Abstract
Objectives
To review the electrophysiological and clinical characteristics of 32 patients with orthostatic myoclonus (OM), a relatively newly identified movement disorder, and compare these characteristics to those of primary orthostatic tremor (OT) patients and patients with similar gait and balance complaints without either hyperkinesia diagnosed during the same 30-month period.
Methods
The database of the Mayo Clinic Florida Movement Disorders Electrophysiology Laboratory (MDEL) was searched for all patients referred for possible OM or OT from 6/2010-12/2012. All available clinical records and archived surface electromyographical data for these patients were reviewed and analyzed.
Results
32 patients with OM (mean age 74 years), 8 with primary OT (mean age 71), and 55 with neither orthostatic hyperkinesia (NOH) (mean age 68) were identified. All OT patients and 84% each of OM and NOH patients complained of involuntary leg movements while standing, e.g., “shaking,” “trembling,” or “jerking.” All OM and OT patients experienced symptomatic and electrophysiological abatement or attenuation of their leg hyperkinesias by leaning forward onto an object while standing.
Conclusions
OM has some similarities to OT, including causing “shaky legs” subjectively in standing older patients. Novel data from this work include that, as in OT, OM essentially abates when patients remove their weight from their legs. This shared isometric phenomenon may reflect that OT and OM are on a pathophysiological continuum. Further, many patients who complain of their legs “shaking” while standing may have neither OT nor OM. Surface electromyography may be a useful adjunct in extrapolating patients complaining of “shaky legs.”
Keywords: orthostatic myoclonus, orthostatic tremor, freezing of gait, Parkinson disease, higher-level gait disorder, electromyography, neurophysiology
Introduction
Orthostatic myoclonus (OM) is a recently recognized hyperkinesia that may exacerbate gait instability.1, 2 Like orthostatic tremor (OT), OM is primarily noted in patients over 65 years of age in leg muscles while standing, may produce “shaky legs,” and is diagnosed definitively with surface electromyography (SEMG).1-3 However, unlike “primary” OT patients, those with OM (and “OT-plus”) have abnormal gaits.1, 3-5 Most reported OM patients, as well as those with OT-plus, have had some parkinsonian gait and balance characteristics (e.g., stride reduction and/or freezing of gait, postural instability on “pull” testing, etc.), although their clinical diagnoses have been variable.1,2,4,5
Since the original two reports on OM describing 18 patients1,2, no others have been published. This article is a retrospective study of observations garnered over two-and-a-half years of assessing 95 patients for possible OT or OM clinically and with SEMG. Analysis of this cohort sheds light on similarities and disparities between OM, primary OT, and neither orthostatic hyperkinesia (NOH), prompting some provocative questions regarding gait and balance dysfunction in older patients, and potentially offering insights into gait and balance pathophysiology.
Methods
The author searched the Mayo Clinic Florida Movement Disorders Electrophysiology Laboratory's (MDEL's) database for all patients referred for possible OT or OM from 6/2010 through 12/2012, and each patient's chart was reviewed. OM was identified using the electrophysiological criteria established by Matsumoto and colleagues. These include semi-rhythmical bursts of motor activity with durations less than 100 ms that occur consistently over several leg muscles simultaneously during standing and are not present during sitting or a “marked increase of myoclonic burst frequency in leg muscles upon standing.1” OT, like other true tremors, has a consistent frequency, i.e., is rhythmical, and was diagnosed based on the guidelines published by the Movement Disorders Society.4,5
Surface 8-channel EMG recordings were made with 1.5 meter lead CareFusion (Middleton, WI) disposable, adhesive disk electrodes that were applied, then taped to the skin 2-4 centimeters apart over arm and leg muscle bellies after the overlying skin had been lightly abraded with rubbing alcohol and, if necessary, shaved. Signals were amplified and filtered at a bandpass of 30 Hz to 3 kHz using a Nicolet Viking (Madison, WI) machine. Twenty divisions per screen were present, and the sweep speed was 50 or 100 milliseconds (ms), with the gain set typically at 100-200 microvolts (uV). Surface EMG (SEMG) waveforms were visualized and heard continuously throughout the recordings. The auditory signature of OT is similar to the sound of a helicopter's rotating blades4 (video 1). In isolation, myoclonic bursts sound as fasciculations do on needle electromyography, and when occurring semi-rhythmically in multiple muscles, they resemble the sound of popcorn heating (video 2). Both OM and OT sound very different from non-specific tonic and semi-rhythmical activity lasting hundreds of milliseconds. Each study lasted a minimum of 15 minutes. The standard montage used consisted of the following muscles: unilateral triceps, wrist extensors (WEs), abductor digiti minimi (ADM), vastus lateralis (VL), and bilateral tibialis anteriors (TAs) and medial gastrocnemii (MGs). In each case, activity was recorded with the patient sitting comfortably on the edge of an examination table with their arms in their lap and their legs dangling above the floor. They were then asked to position themselves with their shoulders, arms, wrists, and knees extended, and their feet dorsiflexed, simultaneously. The patients then were instructed to perform finger-nose testing with the limb attached to recording electrodes. With the patients' hands at rest and extended, the examiner tapped on their phalanges to assess for stimulus-sensitive myoclonus. Prior to the recordings, the same maneuvers were examined to aid in choosing the upper extremity to record from, i.e., if jerks and/or tremulous activity were more predominant on one side, then those limbs were selected as the primary recording side. Finally, recordings were made with the patient standing for at least five minutes and then leaning forward onto a chair or walker while continuing to stand. Patients were queried whether they felt involuntary leg movements, and if so, whether these were only apparent while standing, and if these changed when they leaned onto the object. Multiple representative SEMG tracings were printed and saved with each report. Myoclonic bursts were identified per muscle per page and tabulated. Synchronous bursts were defined as two or more bursts occurring in separate muscles within 15 ms of one another.1 Averages of synchrony and frequency were calculated from each patient's recordings.
A Mayo Clinic staff neurologist evaluated each patient. Provisional neurological diagnoses for each patient's gait disorder were made based on current published diagnostic criteria (see Supplemental Tables 1 and 2).6-22 None of the subjects has undergone a brain autopsy; all are still alive. If the diagnostic certainty was less than probable, a question mark in parenthesis was listed after the proposed diagnosis (Supplemental Tables 1 and 2). “Higher level gait disorders” (HLGDs) merit particular explication as there are various designations in the literature of “lower body parkinsonism,” “vascular parkinsonism,” “cautious gait of the elderly,” HLGDs, etc.,13, 19, 22 which are often ambiguous. Here, following Nutt,23 HLGD refers to a gait syndrome characterized by varying degrees of hypokinesia (legs ≫ arms), including potentially freezing of gait (FOG), as well as ataxia and postural instability that is not associated with limb bradykinesia, ataxia, or rigidity on seated or supine testing. The gait ataxia may only manifest on tandem-walking. If neuroimaging of a patient with a HLGD was unremarkable (e.g., does not clearly show extensive white matter disease, frontal lobe atrophy, hydrocephalus, etc., which are known etiologies of this gait disorder), then the diagnosis, as well as the gait, are classified as “HLGD” (Supplemental Tables 1 and 2).
Medications known to cause myoclonus8 were tabulated for each patient to ascertain whether these might have contaminated the SEMG results. With rare exceptions, these medications were initiated before the patients were evaluated at Mayo Clinic Florida.
The study was approved by the Mayo Clinic Institutional Review Board (IRB).
Results
The author clinically examined 30/32 (94%) of the OM patients; 6/8 with OT; 51/55 (93%) with NOH; and performed all of the SEMGs. The data from each cohort are summarized in Supplemental Tables 1-3. Regarding OM, a variety of electrophysiological patterns was evident. Approximately 70% of OM bursts occurred synchronously, with the bilateral TAs being the most commonly affected muscles. Sometimes, synchrony between homologous muscles and a unilateral antagonist was also present. Synchrony between the bilateral TAs and MGs was rare. Also, unilateral, intermittent, semi-rhythmical bursts of motor activity alternating between muscle antagonists at approximately 7 Hz or less, were observed in 53% of patients (present in 21% of the total tracings overall), reminiscent of the firing pattern typical of OT3 (see Figure 1). The OM frequency range was 4-11 Hz in 88% of patients. The median frequency was 7 Hz, but as Matsumoto et al. found, there was great variability in the frequency of myoclonus between lower limb muscles, whether ipsilateral or homologous to each other, as well as in the same muscle over time. Stimulus-sensitive myoclonus was present in only two OM patients. One of these has an ipsilateral 4 Hz rest hand tremor, likely secondary to Parkinson Disease (PD), and the other had normal cortical somatosensory evoked potentials (SEPs) and no long latency reflexes (LLRs) at rest during median nerve stimulation6. Overall, three OM subjects (14, 16, 27) have had repeat SEMGs, separated by over 1.5 years. OM was evident on all studies.
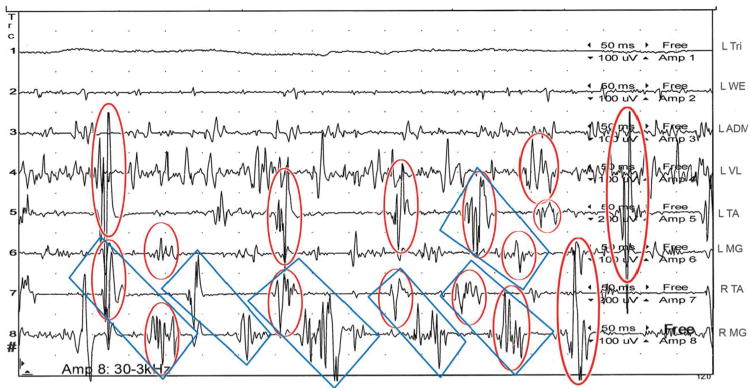
Figure 1.
SEMG tracing lasting one second of Subject 30 standing, intermittently feeling involuntary, “little, unsteady movements, like I am on a boat” within his legs. Semi-rhythmical bursts of motor activity lasting less than 50 ms are ubiquitous, often firing synchronously, particularly in the bilateral TAs (see circled in red). Additionally, semi-rhythmical activity, alternating primarily between the right TA and MG at approximately 7 Hz, is present, and indicated by blue rectangles. Time between each horizontal dot is 50 ms.
L= left; R= right; Tri= triceps; WE= wrist extensors; ADM= abductor digiti minimi; VL= vastus lateralis; TA= tibialis anterior; MG= medial gastrocnemius; ms= milliseconds, uV= microvolts
All OT patients had tremor frequencies between 12-17 Hz, with very consistent synchrony between homologous muscles and the typical alternating firing pattern between muscle antagonists. The tremor frequency in each muscle was virtually identical (i.e. strongly coherent), as has been reported in OT.3 No OT-plus patients were identified
All OT patients and 84% each of the OM and NOH patients reported involuntary leg movements only while standing, usually describing these as “shaky,” “trembly,” or “jerky.” Complaints of their legs feeling like they would “give-out” or “will not hold me up” were described by 28% of OM patients, almost half of whom have suffered idiopathic drop attacks. One OT and NOH patient each complained that their legs would “give-out,” although no drop attacks have been noted in either of these cohorts. All OT and OM patients experienced either utter cessation of their leg hyperkinesias while standing and leaning forward onto an object, or a marked attenuation thereof (see Figure 2); however, whereas 100% of the OT patients then developed the same hyperkinesia in their arms during this maneuver (video 3), only 13% of the OM patients did (video 4).
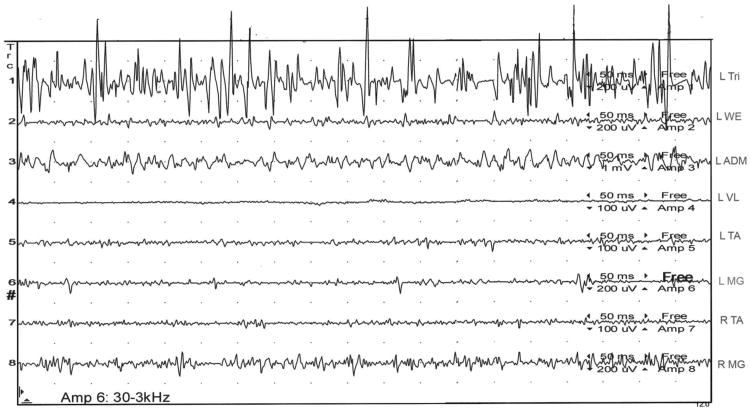
Figure 2.
Subject 30 is now standing and leaning forward on a chair, taking much of his weight off his legs. He asserts his legs feel, “steady and secure” and the “little, unsteady movements and jerks” within them have utterly, subjectively abated. The SEMG tracing lasting one second reveals high amplitude, tonic motor activity in his left triceps and only very low amplitude, tonic activity in his legs.
L= left; R= right; Tri= triceps; WE= wrist extensors; ADM= abductor digiti minimi; VL= vastus lateralis; TA= tibialis anterior; MG= medial gastrocnemius; ms= milliseconds, uV= microvolts
In comparing diagnoses between the OM and NOH patients, 19% of the OM patients had PD, whereas only 7% of the NOH patients did. A Parkinsonism-plus syndrome was present in 27% of the NOH group and 16% of the OM group. Thus, each group had about one-third of patients with either PD or a parkinsonism-plus syndrome. Also, about one-third in each group had microvascular encephalopathy (MVE) (34% of OM and 25% of NOH patients, respectively). A functional gait disorder was at least probable in 16% of NOH patients.20, 21 Finally, the use of medications overall known to potentially cause myoclonus was similar between the OM and NOH cohorts. A full list of the medications taken by the patients in this study can be found in Supplementary Table 2.
Discussion
Over 30 months, 32 patients were diagnosed with OM in the Mayo Clinic Florida MDEL, and 8 were diagnosed with “primary” OT. As noted by other investigators, both conditions are uncommon before the fifth decade of life.1,4-5 OM may be more prevalent than OT, which is considered “rare”3; however, whether OM is actually four times as common as OT undoubtedly will require much larger, prospective, longitudinal studies to ascertain. However, it is possible that OM is more prevalent than OT and may be at least as common in men. Also, unlike “primary” OT, OM typically co-exists with an abnormal gait and impaired postural reflexes.1-4 Four of the OM patients have suffered drop attacks, and none of the OT or NOH patients has, raising the possibility that OM may entail negative, as well as positive myoclonus. Except in the case of asterixis, identifying negative myoclonus on SEMG may prove difficult (Caviness J, personal communication). Nonetheless, theoretically, synchronous, bilateral lower extremity negative myoclonus may produce drop attacks, as has been described in post-anoxic myoclonus patients.24 Patient 10 of Matsumoto and colleagues' series had “frequent paroxysmal collapses while standing,” which perhaps reflected this phenomenon.1
As in patients with OT 4, 5, those with OM typically have symptoms of involuntary leg movements while standing, which do not abate with prolonged standing, and produce increasing instability. Also, like OT patients, the OM patients experience utter or virtually complete abatement of their orthostatic hyperkinesia promptly when leaning forward onto an object, taking weight off of their legs (see Figure 2 and video 4); however, most OM patients do not develop myoclonus in their arms while leaning forward (only 13% in this cohort), unlike OT patients who usually manifest rapid tremor in their arms while doing leaning forward onto an object4, which was universal in this OT group (video 3). Perhaps this is a reflection of the strong coherence and synchrony of motor activity in OT, but not in OM. Also, the intermittent, semi-rhythmical bursts alternating between muscle antagonists that occur at least occasionally in about half of the OM patients is reminiscent of the typical firing pattern of OT, albeit at approximately half the frequency (see Figure 1). Although no patient with both OM and primary OT was recognized, the clinical and electrophysiological similarities between OT and OM raise the possibility that they may share a common, underlying pathophysiological mechanism, involving isometric contraction, supporting Caviness' hypothesis that abnormal hyperkinetic physiology may pass through stages from tremor to myoclonus, depending upon the natural history of the underlying disease.7 Patients with OT-plus syndromes (i.e., a combination of OT and neurological disorders, e.g., progressive supranuclear palsy) 5 may reflect this continuum. Serial SEMGs of OT-plus patients might reveal both tremor and myoclonus, but have not been performed yet (Chen R, personal communication). Future such studies could prove illuminating.
Although there are many uncertainties about the diagnoses of the OM patients in this cohort, each had neurological abnormalities, which could be relevant regarding their gait and station dysfunction. Why certain patients with a parkinsonian gait disorder manifest OM and others do not is currently an enigma. The overall similar use of medications known to induce myoclonus within the OM and NOH groups suggests iatrogenesis is unlikely the reason. It is also unlikely that OM is merely an involuntary, compensatory balance “sway” mechanism in patients with parkinsonian gait disorders, just as it is improbable that primary OT is a normal, compensatory phenomenon.3-5
This work contains several limitations, some of which are inherent in any retrospective study. The overall number of OM patients is small; however, it is twice the number in the original study by Matsumoto and colleagues, and only eighteen OM patients have been reported to date.1, 2 Also, the author performed all of the SEMGs and clinically examined 92% of the patients, which hopefully enhanced the uniformity of their evaluations. On the other hand, this may have contributed to patient selection bias, as the author's practice is heavily-weighted toward patients with gait disorders over the age of 65 years. In addition, given that OT was identified almost thirty years ago,4 and OM is likely far less well known, perhaps fewer OT patients are being referred to tertiary care centers, thus artificially raising the percentage of OM patients with an orthostatic hyperkinesia at our center.
The absence of autopsy-verified diagnoses and the overall diagnostic uncertainty of the OM and non-OM patients, many of whom likely have multi-factorial neurological disease, complicate drawing firm conclusions about the pathophysiology of OM. Also, only some of the more recently studied OM patients have had more comprehensive neurophysiological studies (e.g., LLRs and cortical SEPs). Neither of the OM patients (subjects 29 and 30) studied had “giant” cortical SEPs or a “C” response at rest, making it less likely that their myoclonic generator is cortical6, which is also consistent with the rarity of stimulus-sensitive myoclonus in OM patients. However, far more OM patients will require in-depth neurophysiological evaluations to help establish whether OM has more than one potential generator. Additionally, comparing the findings from computerized dynamic posturography (CDP) between OT, OM, and NOH patients may provide insights into underlying pathophysiological mechanisms of imbalance. For example, the leg “trembling” of the OM and OT patients is reminiscent of that experienced by patients with FOG, which may represent multiple anticipatory postural adjustments (APAs) 25. From extant descriptions, these latter movements are likely a form of myoclonus25, which is perhaps not coincidental.
The current lack of long-term follow-up to ascertain whether OM responds to medications hopefully will also be addressed in future prospective studies. The hypothesis that OM and OT share similar pathophysiological mechanisms may indicate that clonazepam could be useful in some patients, as found by Matsumoto et al.1 Interestingly, anecdotal evidence suggests levetiracetam may ameliorate OM, which may be beneficial given its more attractive side effect profile and pharmacokinetics.26 Four OM patients in this cohort (subjects 13, 16, 19, 27) have subjectively benefited from levetiracetam; none of the subjects in this study concluded that levetiracetam was unhelpful. However, only one (subject 27) has been followed for over a year.
An alternative, non-pharmacological strategy is to have patients with OM utilize a gait-assistive device, given that their lower limb hyperkinesias abate when they lean forward, taking some of their weight off their legs. Indeed, during their initial consultation, OM patients within this cohort commonly reported a substantial improvement in their gait and balance, including a subjective feeling of heightened security when using a shopping cart. Many of these individuals are now using a four-wheeled walker and report a substantial increase in their daily ambulation and reduction in falls. Prospective studies of OM and OT patients utilizing this simple intervention could prove useful.
In conclusion, OM may be a more common cause of “shaky legs” in older patients than primary OT, and NOH actually may produce these symptoms more commonly than either. OM is likely under-diagnosed given that its recognition requires SEMG, which is not routinely utilized in the evaluation of patients with gait and balance disorders. Perhaps, as Nutt suggests, diagnostic techniques such as SEMG should be used more often in evaluating patients with complex gait and balance disorders.27 Fortunately, many standard EMG machines now contain the software for SEMG, allowing such studies to be performed by clinicians with expertise in neurophysiology, who do not have access to sophisticated gait and balance laboratories.28 Hopefully, by heightening awareness of OM among clinicians that evaluate patients with gait disorders, this preliminary work will spur an increase in the utilization of SEMG in elucidating the cause(s) of gait and balance dysfunction and idiopathic drop attacks, as well as promote research into the etiology, pathophysiology, and treatment of orthostatic hyperkinesias.
Supplementary Material
Video 1: A patient with OT manifests a continuous 15 Hz lower extremity tremor just after standing. The “helicopter” sound is readily audible.
Video 2: A patient with OM demonstrates multi-focal lower extremity myoclonus upon standing. The intermittent myoclonic bursts sound very different from OT and resemble fasciculation potentials on needle EMG.
Video 3: The OT patient has immediate cessation of the 15 Hz leg tremor after leaning forward onto a chair, and the 15 Hz tremor re-emerges in her upper limbs, particularly in the triceps (channel one).
Video 4: The OM patient has abatement of the lower limb myoclonus while leaning onto her walker, with the motor activity devolving into primarily tonic bursts lasting hundreds of milliseconds. There are rare myoclonic bursts in the triceps (channel one).
Highlights.
As in orthostatic tremor (OT), orthostatic myoclonus (OM) essentially abates when patients remove their weight from their legs.
OM may be a more common cause of “shaky legs” in older patients than primary OT.
Many older adults with “shaky legs” will not have OT or OM
Diagnostic techniques such as SEMG should be used more often in evaluating patients with complex gait and balance disorders.
Acknowledgments
Funding: The author is grateful for the critical reviews of this manuscript by Dr. John Caviness and Dr. Mark Hallett. He is also much indebted to Mrs. Kelly Viola, ELS who provided editing of the highest caliber.
Dr. van Gerpen is the sole author of this manuscript. He was responsible for the entire manuscript.
Footnotes
Competing Interests: Dr. van Gerpen has no competing interests associated with this manuscript.
Financial Disclosures: Dr. van Gerpen receives research funds from the Mayo Clinic CR program and NIH/NINDS P50 NS072187. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Glass GA, Ahlskog JE, Matsumoto JY. Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology. 2007;68:1826–1830. doi: 10.1212/01.wnl.0000260225.46732.af. [DOI] [PubMed] [Google Scholar]
- 2.Leu-Semenescu S, Roze E, Vidailhet M, et al. Myoclonus or tremor in orthostatism: an under-recognized cause of unsteadiness in Parkinson's disease. Mov Disord. 2007;22:2063–2069. doi: 10.1002/mds.21651. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD, Rothwell JC, Day BL, et al. The physiology of orthostatic tremor. Arch Neurol. 1986;43:584–587. doi: 10.1001/archneur.1986.00520060048016. [DOI] [PubMed] [Google Scholar]
- 4.Jones L, Bain PG. Orthostatic tremor. Pract Neurol. 2011:240–243. doi: 10.1136/practneurol-2011-000022. [DOI] [PubMed] [Google Scholar]
- 5.Mestre TA, Lang AE, Ferreira JJ, et al. Associated movement disorders in orthostatic tremor. J Neurol Neurosurg Psychiatry. 2012;83:725–729. doi: 10.1136/jnnp-2012-302436. [DOI] [PubMed] [Google Scholar]
- 6.Shibasaki H, Hallett M. Electrophysiological studies of myoclonus. Muscle Nerve. 2005;31(2):157–174. doi: 10.1002/mus.20234. [DOI] [PubMed] [Google Scholar]
- 7.Caviness JN. Myoclonus and neurodegenerative disease-what's in a name? Parkinsonism Relat Disord. 2003;9:185–192. doi: 10.1016/s1353-8020(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 8.Caviness JN. Pathophysiology and treatment of myoclonus. Neurol Clin. 2009;27:757–777. doi: 10.1016/j.ncl.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status: correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 10.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Radford NR. Normal pressure hydrocephalus. Neurol Clin. 2007;25:809–832. doi: 10.1016/j.ncl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Zijlmans JC, Daniel SE, Hughes AJ, Révész T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 14.Daniel SE, de Bruin VM, Lees AJ. The clinical and pathological spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain. 1995;118:759–770. doi: 10.1093/brain/118.3.759. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford C, Lees AJ. Accuracy of clinical diagnosis of idiopathic PD: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz CG, Stebbins GT, Chmura TA, Fahn S, Poewe W, Tanner CM. Teaching program for the Movement Disorder Society-sponsored revision of unified Parkinson's disease rating scale (MDS-UPDRS) Mov Disord. 2010;25:1190–1194. doi: 10.1002/mds.23096. [DOI] [PubMed] [Google Scholar]
- 17.Sorbi S, Hort J, Erkinjuntti T, et al. EFNS-ENS guidelines on the diagnosis of and management of disorders associated with dementia. Eur J Neurol. 2012;9:1159–1179. doi: 10.1111/j.1468-1331.2012.03784.x. [DOI] [PubMed] [Google Scholar]
- 18.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 19.Huber-Mahlin V, Giladi N, Herman T, Perez C, Gurevich T, Hausdorff JM. Progressive nature of a higher level gait disorder: a 3-year prospective study. J Neurol. 2010;257:1279–1286. doi: 10.1007/s00415-010-5507-6. [DOI] [PubMed] [Google Scholar]
- 20.Lang AE, Voon V. Psychogenic movement disorders: past developments, current status, and future directions. Mov Disord. 2011;26:1175–1182. doi: 10.1002/mds.23571. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S, Williams PJ. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 22.Van Gerpen JA. Office assessment of gait and station. Semin Neurol. 2011;31:78–84. doi: 10.1055/s-0031-1271309. [DOI] [PubMed] [Google Scholar]
- 23.Nutt JG. Higher-level gait disorders: an open frontier. Mov Disord. 2013;28:1560–1565. doi: 10.1002/mds.25673. [DOI] [PubMed] [Google Scholar]
- 24.Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord. 2000;15(Suppl 1):8–13. doi: 10.1002/mds.870150703. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankovic SM, Dostic M. Choice of antiepileptic drugs for the elderly: possible drug interactions and adverse effects. Expert Opin Metab Toxicol. 2012;8:81–91. doi: 10.1517/17425255.2012.645535. [DOI] [PubMed] [Google Scholar]
- 27.Nutt JG. Classification of gait and balance disorders. Adv Neurol. 2001;87:135–141. [PubMed] [Google Scholar]
- 28.McKeon A, Pittock SJ, Glass GA, et al. Whole-body tremulousness: isolated generalized polymyoclonus. Arch Neurol. 2007;64:1318–1322. doi: 10.1001/archneur.64.9.1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: A patient with OT manifests a continuous 15 Hz lower extremity tremor just after standing. The “helicopter” sound is readily audible.
Video 2: A patient with OM demonstrates multi-focal lower extremity myoclonus upon standing. The intermittent myoclonic bursts sound very different from OT and resemble fasciculation potentials on needle EMG.
Video 3: The OT patient has immediate cessation of the 15 Hz leg tremor after leaning forward onto a chair, and the 15 Hz tremor re-emerges in her upper limbs, particularly in the triceps (channel one).
Video 4: The OM patient has abatement of the lower limb myoclonus while leaning onto her walker, with the motor activity devolving into primarily tonic bursts lasting hundreds of milliseconds. There are rare myoclonic bursts in the triceps (channel one).