLMIXED in SAS. The contribution of left-censored values to the maximum likelihood function was determined by the cumulative-distribution function, while the contribution of non-left-censored values was determined by the probability-density function.
For comparing excretion by genotype, the geometric means and interquartile ranges (IQR) of each air pollutant were calculated by day for each genotype-treatment strata and displayed graphically. As a summary of overall levels, the geometric means were calculated within each individual from days 1 through 84. The Wilcoxon rank sum test compared these individual levels by genotype class.
SF pharmacokinetics was described by fitting individual linear regressions of the excreted SF conjugates in the log scale. The slopes from these regressions describe the average change per week. The sign rank test compared whether the average change per week was significantly different than 0% (Egner et al., 2011). Statistical significance was assessed at the α = 0.05 level. All analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC).
Results
Compliance, data collection completeness, and tolerability
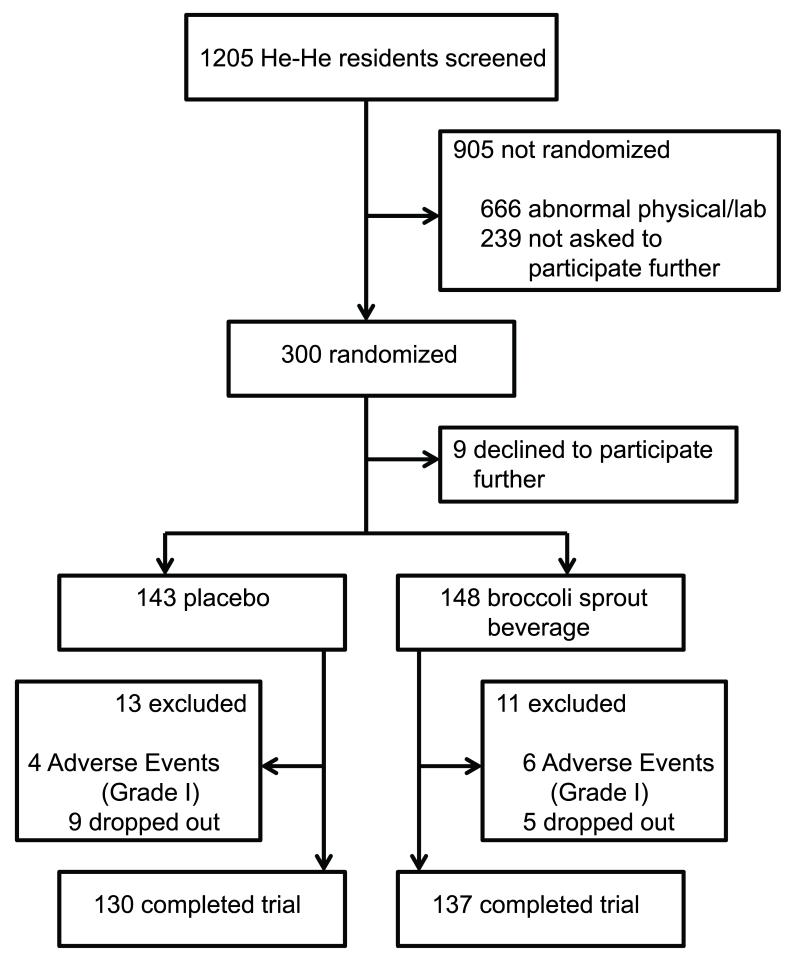
As indicated in Figure 1, 300 individuals were randomized into the two intervention arms; 9 declined to participate shortly thereafter. The intervention groups did not differ significantly (p > 0.05) by gender, age, or body mass index (Table I). Of the 24 drop-outs, 13 were assigned to the placebo arm while 11 were assigned to the broccoli sprout beverage arm. Two-hundred and sixty-seven out of 291 participants (92%) completed the trial: 53% drank every beverage while the rest consumed at least 80 out of 84 assigned beverages. Ten grade I adverse events were reported; all occurred in the first week of the trial and were distributed as 4 (2.8%) participants drinking the placebo and 6 (4.1%) the broccoli sprout beverage. Unacceptable taste and mild stomach discomfort were the common complaints. One individual, assigned to the broccoli beverage, reported mild vomiting. Most of the remaining 14 drop-outs left the study because of the inconvenience of meeting on a daily basis for supervised beverage consumption. The tolerability of the broccoli beverage was vastly improved due to the water:pineapple juice:lime juice formulation recommended by Sensory Spectrum (New Providence, NJ) (29) compared to earlier trials in which broccoli sprout extracts were delivered in water (21) or a 50:50 water:mango juice mixture (10). Furthermore, of the 267 participants who completed the trial, only 18 of 4,539 possible urine samples were not collected (0.3%); 99.8%% of the blood samples were collected. There were no abnormal clinical chemistry values for blood samples collected on the last day of the intervention.
Figure 1.
Intervention trial profile.
TABLE I.
Demographic distribution (% (n) or median [ICR]) of screened population and enrolled participants by treatment
| Treatment group | |||
|---|---|---|---|
|
| |||
| Variable | Screened Population (n= 1205) |
Placebo (n= 143) |
Broccoli Sprout (n= 148) |
| Female | 74% (889) | 78% (111) | 80% (118) |
| Age, years | 54 [48, 59] | 53 [48, 59] | 52 [46, 58] |
| Body mass index | 23.9 [21.8, 26.2] | 23.8 [21.9, 35.8] | 23.4 [21.3, 25.1] |
| Among women | 23.9 [22.0, 26.1] | 23.8 [22.1, 25.4] | 23.6 [21.8, 25.1] |
| Among men | 23.8 [21.6, 26.5] | 23.6 [21.5, 26.7] | 22.5 [20.3, 25.3] |
| Current smoker | 12% (146) | 13% (18) | 9% (14) |
| Among women | 0% (0) | 0% (0) | 0% (0) |
| Among men | 46% (146) | 56% (18) | 47% (14) |
Levels of air pollutant biomarkers at baseline
Levels of SPMA, 3-HPMA and HMPMA were measured in all study participants in the 12-hour overnight urine samples collected on the morning prior to consumption of the first beverage. These analytes serve as biomarkers of internal dose from ambient exposures to these pollutants. Since the three biomarkers exhibited strong skewness (7.2, 6.9 and 6.4 for SPMA, 3-HPMA and HMPMA, respectively) all analyses were performed on the log-transformed scale which reduced the skewness to −0.2, 0.7 and 1.4, respectively. Table II presents the geometric means and IQR for these day 0 values segregated by treatment arm assignment. There were no significant differences in biomarker levels in the participants upon entry into the placebo and broccoli sprout beverage arms of the trial.
TABLE II.
Geometric mean levels [IQR] of benzene, acrolein and crotonaldehyde mercapturic acids at day 0 by treatment assignment
| Mercapturic Acids a | Placebo (n= 143) | Broccoli Sprout (n= 148) | P-value |
|---|---|---|---|
| Carcinogen | |||
| Benzene [SPMA] | 0.709 [0.326, 1.542] | 0.745 [0.395, 1.407] | 0.885 |
| Irritants | |||
| Acrolein [3-HPMA] | 3361 [1686, 5486] | 3569 [1703, 6186] | 0.779 |
| Crotonaldehyde [HMPMA] | 1510 [880, 1959] | 1312 [829, 1790] | 0.112 |
pmol/mg creatinine
Effects of broccoli sprout beverage on air pollutant biomarkers
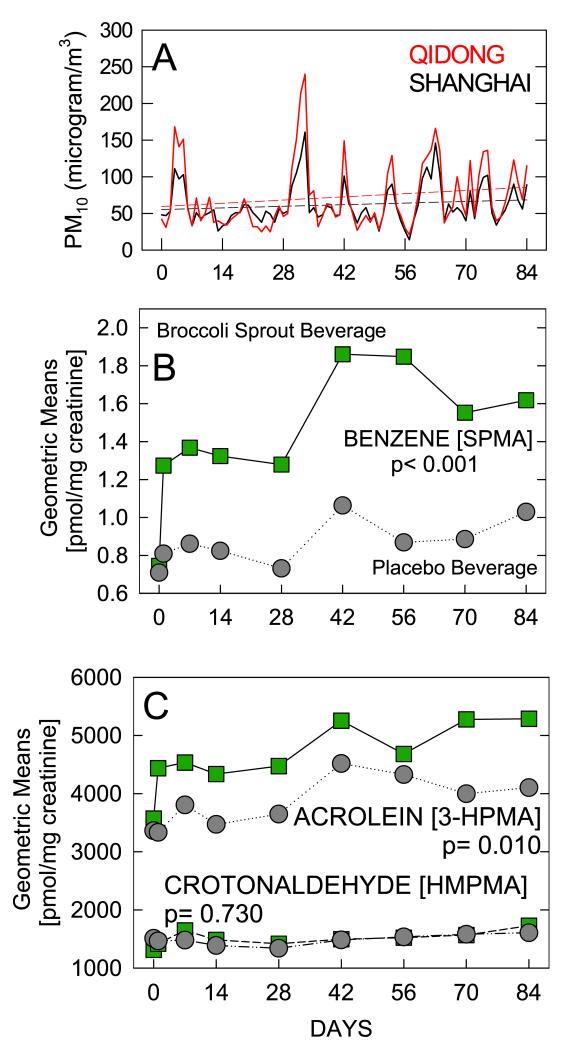
No routine monitoring of airborne concentrations of volatile organic chemicals is conducted in China, and was not undertaken as part of this study. However, daily tracking of the concentration of PM10 was recorded for many Chinese cities including Shanghai and Qidong at the time of the study. Presented in Figure 2A are the daily, 24-hour averaged concentrations of PM10 recorded in central Shanghai and in Qidong during the study period. The means of two Qidong monitoring sites, each within 0.5 km of the Qidong Liver Cancer Institute, are presented. The excellent concordance in the daily fluctuations over the 84-day period between the Shanghai and Qidong sites highlights the regional nature of the pollution in the Yangtze River delta area. Exposures were consistently but moderately higher in Qidong compared to Shanghai. Moreover, the rates of seasonal increase in PM10 levels were consistent (+2.0%/week) between the monitoring sites.
Figure 2.
Geometric means for biomarker levels on days 0, 1, 7, 14, 28, 42, 56, 70, and 84 of the intervention. A. Daily average levels for PM10 in Shanghai (black) and Qidong (red) during the study period. B. Urinary benzene mercapturic acid levels. C. Urinary acrolein and crotonaldehyde mercapturic acid levels. ( ) Broccoli sprout beverage arm; (•), placebo beverage arm. The geometric means for benzene appropriately accounted for left-censoring using the flexible PROC NLMIXED command in SAS 9.2.
) Broccoli sprout beverage arm; (•), placebo beverage arm. The geometric means for benzene appropriately accounted for left-censoring using the flexible PROC NLMIXED command in SAS 9.2.
Isotope dilution mass spectrometry was used to quantify the urinary excretion of the mercapturic acids of benzene, acrolein, and crotonaldehyde from the eight overnight 12-hour urine samples collected from each participant over the 12-week intervention period. Based on the mixed model, the estimated SPMA excretion for the placebo group was 0.818 pmol/mg creatinine (95%CI: 0.725, 0.922) at day 1. As shown in Figure 2B, those receiving broccoli sprout beverage had 60.6% higher excretion (95%CI: +35.8%, +89.8%: p< 0.001) at day 1, and this effect persisted over time. The average change per week for the placebo arm was +1.7% (95%CI: +0.7%, +2.6%) and it was similar (p = 0.204) to the average change per week for the broccoli sprout beverage (+2.5%: 95%CI: +1.6%, +3.5%). Similarly, as shown in Figure 2C, the broccoli sprout beverage group had a +22.7% higher urinary excretion of 3-HPMA (95%CI: +5.0%, +43.4%: p= 0.010) at day 1 than the placebo group, whose average level was 3548 pmol/mg creatinine (95%CI: 3173, 3968). This significantly higher level persisted over time, while each group had modest increase of 1.7% per week (p-value for difference in change per week between arms = 0.877). Lastly, the urinary excretion of HMPMA for the placebo arm at day 1 was 1412 pmol/mg creatinine and the broccoli sprout beverage was practically identical (p = 0.531).
Restriction of the analyses to the 211 women who completed the trial, all of whom were non-smokers, yielded the same results as seen with all participants. In this subgroup analysis, the increases in the excretion of the mercapturic acids of benzene, acrolein, and crotonaldehyde for the broccoli sprout versus placebo group were +54.7% (+27.2%, +88.1%), +21.7% (+1.8%, 45.5%), and +2.0% (−13.7%, +20.4%), respectively.
Effect of GST genotypes and Nrf2 rSNP-617 on biomarker levels
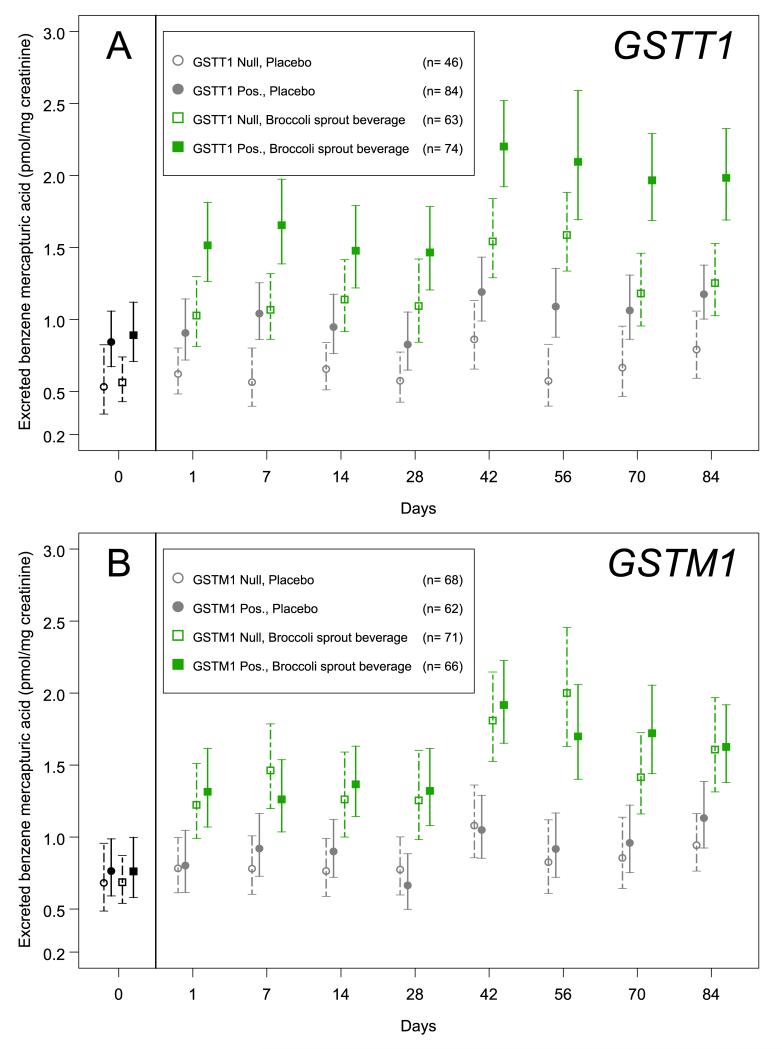
The absence of the GSTT1 allele is known to diminish SPMA excretion in settings of occupational exposures (17); an effect of GSTM1 is far less certain (16). The 267 participants who completed this study were genotyped for presence of these two GST alleles. The distributions of the null genotype for GSTT1 (41.9%) and GSTM1 (52.1%) were in accord with our earlier determinations in this population (10, 21). As shown in Figure 3A, on day 0 there was a significant 59% elevation of SPMA excretion in those individuals positive for the GSTT1 allele compared to those who were null (p< 0.01). Within the placebo arm there was a consistent > 50% increase in SPMA excretion at each time-point evaluated based on a positive GSTT1 genotype. A similar differential effect of null and positive GSTT1 genotype was seen in the treatment arm, but starting from a higher baseline value reflecting the intervention effect. Thus, although GSTT1 genotype is an important modifier of benzene metabolism, the broccoli beverage-induced effects on increased excretion of SPMA appear to be independent of GSTT1 status. By contrast, shown in Figure 3B, on day 0 and throughout the trial, the presence or absence of the GSTM1 alleles had no effect on rates of excretion of SPMA in the overnight voids (day 0; p= 0.501). Moreover, the effect of treatment was evident at all time-points, irrespective of GSTM1 genotype.
Figure 3.
Effect of GSTM1 and GSTT1 genotypes on urinary excretion of benzene-mercapturic acid. Distributions of benzene-mercapturic acid levels (geometric means and 95% confidence intervals) in participants either null or positive for the GSTT1 gene (panel A) or the GSTM1 gene (panel B) by assignment group (placebo or treated and day of study). Bars highlighted in green indicate those receiving the broccoli beverage. Open symbols represent medians for the participants null for the genotype and solid symbols indicate the geometric means of those who were positive.
There is a functional polymorphism in an Antioxidant Response Element-like sequence at −617 of the promoter of the transcription factor NRF2 in which A is substituted for C (30). Nrf2 is known to regulate the expression of genes, including GSTs, involved in the detoxication of toxicants and carcinogens (31). In an exploratory analysis, among the 267 participants completing the trial, 123 were homozygous for the C allele (C/C), 115 heterozygous (C/A), and 29 A/A. There was no significant effect of this SNP (A/A or A/C versus C/C) on the excretion of SPMA at baseline (day 0) (p= 0.203). Moreover, no change was seen in the placebo arm throughout the intervention period. The median [IQR] of individual geometric mean levels in pmol/mg creatinine for days 1 through 84 of the trial were 0.750 [0.461, 1.140] for C/C and 0.860 [0.443, 1.119] for C/A or A/A (p= 0.896). However, a significant effect on benzene metabolism and excretion was seen following treatment with the broccoli beverage, indicating a potential, partial role for NRF2 in the actions of SF in this setting. The median [IQR] levels in pmol/mg creatinine were 1.104 [0.797, 1.519] for C/C and 1.352 [0.938, 1.833] for C/A or A/A (p= 0.029).
Sulforaphane pharmacokinetics
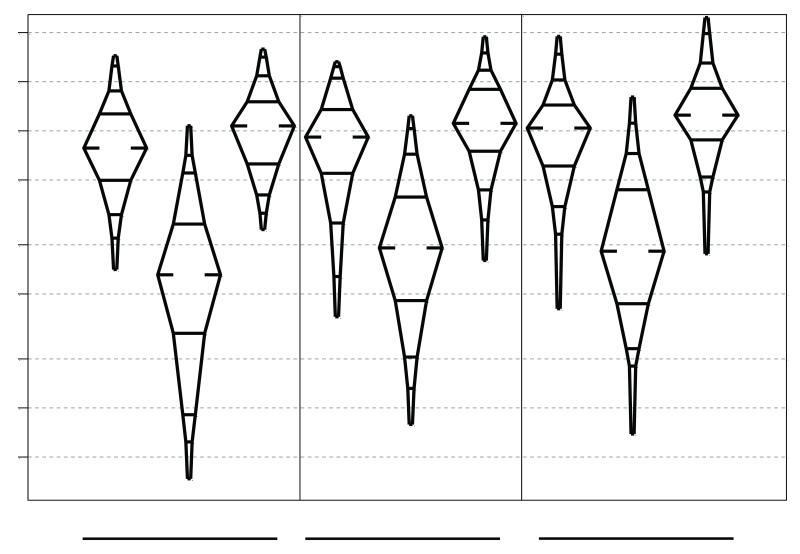
Isotope-dilution mass spectrometry was used to measure the levels of glutathione-derived conjugates of SF excreted in the urine during consecutive 12-hour collections on days 1, 42, and 84, which are shown in Figure 4. SF-N-acetylcysteine (80-81%), SF-cysteine (12-14%) and free SF (5-7%) are the major urinary metabolites; the other glutathione-derived conjugates SF-glutathione and SF-cysteinyl-glycine account for < 1%, as seen previously (28). The distributions of individual urinary metabolites did not change over the course of the intervention. Even with the blended GR and SF formulation designed to extend the biological half-life of each dose, the majority of the SF metabolites were excreted in the first 12 hours following administration of each dose, although the percentage of total 24 hour excretion increased from 72% to 82% to 84% on days 1 to 42 to 84. Median levels of the 24-hour excretion of SF metabolites increased from 54 to 56 to 62 μmole. These amounts represent 8.4, 8.8 and 9.7% of the administered daily dose of SF (600 μmol GR+ 40 μmol SF). In order to summarize the within subject changes in SF metabolites over time, regression lines were fit to each individual’s data. The median of the 136 subject-specific slopes, expressed as percent change, was +10.1% [IQR: −13.7%, +43.9%]. This average change was significantly greater than 0 (sign rank p= 0.01). The intra-class correlation coefficient for the repeated measures of SF urinary metabolites was 0.35.
Figure 4.
Urinary excretion of sulforaphane and its metabolites (sulforaphane-cysteine and sulforaphane-mercapturic acid) on days 1, 42, and 84 as measured by isotope dilution mass spectrometry in participants randomized to the broccoli sprout beverage arm.
All individuals assigned to the placebo arm had urinary SF metabolite levels below 1 μmol/mg creatinine with the exception of 6 individuals at day 42 and 4 individuals at day 84. The highest value amongst these individuals was 14 μmol/mg creatinine per 24 hr. These excursions into the detectable range likely reflected the consumption of broccoli, which was being harvested from the local fields during the second half of the study, as participants were under no dietary restrictions throughout the trial.
Discussion
The key finding from this clinical trial was the observed rapid and highly durable elevation of the detoxication of benzene, a known human carcinogen, and acrolein metabolites in the participants randomized to the blended GR- and SF-rich broccoli beverage. In this regard the study demonstrated the persistent effects over the course of 12 weeks, extending the findings first described in our a small, short-term cross-over trial in which daily consumption of either a GR- or SF-rich broccoli beverage enhanced the excretion of these analytes at a 7-day endpoint (10, 28). Selection of a proper dose is especially difficult with a food-based intervention. In the cross-over trial we reported that 104 μmol of SF equivalents were excreted in the urine following the initial dose of SF (150 μmol) and 32 μmol following the initial dose of GR (800 μmol). Either intervention resulted in comparable increases in SPMA or acrolein-mercapturic acid excretion, suggesting that the dose-response effects were saturated. In the current trial, using a blend of 600 μmol of GR and 40 μmol of SF, we observed a median excretion of 54 μmol of SF equivalents over the 24 hours following the first dose (day 1). The magnitude of increased SPMA and acrolein-mercapturic acid excretion were nearly identical at the common time-point of day 7 in the two studies. The dose levels used in these two studies effectively defined the maximum tolerated doses of GR, SF or the two combined. They reflect levels of intake beyond that typically associated with broccoli consumers. Thus, future efforts should evaluate the efficacy of lower doses. Formulations with more consistent bioavailability need to be considered as well. In our initial 7-day cross-over study, the conversion of the GR following hydrolysis, absorption and conjugation with glutathione varied from 2% to 50% amongst study participants. Yield of SF equivalents in the current study ranged from 6.4% to 48.9%, buttressed at the lower end by the inclusion of some SF in the formulation. It is well established that SF-rich beverages provide higher and more consistent levels of SF than do GR-rich beverages (28, 32). Conversion efficiency is likely determined by the composition of the gut microflora of individual participants (32). Interestingly, the bioavailability of the GR contribution to systemic SF appeared to increase over the 84-day period, perhaps due to changes in the composition of the microflora.
The mechanisms underlying the actions of SF on benzene metabolism or its myriad of other protective effects are unclear. Nonetheless, activation of the NRF2 cytoprotective signaling pathway is a hallmark of SF mode of action (33, 34). In mice, disruption of Nrf2 signaling obviates the cancer chemopreventive actions of SF (35). In this study, GSTT1 genotype had a dramatic effect on rates of SPMA excretion, but this effect appeared to be independent of the broccoli beverage intervention. GSTT1 is not known to be a transcriptional target of NRF2 in humans. The nature of the inducible factors contributing to the broccoli-enhanced detoxication of benzene is not resolved. Acrolein is principally conjugated with glutathione through the catalytic actions of GSTP1 (14). Allelic variants in human GSTP1 are known to influence rates of conjugation of acrolein (15). By contrast, the specific activity of crotonaldehyde with human GSTs is minimal (14), perhaps explaining the absence of modulating effect by the broccoli sprout beverage on excretion of HMPMA.
SF-rich broccoli sprout preparations can induce NRF2-regulated gene expression in the upper airway of human subjects (36) and attenuate nasal allergic response to diesel exhaust particles (37). In mice, disruption of Nrf2 enhances susceptibility to airway inflammatory responses and DNA damage induced by diesel exhaust particles (38, 39). Moreover the acute toxicity or carcinogenicity of many of the metals and organic molecules adsorbed onto air pollution particles has been shown individually to be exacerbated in Nrf2-disrupted mice (35, 40, 41). That NRF2 signaling may be the responsible target for SF is further buttressed by our finding that a functional polymorphism in the proximal promoter of the NRF2 gene (−617) affects rates of SPMA excretion following treatment with the broccoli beverage.
Outdoor air pollution is associated with a wide range of adverse health outcomes, including cardio-respiratory mortality, chronic obstructive pulmonary disease, lung cancer along with increased rates of hospital admissions and exacerbation of chronic respiratory conditions together with decreased lung function (1, 42, 43). The Global Burden of Disease Study of 2010 lists chronic obstructive pulmonary disease as the third leading cause of death in China (44). Two sources of PM, ambient air and indoor air, were listed only below dietary risk levels, high blood pressure, and tobacco smoke as the prime risk factors for disability-adjusted life years in China. Clearly, control of ambient and, as possible, indoor air pollution (45) must become public policy priorities. Indeed, significant improvements in air quality in the United States, in part from public policy efforts to control air pollution, have been associated with improvements in life expectancy (46).
A population-based cancer registry has been in place in Qidong since 1972 and documents that the China age-standardized incidence rate for lung cancer has tripled in Qidongese men over the last 40 years, an increase consonant with a 5-fold increase in per capita tobacco sales in Qidong over that time (47). In Qidong, and indeed in many rural areas of China, a majority of men are smokers (~60%), while women are largely non-smokers (<1%) (47). Although smoking was not an exclusion criterion, none of the 211 women enrolled in our study were smokers. Given that there is a several decade lag between cigarette use and development of lung cancer, this increase in smoking from the 1950s into the 1980s and beyond likely drives much of the lung cancer in Qidongese men seen after 1972. There has been a doubling of the China age-standardized incidence rate of lung cancer in women in Qidong, beginning in 2000 (47). Unlikely to be associated with even second-hand smoking, the inflection in lung cancer in women begins a decade or more later than the emergence of economic development in the region. Whether this increase reflects increased exposures to outdoor or indoor air pollutants is not clear at this time, but it is certainly an escalating public health concern and a potential opportunity for evaluation of population-based approaches for chemoprevention.
Acknowledgments
We thank the staff of the He-He Public Health Station and the He-He Medical Clinic, the village doctors, and the residents of He-He for their participation; Kristina Wade (Johns Hopkins University, Baltimore, MD) for the quality control analyses; and the laboratory and clinical staff of the Qidong Liver Cancer Institute for their logistical support throughout the study. We thank Safeway, Inc for donating the lime juice used in this study.
Financial support: This work was supported by the National Institutes of Health (P01 ES006052 and P30 ES003819 to JDG).
Footnotes
Conflicts of interest: none declared.
References
- 1.Loomis D, Groose Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–3. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Hong C, Kan H. Exposures and health outcomes from outdoor air pollutants in China. Toxicology. 2004;198:291–300. doi: 10.1016/j.tox.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Pan D, Davis SJ, Zhang Q, He K, Wang C, et al. China’s international trade and air pollution in the United States. Proc Natl Acad Sci USA. 2014;111:1736–41. doi: 10.1073/pnas.1312860111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Fu JS, Zhuang G, Levy JI. Risk-based prioritization among air pollution control strategies in the Yangze River delta, China. Envirn. Health Perspect. 2010;118:1204–10. doi: 10.1289/ehp.1001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;176:667–72. doi: 10.1164/rccm.200503-443OC. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raaschou-Nielsen O, Andersen ZJ, Beeten R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–22. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 8.Yuan JM, Gao YT, Wang R, Chen M, Carmella SG, Hecht SS. Urinary levels of volatile organic and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis. 2012;33:804–809. doi: 10.1093/carcin/bgs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan JM, Butler LM, Gao YT, Murphy SE, Carmella SG, Wang R, et al. Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis. 2014;35:339–45. doi: 10.1093/carcin/bgt352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Muñoz A, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33:101–7. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht SS, Seow A, Wang M, Wang R, Meng L, Koh WP, et al. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer Epidemiol Biomarkers Prev. 2010;19:1185–92. doi: 10.1158/1055-9965.EPI-09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HEI Air Toxics Review Panel . Mobile-source air toxics: a critical review of the literature on exposure and health effects. Health Effects Institute; Boston: 2007. HEI Special Report 16. [Google Scholar]
- 13.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berhane K, Widersten M, Engström A, Kozarcih JH, Mannervik B. Detoxication of base propenals and other α,β-unsatruated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci USA. 1994;91:1480–84. doi: 10.1073/pnas.91.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal A, Hu X, Zimniak P, Singh SV. Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of α,β-unsaturated aldehydes. Cancer Lett. 2000;154:39–43. doi: 10.1016/s0304-3835(00)00390-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Lan Q, Waidyanatha S, Chanock S, Johnson BA, Vermeulen R, et al. Genetic polymorphisms and benzene metabolism in humans exposed to a wide range of air concentrations. Pharmacogenetics Genomics. 2007;17:789–801. doi: 10.1097/FPC.0b013e3280128f77. [DOI] [PubMed] [Google Scholar]
- 17.Qu Q, Shore R, Li G, Su L, Jin X, Melikian AA, et al. Biomarkers of benzene: Urinary metabolites in relation to individual genotype and personal exposure. Chem-Biol Interac. 2005;153-154:85–95. doi: 10.1016/j.cbi.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Kensler TW, Cho C-G, Posner GH, Talalay P. Anticarcinogenic activity of sulforaphane and structurally related synthetic norbornanyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 21.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthene tetraols in a randomized clinical trial in He Zuo Township, Qidong, PRC. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson LP, Zhang B-C, Zhu Y-R, Wang J-B, Wu Y, Zhang Q-N, et al. Oltipraz chemoprevention trial in Qidong, People’s Republic of China: study design and clinical outcomes. Cancer Epidemiol Biomarkers Prev. 1997;6:257–65. [PubMed] [Google Scholar]
- 23.Wade KL, Garrard IJ, Fahey JW. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J Chrom A. 2007;1154:469–72. doi: 10.1016/j.chroma.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, Fahey JW. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Molec. Cancer Therapeutics. 2006;5:935–44. doi: 10.1158/1535-7163.MCT-05-0476. [DOI] [PubMed] [Google Scholar]
- 26.Fahey JW, Dinkova-Kostova AT, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382(Part B):243–58. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 27.Carmella SG, Chen M, Zarth A, Hecht SS. High throughput liquid chromatrography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers’ urine. J Chromatog B. 2013;936:36–40. doi: 10.1016/j.jchromb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4:384–95. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Civille GV, Oftedal KN. Sensory evaluation techniques – make “good for you” taste “good”. Physiol Behav. 2012;107:598–605. doi: 10.1016/j.physbeh.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–46. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 31.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 32.Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5:603–11. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33:2–9. doi: 10.1093/carcin/bgr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, et al. Keap1-Nrf2 signaling: A target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–78. doi: 10.1007/128_2012_339. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antiobiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA. 2002;99:7610–15. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–51. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, et al. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food & Function. 2014;5:35–41. doi: 10.1039/c3fo60277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, et al. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2008;128:366–73. doi: 10.1016/j.clim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 40.Toyama T, Shinkai Y, Yasutake A, Uchida K, Yamamoto M, Kumagai Y. Isothiocyanates reduce mercury accumulation via an Nrf2-dependent mechanism during exposure of mice to methylmercury. Environ Health Perspect. 2011;119:1117–22. doi: 10.1289/ehp.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu KC, Liu JJ, Klaassen CD. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol Appl Pharmacol. 2012;263:14–20. doi: 10.1016/j.taap.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Ebenstein A, Greenstone M, Li H. Evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River policy. Proc Natl Acad Sci USA. 2013;110:12936–41. doi: 10.1073/pnas.1300018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samet J, Krewski D. Health effects associated with exposure to ambient air pollution. J. Toxicol. Environ. Health. 2007;198:291–300. doi: 10.1080/15287390600884644. [DOI] [PubMed] [Google Scholar]
- 44.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2009. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Zou Y, Li X, Chen S, Zhao Z, He F, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: A 9-year prospective cohort study. PLos Med. 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–86. doi: 10.1056/NEJMsa0805646. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JG, Kensler TW. Changing rates for liver and lung cancers in Qidong, China. Chem Res Toxicol. 2014;27:3–6. doi: 10.1021/tx400313j. [DOI] [PMC free article] [PubMed] [Google Scholar]