Abstract
Epidemiological and laboratory studies indicate that dietary selenium protects against prostate cancer. Results from clinical trials suggest that selenium-enriched yeast (SY) but not selenomethionine (SeMet) may be effective at reducing prostate cancer risk. Our objectives were to directly compare for the first time the effects of SeMet and SY on prostate cancer relevant biomarkers in men. We performed a randomized double blind, placebo-controlled trial of SY (200 or 285 µg/day) and SeMet (200 µg/day) administered for 9 months in 69 healthy men. Primary endpoints included blood levels of selenium-containing compounds and oxidative stress biomarkers (urine 8-hydroxy-2’-deoxyguanosine [8-OHdG] and 8-iso-prostaglandin-F2α [8-iso-PGF2α] and blood glutathione [GSH]). Secondary endpoints included plasma glucose and PSA levels. Compliance was high in all groups (>95%). Plasma selenium levels were increased 93%, 54%, and 86% after 9 months in SeMet and low and high dose SY groups, respectively, and returned to baseline levels after a 3 month washout (P<0.05). Levels of 8-OHdG and 8-iso-PGF2α, were decreased 34% and 28%, respectively, after 9 months in the high dose SY group (P<0.05). These decreases were greatest in individuals with low baseline plasma levels of selenium (<127 ng/ml). No changes in serum PSA or blood glucose and GSH were observed. Overall, we showed for the first time, reductions in biomarkers of oxidative stress following supplementation with SY but not SeMet in healthy men. These findings suggest that selenium-containing compounds other than SeMet may account for the decrease in oxidative stress.
Keywords: selenium-enriched yeast, selenomethionine, prostate cancer, oxidative stress
Introduction
Prostate cancer (PC) is the second leading cause of cancer-related deaths in men (1). Diet-derived agents including selenium have been shown to have chemopreventive potential against PC (2, 3). Epidemiological and laboratory investigations have shown that dietary selenium is protective against the development of cancer at many sites including the prostate (3–7). In the Nutritional Prevention of Cancer (NPC) study (8), supplementation with selenium-enriched yeast (SY) in men was associated with an ~50% reduction in cancer morbidity and mortality, including a 63% decrease in PC incidence, with subjects having low baseline plasma selenium levels (<122 ng/ml) showing the greatest benefit (9). However, in the Selenium and Vitamin E Cancer Prevention Trial (SELECT), which was designed to test the protective effects of selenomethionine (SeMet) and vitamin E individually and in combination against PC (10), no protection was observed, supporting the notion that SeMet is a form of selenium which is not highly active against PC (10). This is in line with laboratory studies which have consistently demonstrated that while multiple organic forms of selenium have anticancer activity, SeMet alone was relatively inactive (11). These results suggest that while SeMet represents a major form of selenium in SY, it is not likely the form responsible for the chemopreventive properties of SY.
While results from previous studies support the chemoprotective efficacy of SY (8, 9) but not SeMet (10), no direct comparison between SY and SeMet supplementation in men has been reported. Direct comparisons in laboratory animals have revealed differing results. In the rat, McCormick et al (12) reported the lack of protective effect of both SeMet and SY against the development of PC. In dogs, no differences were observed in tissue selenium levels or in the levels of several biomarkers of prostate epithelial DNA damage, proliferation or apoptosis between SY and SeMet supplemented groups (13). In contrast to the above mentioned studies, supplementation with different forms of selenium (selenite, SeMet and SY) resulted in clear differences in gene expression profiles in mice; SY was the only form associated with a pattern of decreased DNA damage (14).
In order to directly compare the effects of SY and SeMet for the first time in humans, we conducted a double blind, placebo-controlled, randomized trial in healthy adult men. Our primary objectives were to determine and compare the effects of these different forms of selenium on PC relevant biomarkers including blood selenium levels and blood and urinary biomarkers of oxidative stress. A secondary objective in this trial was to determine the impact of SY and SeMet on PSA and glucose levels.
Methods
Trial design
The study (ClinicalTrials.gov: NCT01112449) was conducted with approval from the Institutional Review Boards of the Penn State University (PSU) College of Medicine and Robert Wood Johnson Medical School. Recruitment, interviewing and sample collections were performed at three sites: Penn State Hershey Cancer Institute, Hershey, PA, PSU Clinical Research Center, State College, PA and the Cancer Institute of New Jersey, New Brunswick, NJ. Subjects were recruited from local surrounding areas using fliers, newspaper and radio advertisements, online announcements and word of mouth. Potentially eligible subjects as assessed by telephone prescreening, were interviewed in the clinic after providing informed consent. Each subject was screened for eligibility based upon the following criteria: Healthy male nonsmokers, 20–79 years of age, normal serum PSA based upon the age- and race-specific cutoffs defined in the 2009 AUA Clinical Guidelines (15), no history or evidence of diabetes, PC, liver or kidney disease, and not taking >50 µg/day selenium as a dietary supplement.
Eligible subjects were randomly assigned with equal probability using a computer-generated list prepared by the biostatistician to one of 4 treatment groups: Placebo (non-selenized-yeast), low dose SY (SY200, 200 µg selenium/d), high dose SY (SY285, 285 µg selenium/d), and SeMet (200 µg selenium/d) (Figure 1) by the investigational pharmacists. The randomization status was blinded to all others in the study except to the Penn State University investigational pharmacists until a patient had finished the study and until near the end of the study upon IRB approval. The dose and form of selenium in the SY200 and SeMet groups were selected to match those used in the NPC (8) and SELECT (10) studies, respectively. The dose of selenium in the SY285 group was selected so that the levels of SeMet would be equivalent to that in the SeMet group, based upon a SeMet concentration in SY of 70% (16). Selenium-enriched yeast (SelenoExcell®) was provided by Cypress Systems, Inc. (Madera, CA); SeMet was purchased from Sabinsa Corporation (Piscataway, NJ) and packaged by Cypress Systems, Inc. Placebo, SeMet, and SY capsules were identical in appearance. At baseline, a structured questionnaire was administered to each subject to collect information on demographics, medical history, medications, dietary supplements, alcohol consumption and cigarette smoking history. Study participants were remunerated with $35 per visit.
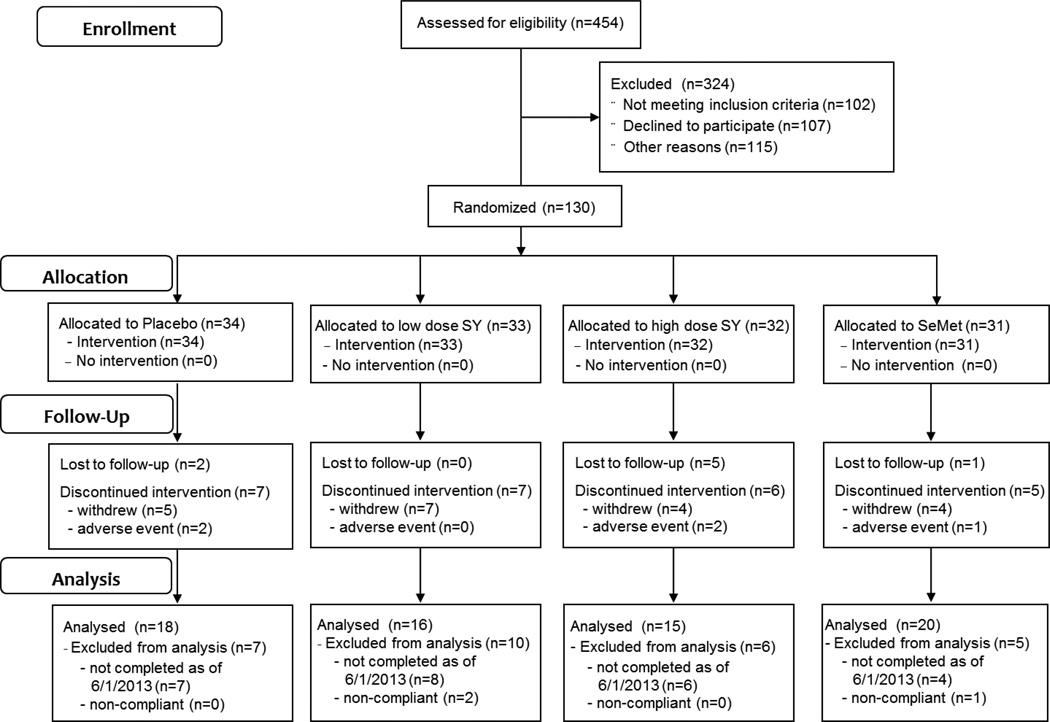
Figure 1.
Subject flowchart summarized according to the Consolidated Standards of Reporting Trials.
After randomization participants were provided their respective capsules along with instructions for usage. After 3, 6, and 9 months participants returned to the clinic to receive new and return unused capsules. At 9 months, all subjects were switched to placebo for the final 3 months of the trial (washout period). At baseline and after 3, 6, 9 and 12 months, biological samples including blood and urine were collected and processed as described below.
Outcome measures included plasma total selenium at baseline, and 3, 9 and 12 months; blood free and protein bound GSH and urinary 8-OHdG and 8-iso-PGF2α at baseline and 9 and 12 months. Secondary outcomes included blood glucose and serum PSA at baseline, and 3, 6, 9 and 12 months as well as selenium speciation in plasma at baseline and 9 months.
Collection and processing of biological samples
Fasting venous blood samples were collected between 9:00 am and 3:30 pm into either plain or EDTA-containing tubes and immediately placed on ice. An aliquot of whole blood was removed from the EDTA tube for analysis of glutathione. Remaining samples were centrifuged at 4°C and resulting plasma or serum was aliquoted and immediately frozen at −80°C. Packed red cells were washed 3× in saline and stored at −80°C. Urine was placed immediately on ice, aliquoted and frozen at −80°C.
Analysis of blood and urine markers
Plasma total selenium levels were determined by atomic absorption spectrophotometry as described previously (17).
Selenium speciation in plasma
Plasma SeMet, methylselenocysteine (MSC), selenate and selenite were analyzed by Ion Chromatography Inductively Coupled Plasma Collision Reaction Cell Mass Spectrometry by Applied Speciation and Consulting, LLC (Bothell, WA).
Glutathione and glutathionylated proteins
Total glutathione (GSH and glutathione disulfide) and protein bound GSH was analyzed as described previously (18, 19). Hemoglobin was determined by spectrophotometrically using Drabkin’s reagent (20).
8-Iso-prostaglandin-F2α (8-iso-PGF2α) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in urine were analyzed by ELISA Cayman Biochemical (Cat. Nos. 516351 and 589320; Ann Arbor, MI, USA). Creatinine was determined by reaction with picrate as described previously (21).
PSA and glucose
Serum PSA was determined using the ADVIA Centaur XP Immunoassay System (Siemens Healthcare Diagnostics Inc., Deerfield, IL). Blood glucose levels were determined using an Ortho Vitros 5600 analyzer.
Statistical analysis
Comparison of plasma selenium levels at each time point between groups was performed by Analysis of Variance with adjustment of multiple comparisons using Tukey’s method. Plasma levels of selenium metabolites were compared between baseline and 9 months using the Welch Two Sample t-test (22). Changes in serum PSA, plasma glucose and blood GSH and protein bound GSH and urine 8-isoprostane and 8-OHdG levels from baseline at different time points were compared between the 4 treatment groups using Analysis of Variance. Changes from baseline for urinary 8-OHdG and 8-isoprostane at 9 months were compared for each treatment group by baseline plasma selenium level tertile using Analysis of Variance.
Results
Study subject characteristics
The clinical phase of the study was conducted from May, 2008 until June, 2013. A total of 130 subjects were randomized, of which 69 were included in the analysis (Figure 1). Of the 61 randomized subjects not included in the analysis, 25 discontinued intervention prior to completion, 8 were lost to follow-up, 25 had not completed the protocol as of June 1, 2013 and 3 were excluded from analysis due to lack of compliance (Figure 1). The characteristics of these study participants are summarized in Table 1. All subjects were non-smokers (nonsmoker for at least for 1 yr prior to entry into the study) and had no history of chronic disease or high dose antioxidant supplement usage. Subjects ranged in age from 23 to 78 years of age (mean ± SD = 51.1 ± 14.0 years). No significant differences were observed between subjects by age, weight or BMI between treatment groups. A total of 70% of subjects fell within the normal to overweight categories with 30% being obese (BMI > 30).
Table 1.
Study Subject Characteristics at Baseline
| Group | N | Age (yr) | Race/Ethnicity n (%) |
BMI (kg/m2) |
|---|---|---|---|---|
| Placebo (plain yeast) | 18 | 48.1 ± 14.6 (22–70) | White: 17 (94%) | 30.0 ± 4.79 (22.0–38.3) |
| Black: 1 (6%) | ||||
| Asian: 0 (0%) | ||||
| SY (200 µg/d) | 16 | 50.7 ± 16.2 (23–78) | White: 15 (94%) | 28.0 ± 3.20 (22.4–34.9) |
| Black: 1 (6%) | ||||
| Asian: 0 (0%) | ||||
| SY (285 µ g/d) | 15 | 51.3 ± 12.0 (25–72) | White: 13 (87%) | 27.8 ± 3.14 (23.7–34.2) |
| Black: 1 (7%) | ||||
| Asian: 0 (0%) | ||||
| SeMet (200 µg/d) | 20 | 54.0 ± 13.4 (30–75) | White: 17 (85%) | 28.5 ± 3.79 (23.0–36.4) |
| Black: 1 (5%) | ||||
| Asian: 2 (10%) |
Values are mean ± SD (range)
Compliance and Adverse Effects
A total 5 of subjects (2 in the placebo group, 2 in the SY285 group and 1 in the SeMet group) were removed from protocol due to adverse events: High PSA (1, SY285 group), PC (1 in SY285 group and 1 in SeMet group), and high blood glucose levels (2 in Placebo group). Among the participants that completed the study, no serious adverse effects were reported regardless of arm. All potential adverse events reported were minor (e.g., headaches and colds) and none were attributed to protocol treatment. Compliance was consistently high when assessed by either pill count or patient diary entries. Percent compliance was 97.0 ± 4.7 (mean ± SD) in all subjects and did not differ between treatment arms (placebo, 95.9 ± 5.4; SY200, 97.8 ± 3.9; SY285, 96.1 ± 6.3; SeMet, 98.0 ± 3.1).
Plasma selenium levels
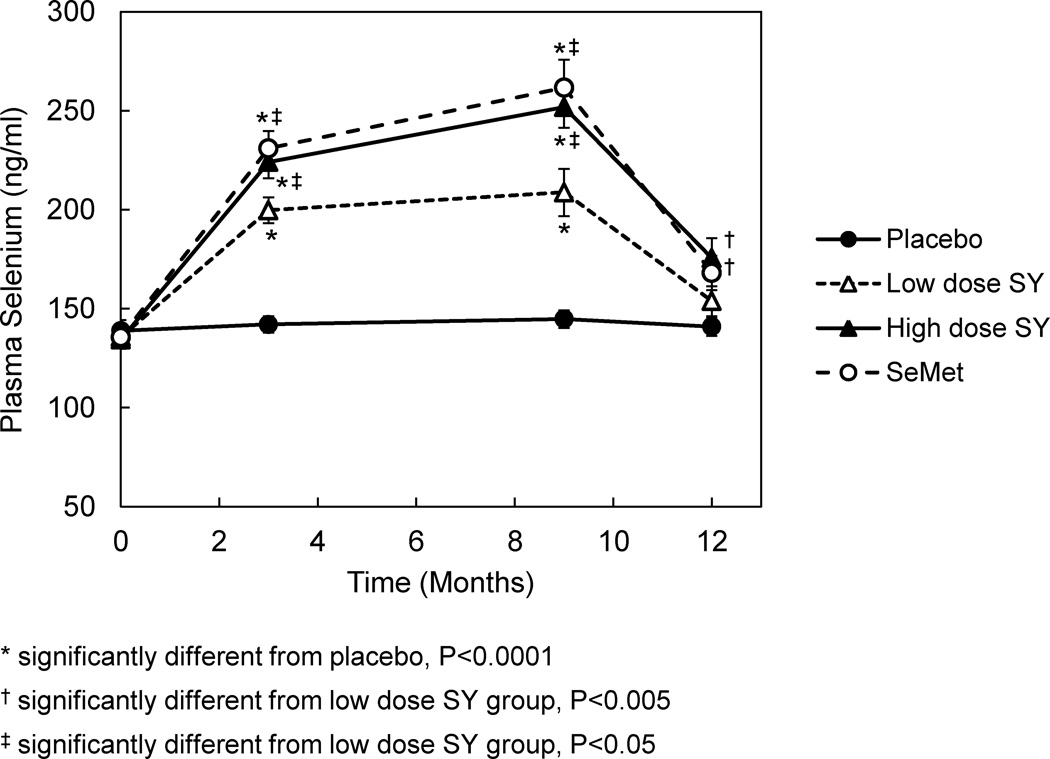
Plasma selenium levels were not different between groups at baseline (Figure 2) and were consistent with those reported in the SELECT trial (10). In all selenium-treated groups, there was a progressive increase in levels from 3 to 9 months followed a return to baseline after the 3-month washout. In the SY groups, the increases were dose dependent reaching a maximum increase above baseline of 86% in the SY285 group and 54% in the SY200 group. When comparing the change from baseline between the four groups for months 3, 9 and 12 separately, significant differences were observed between all 3 selenium groups and placebo (P<0.0001) and between both the SeMet and SY285 groups and the SY200 group, respectively (P<0.05). At 12 months, statistical differences were observed between both SeMet and SY285 groups and placebo only (P<0.001).
Figure 2.
Effect of supplementation with SeMet or SY on plasma selenium levels in adult men. Subjects were randomized to placebo (n=18), 200 µg/d SY (n=16), 285 µg/d SY (n=15) or 200 µg/d SeMet (n=20). Supplementation continued for 9 months followed by a 3 month washout. Plasma total selenium levels were assessed by atomic absorption spectrophotometry. Symbols and bars represent mean and standard error values, respectively.
Plasma Selenium Speciation
Selenium speciation analyses were conducted in plasma samples collected at baseline and after 9 months from a subset of subjects randomly selected from the SeMet and high-dose SY groups (n=5 per group) (Table 2). These groups were selected based upon the equivalent doses of SeMet. Selenite was detected in all samples, whereas, selenate and MSC levels were below the limits of detection. SeMet was not detected in baseline samples but was detected in all 9-month samples. After 9 months of supplementation, significant increases from baseline were observed for SeMet and selenite in both groups (P<0.001). Overall, increases in SeMet and selenite could only account for 0.8% and 5.1%, respectively, of the increases observed for selenium at 9 months. Increases in SeMet levels from undetectable at baseline to 0.74 ng/ml in the SeMet group and 0.55 ng/ml in the SY285 group were not significantly different between groups. Likewise, increases in selenite levels of 166% in the SeMet group and 88% in the SY285 group were not significantly different between groups.
Table 2.
Plasma Selenium Speciation
| Plasma Concentration (ng/ml)* | ||||
|---|---|---|---|---|
| Analyte‡ | Group | Baseline | 9 Months | Change from baseline |
| Selenium | SeMet | 139 ± 7.0 | 256 ± 14.9† | 117 ± 13.2 |
| SY285 | 129 ± 8.7 | 256 ± 26.9† | 128 ± 30.2 | |
| SeMet | SeMet | <0.12 | 0.736 ± 0.16† | 0.616 ± 0.16 |
| SY285 | <0.12 | 0.550 ± 0.07† | 0.430 ± 0.07 | |
| Selenite | SeMet | 2.97 ± 0.22 | 7.90 ± 0.37† | 4.93 ± 0.42 |
| SY285 | 4.47 ± 0.49 | 8.43 ± 0.32† | 3.96 ± 0.24 | |
Values are mean ± SE (n=5).
Significantly different from baseline (P<0.001).
Selenate and methylselenocysteine concentrations were below detection limits (0.12 ng/ml for selenate and 0.16 ng/ml for methylselenocysteine) in all samples.
Biomarkers of oxidative stress
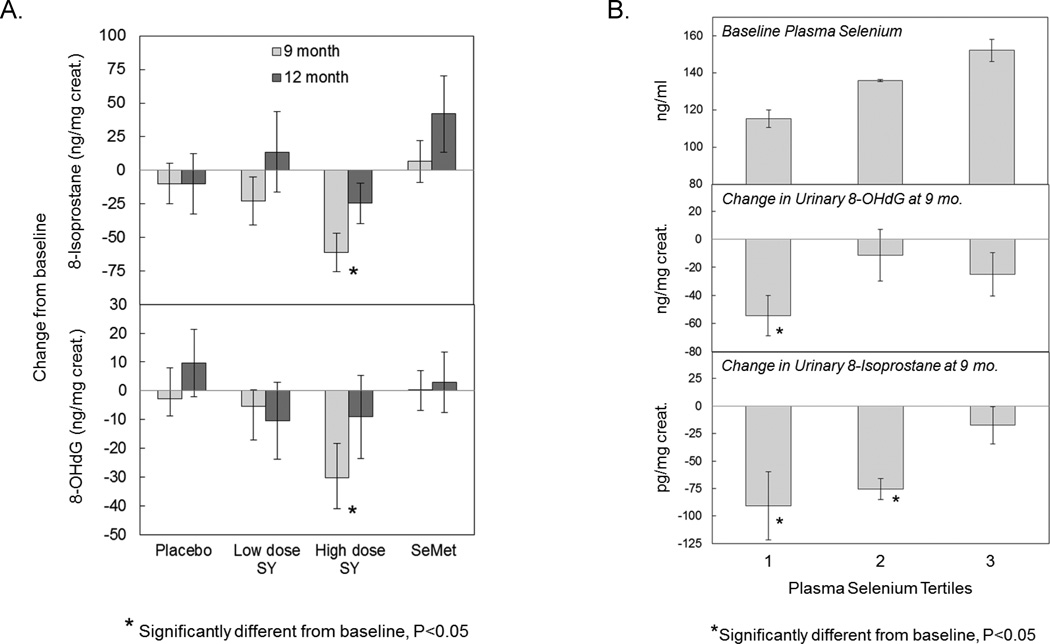
Levels of 8-iso-PGF2α in urine were measured as a biomarker of lipid peroxidation (Figure 3A, upper panel). A dose responsive decrease was observed for the SY groups reaching a maximum decrease of 28% occurring in the SY285 group after 9 months (P<0.05). Levels returned to baseline after the 3 month washout. No significant changes were observed in the SeMet, SY200 or placebo groups.
Figure 3.
Effect of supplementation with SeMet or SY on urinary biomarkers of oxidative stress in healthy men. Subjects were randomized to placebo (n=18), 200 µg/d SY (n=16), 285 µg/d SY (n=15) or 200 µg/d SeMet (n=20). Urinary levels of 8-iso-PGF2α and 8-OHdG were determined by ELISA and corrected for urinary dilution by dividing by urinary creatinine levels, and plasma selenium, determined by atomic absorption spectrophotometry. A. Urinary levels of 8-iso-PGF2α (upper panel) and 8-OHdG (lower panel) by treatment arm. Bars represent mean change in biomarker values from baseline after 9 and 12 months with associated standard error values. Baseline values for 8-iso-PGF2α were 199 ± 96, 180 ± 87, 217 ± 117, and 206 ± 114 mg/mg creatinine for placebo, SY200, SY285 and SeMet groups, respectively. Baseline values for 8-OHdG were 66.8 ± 22, 72.7 ± 43, 90.1 ± 38, and 70.1 ± 38 mg/mg creatinine for placebo, SY200, SY285 and SeMet groups, respectively. B. Association of reductions in oxidative stress biomarker levels by SY with baseline selenium levels in plasma. Urinary levels of 8-iso-PGF2α and 8-OHdG for subjects in the 285 µg/d SY group were examined by baseline plasma selenium tertile. Mean baseline plasma selenium levels by tertile are provided in the top panel. Mean changes in 8-OHdG (middle panel) and 8-iso-PGF2α (bottom panel) from baseline at 9 months are reported by baseline selenium tertile. Bars represent mean and standard error values. Tertile baseline plasma cutpoints (mg/ml) are 127 and 138.
8-OHdG levels in urine were measured as a biomarker of oxidative damage to DNA (Figure 3A, lower panel). A dose responsive decrease was observed for the SY groups reaching a maximum decrease of 34% in the SY285 group after 9 months (P<0.05). Levels returned to baseline after the 3 month washout. No significant changes were observed in the SeMet, SY200 and placebo groups.
The impact of baseline selenium levels on biomarkers of oxidative stress
Mean changes in urinary 8-OHdG and 8-iso-PGF2α levels at 9 months were compared between baseline selenium tertile groups (Supplementary Table 1). Significant associations were observed in the SY285 group only (Figure 3B). In the lowest tertile of baseline selenium (mean ± SD: 115 ± 10.3 mg/ml) levels of both biomarkers were significantly reduced. In the middle tertile (136 ± 1.6 ng/ml), a significant reduction was observed for 8-iso-PGF2α only. No changes were observed in the highest tertile (152 ± 13.1 ng/ml). The largest decreases in both biomarkers were observed for the lowest tertile.
Glutathione and protein glutathionylation
Levels of free and protein bound GSH were measured in whole blood at baseline and after 9 and 12 months (Table 3). There were no changes in either free or bound levels of GSH were observed in any of the 4 study groups.
Table 3.
Effect of selenium-enriched yeast or SeMet on serum PSA and blood glucose and free and bound GSH in healthy men.
| Change from baseline | |||||
|---|---|---|---|---|---|
| Group | Baseline | 3 mo | 6 mo | 9 mo | 12 mo |
| Glucose (mg/dl)* | |||||
| Placebo | 84.7 ± 2.29 | 1.28 ± 2.01 | 3.06 ± 2.32 | 3.72 ± 2.76 | 4.28 ± 1.79 |
| SY (200 µg/day) | 91.6 ± 1.65 | 0.12 ± 2.08 | 2.69 ± 2.62 | −1.44 ± 2.75 | −1.06 ± 2.70 |
| SY (285 µg/day) | 82.7 ± 2.45 | 0.40 ± 1.89 | −3.67 ± 2.57 | 1.60 ± 5.31 | 1.00 ± 3.09 |
| SeMet (200 µg/day) | 91.0 ± 2.07 | −0.50 ± 0.96 | −1.45 ± 1.03 | −1.75 ± 2.46 | −2.20 ± 2.49 |
| PSA(ng/ml)* | |||||
| Placebo | 1.03 ± 0.12 | 0.07 ± 0.03 | 0.32 ± 0.22 | 0.37 ± 0.26 | 0.05 ± 0.06 |
| SY (200 µg/day) | 1.40 ± 0.29 | 0.14 ± 0.09 | 0.22 ± 0.13 | 0.34 ± 0.30 | 0.74 ± 0.50 |
| SY (285 µg/day) | 0.59 ± 0.06 | 0.05 ± 0.03 | 0.09 ± 0.04 | 0.08 ± 0.04 | 0.06 ± 0.05 |
| SeMet (200 µg/day) | 1.14 ± 0.16 | −0.08 ± 9.09 | 0.06 ± 0.07 | 0.02 ± 0.08 | 0.02 ± 0.07 |
| GSH (µmol/ml) | |||||
| Placebo | 0.94 ± 0.08 | ND | ND | −0.12 ± 0.08 | −0.09 ± 0.09 |
| SY (200 µg/day) | 0.90 ± 0.07 | ND | ND | −0.05 ± 0.08 | −0.01 ± 0.12 |
| SY (285 µg/day) | 0.94 ± 0.09 | ND | ND | −0.02 ± 0.09 | −0.10 ± 0.07 |
| SeMet (200 µg/day) | 0.99 ± 0.07 | ND | ND | −0.22 ± 0.07† | −0.11 ± 0.08 |
| Protein bound GSH (% of total GSH) | |||||
| Placebo | 17.7 ± 1.85 | ND | ND | −1.37 ± 2.40 | −1.14 ± 2.31 |
| SY (200 µg/day) | 16.5 ± 1.64 | ND | ND | −0.39 ± 1.97 | 0.54 ± 2.43 |
| SY (285 µg/day) | 15.7 ± 1.33 | ND | ND | 1.86 ± 2.57 | 4.17 ± 2.09 |
| SeMet (200 µg/day) | 16.8 ± 1.61 | ND | ND | 1.25 ± 1.79 | −0.51 ± 2.34 |
Subjects were randomized to placebo (n=18), 200 µg/d SY (n=16), 285 µg/d SY (n=15) or 200 µg/d SeMet (n=20). Values are mean ± SE.
Eligibility criteria: Glucose <106 mg/dl.; normal PSA based on age at enrollment ≤4.0 ng/ml
Statistically significant from baseline (P<0.05).
ND: not determined
Glucose and PSA
At baseline, all subjects had normal fasting blood glucose and serum PSA levels; no differences were observed for either glucose or PSA between groups (Table 3). In all groups, glucose and PSA levels were each unchanged during the course of the study.
Discussion
This is the first clinical trial aimed at comparing the effect of two different forms of selenium on biomarkers that are likely to influence PC risk in men. In a randomized double-blind, placebo-controlled trial in healthy men, we have observed that SY but not SeMet (the formulation used in the largest PC chemoprevention study (10) ever conducted) was effective at reducing the levels of oxidative stress as assessed by reductions in biomarkers of lipid peroxidation (8-iso-PGF2α) and oxidative damage to DNA (8-OHdG), despite inducing similar increases in plasma selenium levels. Although the mechanisms of chemoprevention by selenium remain unclear, enhanced protection against oxidative stress is thought to be involved (23–27). Selenium supplementation in the form of sodium selenite was previously shown to decrease biomarkers of oxidative stress (lymphocyte 8-oxodG and urinary excretion 8-oxoGua) in individuals carrying the BRCA1 mutation (28). This difference in effectiveness of SY vs. SeMet in protecting against oxidative stress may, in part, help explain the results of previous trials which demonstrated the effectiveness of SY (NPC) (8), but not SeMet (SELECT) (10), at reducing the risk for PC. In fact SY was the only form associated with a pattern of decreased DNA damage in rodents (14).
In the present study we observed that both SeMet and SY increased plasma selenium levels and, for SY, the increases were dose-dependent. The increases observed for the SY285 group were comparable to the SeMet group, despite the total dose of selenium being 85 µg/day greater in the SY285 group. Since the dose of SeMet were the same in both the SeMet and SY285 supplements, these results suggest that SeMet is the form of selenium in yeast that is responsible for enhancing plasma total selenium levels. These results are consistent with previous findings which indicate that SeMet-containing proteins (where SeMet is incorporated non-specifically in place of Met), the most abundant source of selenium in plasma (29–32), are the major source for selenium variation in plasma and that selenoproteins or other forms of selenium (eg. MSC) account for very little of the variation of selenium in plasma (33–35). While we were not able to measure the impact of SY or SeMet on prostate selenium status, in a recent clinical study, SY supplementation was associated with a dose-dependent increase in total selenium levels in prostatic tissues that appeared to be greater than that previously observed with SeMet (36). Collectively, these results indicate that the selenium disposition in plasma may differ from that in the target organ (prostate).
Our current finding that the impact of SY on oxidative stress biomarkers was greatest in men with low baseline plasma selenium levels are consistent with a recent report of SY supplementation in healthy men (37) and provides further support for the importance of baseline selenium in predicting the efficacy of supplementation. Epidemiological investigations have linked low selenium intake and levels with increased risk for PC (38–40) including the recent prospective study in the Netherlands, where a strong association between toenail selenium levels and advanced PC was observed in a population with relatively low levels selenium (6). In the NPC, the greatest protective effects were observed in individuals with low baseline selenium and no beneficial effects were observed in individuals with levels above 122 µg/L (9). This is consistent with the present trial where optimal beneficial effects on oxidative stress biomarkers were observed in individuals in the lowest tertile for baseline plasma selenium (≤127 µg/L). The higher baseline levels of selenium in SELECT participants compared to those in NPC may contribute to the lack of a protective effect of selenium observed in that trial. In a recent reevaluation of SELECT, no association of PC risk was observed with baseline toenail selenium levels (41). However, the baseline levels of toenail selenium in this trial were on average >60% higher than in those in the Netherlands trial such that >80% of the subjects in the Netherlands trial would fall within the lowest quintile of SELECT. Considering the baseline selenium levels observed in these trials, Rayman et al. pointed out that the results of NPC are consistent with those of SELECT (42). The lack of a protective effect of SY (200 µg/day) on PC in high risk men in the recently completed Negative Biopsy Trial may be due to the late stage of disease when intervention was started but may also reflect the relatively higher baseline levels of plasma selenium observed in this trial compared to NPC (43).
While SeMet does not appear to be the active chemopreventive agent in SY, it is currently not known which agents may be responsible for this activity. Interest in MSC as one such agent stems from preclinical studies which show that it is more effective than SeMet at impacting PC related pathways in preclinical models (5). We recently identified a number of proteins that are differentially expressed as a result of selenium enrichment of yeast (44), including the selenium-containing protein elongation Factor 2 (45) and others have identified up to 27 selenium containing compounds in SY (46) which may also be playing a role.
A limitation of this study was the relatively small sample size, especially among men with low selenium at baseline, although adequately powered as a randomized phase II study that can support future larger studies. Also, outcome measures did not include PC development or biomarkers specific for PC risk. Strengths of the study include the randomized double-blind, placebo-controlled design in healthy men and the use of selenium supplements at doses which mimic previously conducted disease outcome trials and also allow for comparison between different selenium forms.
Overall, our study highlights the differences between the effects of SY and SeMet in preventing oxidative stress and support the continued development of SY but not SeMet as a chemopreventive agent. Because the effects of SY appear to be based on baseline levels of plasma selenium, such a chemopreventive approach may be best served if applied to men with low selenium levels as suggested in a recent study (6).
Supplementary Material
Acknowledgements
The authors acknowledge the assistance of the staff of the Community Sciences and Health Outcomes Core and Clinical Trials Office of the Penn State Hershey Cancer Institute in this research. We also thank the nursing staff in the Clinical Research Center, Christopher Hamilton in the Core Endocrine Laboratory, Cheryl Reitzel in the Clinical Trials Office for her assistance with Oncore, and Heather Heisey and Alyse Fazzi in the Investigational Drug Service of the Department of Pharmacy, Penn State University College of Medicine. We also acknowledge the contribution of the staff of the Office of Human Research Services at the Rutgers Cancer Institute of New Jersey and the nursing staff at Clinical Research Center, Penn State University, University Park, PA. We thank Dr. Telih Boyiri for his assistance with all regulatory aspects of this trial.
Financial Support: This work was supported a National Cancer Institute grant (R01CA127729) (PI: KE-B). Selenium speciation analyses were supported by Cypress Systems, Inc. (Madera, CA). Additional clinical support was provided by the Penn State Hershey Cancer Institute through its Clinical Trials Office and Community Sciences and Health Outcomes Core and the Office of Human Research Services at the Cancer Institute of New Jersey.
Abbreviations
- SY
selenium-enriched yeast
- SeMet
selenomethionine
- GSH
glutathione
- 8-OHdG
8-hydroxy-2’-deoxyguanosine
- 8-iso-PGF2α
8-iso-prostaglandin-F2α
- PC
prostate cancer
- NPC
Nutritional Prevention of Cancer
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- SY200
200 µg selenium/d
- SY285
285 µg selenium/d
- MSC
methylselenocysteine
Footnotes
The authors’ responsibilities were as follows: JPR, KE-B, RS, and JL conceived and designed the study with input from all authors; AD, AC, RS, WN, AC, WN, SN, and AMC, conducted the research; JPR, RS, JL, AB, KE-B, and TJH analyzed the data and performed statistical analyses; JPR, RS and KE-B wrote the paper with help from all authors; all authors read and approved the final manuscript.
Conflict of Interest: Selenium yeast supplements (SelenoExcell®) and partial research support for selenium speciation analyses were provided by Cypress Systems, Inc. (Madera, CA). Cypress Systems, Inc. had no role in the study design and conduct, collection, management, analysis, and interpretation of the data, or writing of the report. They also had no access to the database. None of the other authors have any conflicts of interest to disclose.
Contributor Information
John P. Richie, Jr, Departments of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA.
Arun Das, Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA.
Ana M. Calcagnotto, Departments of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA
Raghu Sinha, Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA.
Wanda Neidig, Penn State Hershey Cancer Institute Clinical Trials Office, Pennsylvania State University College of Medicine, Hershey, PA.
Jiangang Liao, Departments of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA.
Eugene J. Lengerich, Departments of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA
Arthur Berg, Departments of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA.
Terryl J. Hartman, Department of Epidemiology, Rollins School of Public Health, Emory University
Amy Ciccarella, Center for Clinical Research, Pennsylvania State University, State College, PA.
Aaron Baker, Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA.
Matthew G. Kaag, Division of Urology, Penn State University College of Medicine, Hershey, PA
Susan Goodin, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ.
Robert S. DiPaola, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
Karam El-Bayoumy, Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: implications for cancer survivors. Cancer Res. 2009;69:2699–2703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutation research. 2001;475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 5.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutation research. 2005;591:224–236. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Geybels MS, Verhage BA, van Schooten FJ, Goldbohm RA, van den Brandt PA. Advanced prostate cancer risk in relation to toenail selenium levels. J Natl Cancer Inst. 2013;105:1394–1401. doi: 10.1093/jnci/djt186. [DOI] [PubMed] [Google Scholar]
- 7.Hurst R, Hooper L, Norat T, Lau R, Aune D, Greenwood DC, et al. Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr. 2012;96:111–122. doi: 10.3945/ajcn.111.033373. [DOI] [PubMed] [Google Scholar]
- 8.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 9.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU international. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 10.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozten N, Horton L, Lasano S, Bosland MC. Selenomethionine and alpha-tocopherol do not inhibit prostate carcinogenesis in the testosterone plus estradiol-treated NBL rat model. Cancer Prev Res (Phila) 2010;3:371–380. doi: 10.1158/1940-6207.CAPR-09-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick DL, Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE. Null activity of selenium and vitamin e as cancer chemopreventive agents in the rat prostate. Cancer Prev Res (Phila) 2010;3:381–392. doi: 10.1158/1940-6207.CAPR-09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters DJ, Shen S, Kengeri SS, Chiang EC, Combs GF, Jr, Morris JS, et al. Prostatic response to supranutritional selenium supplementation: comparison of the target tissue potency of selenomethionine vs.selenium-yeast on markers of prostatic homeostasis. Nutrients. 2012;4:1650–1663. doi: 10.3390/nu4111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barger JL, Kayo T, Pugh TD, Vann JA, Power R, Dawson K, et al. Gene expression profiling reveals differential effects of sodium selenite, selenomethionine, and yeast-derived selenium in the mouse. Genes & nutrition. 2012;7:155–165. doi: 10.1007/s12263-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M, et al. Prostate specific antigen best practice statement: 2009 update. The Journal of urology. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 16.Larsen EH, Hansen M, Paulin H, Moesgaard S, Reid M, Rayman M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. Journal of AOAC International. 2004;87:225–232. [PubMed] [Google Scholar]
- 17.El-Bayoumy K, Richie JP, Jr, Boyiri T, Komninou D, Prokopczyk B, Trushin N, et al. Influence of selenium-enriched yeast supplementation on biomarkers of oxidative damage and hormone status in healthy adult males: a clinical pilot study. Cancer Epidemiol Biomarkers Prev. 2002;11:1459–1465. [PubMed] [Google Scholar]
- 18.Richie JP, Jr, Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin Chem. 1996;42:64–70. [PubMed] [Google Scholar]
- 19.Kleinman WA, Komninou D, Leutzinger Y, Colosimo S, Cox J, Lang CA, et al. Protein glutathiolation in human blood. Biochem Pharmacol. 2003;65:741–746. doi: 10.1016/s0006-2952(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 20.Fairbanks VF, Klee GG. Fundamentals of clinical chemistry. Philadelphia: WB: Saunders; 1987. [Google Scholar]
- 21.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scandinavian journal of clinical and laboratory investigation. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 22.Welch BL. The generalisation of student's problems when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 24.Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Aspects Med. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Arbogast S, Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxidants & redox signaling. 2010;12:893–904. doi: 10.1089/ars.2009.2890. [DOI] [PubMed] [Google Scholar]
- 26.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, et al. The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106(Suppl 1):289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. The Proceedings of the Nutrition Society. 2005;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 28.Dziaman T, Huzarski T, Gackowski D, Rozalski R, Siomek A, Szpila A, et al. Selenium supplementation reduced oxidative DNA damage in adnexectomized BRCA1 mutations carriers. Cancer Epidemiol Biomarkers Prev. 2009;18:2923–2928. doi: 10.1158/1055-9965.EPI-09-0529. [DOI] [PubMed] [Google Scholar]
- 29.Combs GF, Jr, Hyun T, Gray WP. Nonprotein bound selenium in plasma: relevance in assessing selenium status. In: Centeno J, Collery P, Vernet G, Finkelman R, Gibb H, Eteinne J, editors. Metal Ions in Biology and Medicine. Montrouge, France: John Libbey Eurotext Ltd.; 2000. pp. 237–240. [Google Scholar]
- 30.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors. 2001;14:107–114. doi: 10.1002/biof.5520140115. [DOI] [PubMed] [Google Scholar]
- 31.Ogra Y, Kitaguchi T, Suzuki N, Suzuki KT. In vitro translation with [34S]-labeled methionine, selenomethionine, and telluromethionine. Analytical and bioanalytical chemistry. 2008;390:45–51. doi: 10.1007/s00216-007-1546-y. [DOI] [PubMed] [Google Scholar]
- 32.Butler JA, Beilstein MA, Whanger PD. Influence of dietary methionine on the metabolism of selenomethionine in rats. J Nutr. 1989;119:1001–1009. doi: 10.1093/jn/119.7.1001. [DOI] [PubMed] [Google Scholar]
- 33.Combs GF, Jr, Watts JC, Jackson MI, Johnson LK, Zeng H, Scheett AJ, et al. Determinants of selenium status in healthy adults. Nutrition journal. 2011;10:75. doi: 10.1186/1475-2891-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–810. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, Jovanovic B, et al. Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer Prev Res (Phila) 2011;4:1938–1944. doi: 10.1158/1940-6207.CAPR-10-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algotar AM, Stratton MS, Xu MJ, Dalkin BL, Nagle RB, Hsu CH, et al. Dose-dependent effects of selenized yeast on total selenium levels in prostatic tissue of men with prostate cancer. Nutr Cancer. 2011;63:1–5. doi: 10.1080/01635581.2010.516476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karunasinghe N, Han DY, Zhu S, Duan H, Ko YJ, Yu JF, et al. Effects of supplementation with selenium, as selenized yeast, in a healthy male population from new zealand. Nutr Cancer. 2013;65:355–366. doi: 10.1080/01635581.2013.760743. [DOI] [PubMed] [Google Scholar]
- 38.Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, Schoenberg JB, et al. Serum selenium and risk of prostate cancer in US blacks and whites. Int J Cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 39.Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies--III: statistical associations with dietary selenium intakes. Bioinorganic chemistry. 1977;7:23–31. doi: 10.1016/s0006-3061(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 40.Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer. 2006;42:2463–2471. doi: 10.1016/j.ejca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt456. djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rayman MP, Combs GF, Jr, Waters DJ. Selenium and vitamin E supplementation for cancer prevention. Jama. 2009;301:1876. doi: 10.1001/jama.2009.625. author reply 7. [DOI] [PubMed] [Google Scholar]
- 43.Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Bayoumy K, Das A, Russell S, Wolfe S, Jordan R, Renganathan K, et al. The effect of selenium enrichment on baker's yeast proteome. Journal of proteomics. 2012;75:1018–1030. doi: 10.1016/j.jprot.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tastet L, Schaumloffel D, Lobinski R. ICP-MS-assisted proteomics approach to the identification of selenium-containing proteins in selenium-rich yeast. J Anal Atom Spectrom. 2008;23:309–317. [Google Scholar]
- 46.Casal SG, Far J, Bierla K, Ouerdane L, Szpunar J. Study of the Se-containing metabolomes in Se-rich yeast by size-exclusion-cation-exchange HPLC with the parallel ICP MS and electrospray orbital ion trap detection. Metallomics : integrated biometal science. 2010;2:535–548. doi: 10.1039/c0mt00002g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.