Abstract
Objective
Determine factors impacting the uptake of genetic counseling and results of genetic testing following universal tumor testing for Lynch syndrome in patients with endometrial cancer.
Methods
The study population consisted of two unselected cohorts of endometrial cancer patients, 408 identified retrospectively and 206 identified prospectively. Immunohistochemistry for mismatch repair protein expression and/or microsatellite instability analysis was performed on these tumors. MLH1 methylation analysis was performed on tumors with loss of MLH1 protein. Tumor studies were considered suggestive of Lynch Syndrome if they showed immunohistochemical loss of MSH2, MSH6, or PMS2, loss of MLH1 without MLH1 promoter methylation, and/or microsatellite instability. Participants with suggestive tumor studies were contacted and offered genetic counseling and testing.
Results
In the retrospective cohort, 11% had tumor studies suggestive of Lynch syndrome. 42% were seen for genetic counseling. A germline mutation was detected in 40%, and one had a variant of uncertain significance. In the prospective cohort, 8.7% of patients had tumor testing suggestive of Lynch syndrome. 72% were seen for genetic counseling. Germline mutations were found in 40%, and one had a variant of uncertain significance. Common challenges included timing of re-contact, age, perceived lack of relevance, inability to travel, and limited insurance coverage.
Conclusions
There are several barriers to genetic counseling and testing follow up after universal tumor testing, and uninformative genetic test results present a management challenge. It is important to consider these limitations when implementing an approach to screening endometrial cancer patients for Lynch syndrome.
Background
Lynch syndrome is a hereditary cancer syndrome due to germline mutations in mismatch repair (MMR) genes and is primarily characterized by an increased risk for colorectal and endometrial cancer. Lynch syndrome is thought to account for 2-3% of all endometrial cancers, and approximately 50% of patients with Lynch syndrome will present with a gynecologic malignancy as their sentinel cancer (1, 2) . The identification of Lynch syndrome-associated endometrial cancer has significant implications both for the patient and her relatives. As these patients have an increased risk to develop subsequent primary colorectal cancer (CRC), it is important to identify EC patients with Lynch syndrome to implement heightened CRC screening. A diagnosis of Lynch syndrome also provides the opportunity for cancer prevention in first-degree relatives, who have a 50% chance to have Lynch syndrome themselves.
The optimal approach to detecting Lynch syndrome in women who present with endometrial cancer is unclear. The Amsterdam criteria and Bethesda criteria, which rely on a combination of young age of onset of cancer and family history factors, can be used to identify a subgroup of cancer patients that are more likely to have Lynch syndrome (3, 4). However, these criteria were developed with a primary focus on colorectal cancer and have not been as extensively assessed in the endometrial cancer population. Thus, the Society of Gynecologic Oncologists developed clinical screening criteria with a focus on gynecologic cancer to assist providers in determining which patients should be considered for further Lynch syndrome evaluation (5). Regardless of which criteria are used, a selective approach has the potential to miss high-risk patients and families (6). In particular, individuals with MSH6 mutations typically present with endometrial cancer at older ages and have less extensive family histories of cancer (1). The penetrance of PMS2 mutations also appears to be lower than that of MLH1 and MSH2 mutations. Thus, individuals with mutations in these two genes are more likely to be missed by a selective screening strategy than those with MLH1 and MSH2 mutations (7, 8). Given the importance of identifying families with Lynch syndrome, a universal approach has been recently proposed and implemented at several institutions (1, 9-11).
There are several important factors to consider when deciding on an approach to screen endometrial cancer patients for Lynch syndrome. Whether one chooses to utilize a universal versus a selective approach, it is vital to consider and ensure optimal follow up with genetic counseling for patients identified as potentially high-risk. Previous studies have shown the uptake of genetic counseling and testing after positive tumor studies via prospective universal screening is low (9, 10). Additionally, of those with abnormal tumor studies that undergo genetic testing, as many as 42-88% will not have an identifiable germline mutation, which presents a clinical challenge (11-13). Management guidelines for these patients are less clear than for those with a germline MMR mutation, and predictive genetic testing for relatives is not available. Several barriers to genetic counseling and testing have been identified in both endometrial and colorectal cancer patients, including insufficient family history collection, lack of referral, insufficient insurance coverage/cost of the appointment, anxiety for the results, lack of interest, patient/family not wanting to know information regarding cancer risks, and lack of understanding regarding benefits of genetic testing and available preventive measures (10, 14, 15).
Recently, investigators at our institution have identified potential Lynch Syndrome-associated endometrial cancer via two different approaches, neither of which depended on clinical criteria such as age of cancer diagnosis or family history for selection. In the first approach, a retrospective IRB-approved research protocol used tumor testing to identify potential patients with Lynch Syndrome-associated endometrial cancer. In the second approach, tumor testing was prospectively performed on all newly diagnosed endometrial cancer patients as part of standard clinical care. For both approaches, genetic counseling of potential Lynch Syndrome patients was indicated. The purpose of this study was to determine factors impacting the uptake of genetic counseling and results of genetic testing following universal tumor testing for Lynch syndrome in patients with endometrial cancer.
Materials and Methods
Study Populations and Procedures
Participants in cohort 1 (retrospective cohort; n=408) were identified retrospectively. After obtaining Institutional Review Board approval, cases of endometrial cancer involving women who underwent hysterectomy at MD Anderson Cancer Center through 2011 were identified. Beginning with the most recent cases, endometrial cancers were included if the patient was 18 years of age or greater and sufficient tissue from the surgery was available for molecular analysis. Endometrioid and non-endometrioid histologies of endometrial carcinoma were included. All hysterectomies were pathologically reviewed by a gynecologic pathologist (RRB). Immunohistochemistry (IHC) for DNA MMR protein expression (MLH1, MSH2, MSH6, and PMS2) was performed on all cases. MLH1 methylation analysis was performed on tumors with IHC loss of MLH1. Microsatellite instability (MSI) analysis was performed on two participants with normal IHC results (positive tumor expression of MLH1, MSH2, MSH6, and PMS2) but personal and/or family histories particularly suggesting Lynch Syndrome. Tumor studies were considered suggestive of Lynch syndrome if they showed IHC loss of MSH2, MSH6, or PMS2, loss of MLH1 without MLH1 promoter methylation, and/or if the tumor was MSI-low or MSI-high. Participants with tumor studies suggestive of Lynch syndrome were contacted by mail and follow-up telephone calls. All were offered genetic counseling and IHC-directed germline genetic testing. Genetic testing was performed at Mayo Medical Laboratories, ARUP Laboratories, or Myriad Genetic Laboratories. Clinicopathologic data collected from medical records were reviewed retrospectively. Barriers to re-contacting and genetic counseling in the retrospective cohort were identified by both the investigative team based on clinician interactions during the re-contacting process and by participant verbal report. Due to the length of time to re-contact (measured in years from time of cancer diagnosis to time of re-contact) and low uptake of genetic counseling in this cohort, we also examined possible demographic differences between participants who attended genetic counseling versus those who did not. T-tests (for continuous variables) and Fisher's exact tests (for categorical variables) were conducted to determine demographic differences between those who attended genetic counseling vs. those who did not. Participants who had previously consulted with genetic counseling or who were deceased at the time of re-contact were excluded from these analyses.
Participants in cohort 2 (prospective cohort; n=206) were identified prospectively through the MD Anderson Gynecologic Oncology Center. These participants had hysterectomy for endometrial cancer performed at our institution between 08/01/2012 and 08/31/2013. On 08/01/2012, a clinical initiative to improve the identification of Lynch syndrome-associated endometrial cancer was initiated. For all patients undergoing endometrial cancer surgery at our institution, microsatellite instability analysis and IHC for MLH1, MSH2, MSH6, and PMS2 was performed. For tumors with IHC loss of MLH1, reflex MLH1 methylation analysis was performed. Patients were provided with an education document in their pre-operative packet explaining the rationale for performing these tumor studies. Patients with tumor testing results suggestive of Lynch Syndrome were informed of these results and offered a referral for genetic counseling by their gynecologic oncologist. Attempts were made to schedule genetic counseling appointments in conjunction with patients' other clinic appointments. All patients attending genetic counseling were offered IHC-directed germline genetic testing. Patients with MSI-low tumors but positive IHC expression of all 4 MMR proteins were offered germline MSH6 genetic testing. Clinicopathologic data collected from medical records were reviewed retrospectively.
Results
Both endometrial cancer patient cohorts had similar clinical and pathological characteristics, including age, BMI, tumor histology, tumor stage, and family history characteristics (Table 1).
Table 1.
Study Population Demographics.
| Clinical Features | Retrospective Cohort (n=408) |
Prospective Cohort (n=206) |
|---|---|---|
| Median age at diagnosis (years) | 61 | 62 |
| Range | (18-92) | (30-86) |
| BMI (kg/m2) mean (SD) | 34.7 (9.4) | 35.2 (10.7) |
| Histology N (%) | 336 (82.4) | 157 (76.2) |
| Endometrioid | 72 (17.6) | 49 (23.8) |
| Non-Endometrioid | ||
| Grade N(%) | ||
| 1 & 2 | 299 (73.3) | 147 (71.3) |
| 3 | 109 (26.7) | 59 (28.6) |
| Stage N (%) | ||
| I & II | 326 (79.9) | 159 (77.2) |
| III & IV | 82 (20.1) | 47 (22.8) |
| Family History of colon or endometrial cancer N(%)* | ||
| Yes | 99 (24.7) | 56 (27.2) |
| No | 302 (75.3) | 150 (72.8) |
7 patients lacked sufficient family history documentation in the medical record
Genetic Testing Outcomes (Retrospective Cohort)
In the retrospective cohort, 45/408 (11%) had tumor studies suggestive of LS (Figure 1). 19/45 (42%) were seen for genetic counseling at our institution, and 15/19 (79%) proceeded with genetic testing. 6/15 (40%) were found to have a deleterious or suspected deleterious mutation (Table 2). One patient was found to have a variant of uncertain significance. 4/6 germline mutation carriers met clinical criteria for a genetic counseling referral based on a personal history of colorectal cancer, a family history of colorectal and/or endometrial cancer in a first-degree relative, a family history of colorectal or endometrial cancer diagnosed before age 50 in a first, second, or third degree relative, or a family history of a germline MMR mutation (16). Of the six patients with a germline mutation, 5 had IHC loss of a MMR protein. The sixth had retained positive IHC expression of MMR proteins but the tumor was MSI-low. Because the patient was young (age 51 years) and the tumor was situated in the lower uterine segment, genetic testing was offered (17). A suspected deleterious MSH6 mutation was identified that was predicted to result in the production of a non-functional MSH6 protein. All other participants with MMR-deficient tumors who underwent germline testing had no detectable mutation (53%).
Figure 1. Retrospective Cohort.
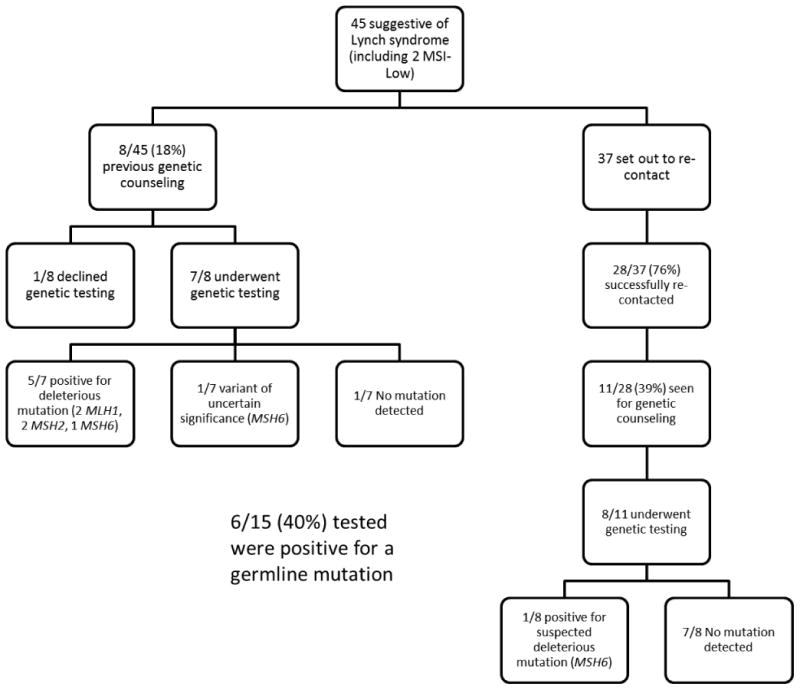
Table 2.
Germline mutations and variants of uncertain significance identified.
| Cohort | Testing Lab | Mutation | Variant Class | Age | Histology | Stage | Grade | IHC | MSI | MLH1 methylation | Family History EC/CRC |
Personal History CRC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Mayo | MSH2 c.1697del A | D | 44 | E | IA | 1 | Loss MSH2/MSH6 | NP | NP | No | No |
| R | Mayo | MLH1 del exons 6-10 | D | 48 | E | IA | 1 | Loss MLH1/PMS2 | NP | No | Yes | Yes |
| R | Mayo | MSH6 3699-3702 del AGAA | D | 56 | E | IA | 1 | Loss MSH6 | NP | NP | No | Yes |
| R | Mayo | MSH2 687delA | D | 42 | M | IIIC2 | 3 | Loss MSH2/MSH6 | NP | NP | Yes | No |
| R | Mayo | MLH1 IVS7+5G>A | D | 46 | E | II | 1 | Loss MLH1/PMS2 | H | No | Yes | No |
| R | Myriad | MSH6 p.F432S, c.1295T>C | SD | 51 | E | II | 1 | Intact | L | No | Yes | No |
| P | Mayo | MSH6 c.3238_3239 delCT, p.L1080VfsX 12 | SD | 65 | M | IB | High | Loss MSH6 | H | NP | Yes | No |
| P | ARUP | PMS2 del exon 10 | D | 49 | E | IA | 2 | Loss PMS2 | H | NP | Yes | No |
| P | Mayo | MSH6 c.2805_2806 delTG, p.D936LfsX2 | SD | 70 | E | IA | 2 | Loss MSH6 | S | NP | Yes | No |
| P | Mayo | MLH1 del exons 2-3 | D | 47 | E | IA | 1 | Loss MLH1/PMS2 | H | No | Yes | Yes |
| R | Mayo | MSH6 c.3724_3726 delCGT | VUS | 52 | E | IA | 1 | Loss MSH6 | NP | NP | Yes | No |
| P | Mayo | MSH6 c.1109T>C, p.L370S | VUS | 53 | E | IB | 3 | Intact | Low | NP | No | No |
IHC=immunohistochemistry; MSI=Microsatellite instability; EC=endometrial cancer; CRC=colorectal cancer; P=prospective; R=retrospective; D=deleterious; SD=suspected deleterious; VUS=variant of uncertain significance; E=endometrioid; M=mixed; H=high; L=low; NP=not performed
Genetic Testing Outcomes (Prospective Cohort)
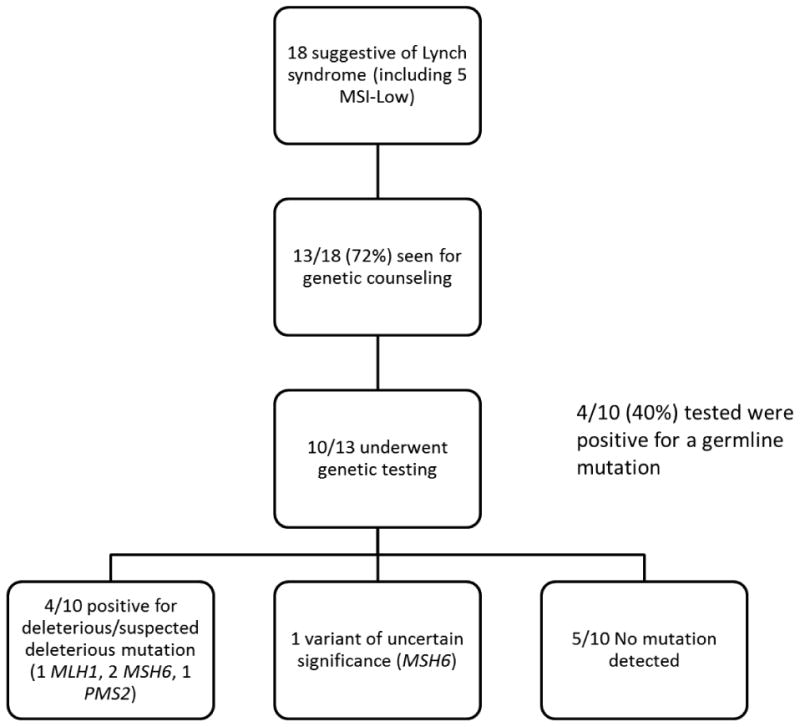
18/206 (8.7%) patients had tumor testing suggestive of Lynch syndrome, including 5 who had MSI-low tumors with intact positive IHC expression of all 4 MMR proteins (Figure 2). Genetic counseling attendance in the prospective cohort compared favorably to what was seen in the retrospective cohort. Of those seen for genetic counseling, 10/13 (77%) underwent germline testing. 4/10 (40%) patients tested were positive for a deleterious mutation (Table 2). 5/10 (50%) had no detectable mutation, and one had a variant of uncertain significance in the MSH6 gene. Excluding those with MSI-low tumors with intact IHC, 4/7 (57%) had a germline mutation and 4/18 (22%) with tumor studies suggestive of Lynch syndrome had a mismatch repair gene mutation.
Figure 2. Prospective Cohort.
Barriers to Re-Contacting and Genetic Counseling (Retrospective Cohort)
The retrospective cohort had a much lower proportion of patients attending a genetic counseling appointment. We next sought to identify possible reasons for the low uptake of genetic counseling in the retrospective cohort. We detected statistically significant differences in age at diagnosis, time to initial re-contact (in months), and age at time of re-contact between participants who attended genetic counseling and those who did not. Participants who were diagnosed with EC at younger ages and those who were re-contacted closer to the time of their initial diagnosis were more likely to attend genetic counseling (Table 3). Barriers to re-contacting included patient death and outdated contact information (Table 4). Barriers to genetic counseling included perceived lack of relevance, inability to travel to Houston, limited insurance coverage, and previous genetic counseling/testing at another institution (Table 4).
Table 3. Demographics of patients seen for genetic counseling vs. not seen (Retrospective Cohort).
| Characteristic | Not seen for genetic counseling (n=23) | Seen for genetic counseling (n=10) | p-value |
|---|---|---|---|
| Age at diagnosis (years) | 0.006 | ||
| Mean (SD) | 63 (8.7) | 54.5 (8.1) | |
| Min-Max | 50 – 87 | 43 - 68 | |
| Time to initial re-contact (years) | 0.002 | ||
| Mean (SD) | 5.3 (2.2) | 2.4 (1.4) | |
| Min-Max | 1.6 - 11.2 | 0.9 - 4.9 | |
| Age at time of re-contact | 0.001 | ||
| Mean (SD) | 69 (9.0) | 57.3 (7.2) | |
| Min-Max | 56.8-93.8 | 50.2-69.8 | |
| BMI (kg/m2) | 0.803 | ||
| Mean (SD) | 31.8 (6.3) | 31.3 (9.8) | |
| Min-Max | 23.6 - 43.7 | 19.4 - 52.8 | |
| Family history of colon or endometrial cancer | 0.646 | ||
| No | 19 (82.6) | 7 (70.0) | |
| Yes | 4 (17.4) | 3 (30.0) | |
| Within greater Houston area (50 miles) | 0.701 | ||
| No | 13 (56.5) | 7 (70.0) | |
| Yes | 10 (43.5) | 3 (30.0) |
4 patients were excluded from this analysis as they were deceased at time of re-contact, and 8 were excluded as they had previously consulted with genetic counseling.
Table 4. Barriers to Re-contacting and Genetic Counseling/Testing in Retrospective Cohort.
| Barriers to re-contacting* (n=37) | Barriers to genetic counseling and testing* (n=28) |
|---|---|
|
|
may not add up to 100% as some participants had multiple barriers identified
Discussion
Most studies of follow up genetic counseling and testing have focused on patients with colorectal cancer. Previous studies in CRC patients undergoing genetic testing for Lynch syndrome have revealed gender differences in both motivations for pursuing genetic testing and psychosocial functioning/distress (18, 19). While these studies did not specifically address barriers to genetic counseling and testing, they do suggest that gender may impact the process of Lynch syndrome evaluation. In addition, there may be differences in rates of referral depending on the syndrome itself or the organ primarily affected by the syndrome. Previous studies assessing other hereditary cancer syndromes have suggested under-referral for Lynch syndrome genetic counseling versus BRCA1/2 genetic testing in patients with gynecologic cancer (20). Additionally, the uptake of genetic counseling has been shown to be lower in patients with EC versus those with ovarian cancer (21). While there are significant differences between Lynch syndrome and hereditary breast and ovarian cancer syndrome, these studies do suggest that gender may impact genetic counseling referral rates through differences in the syndrome itself or the organ primarily affected by the syndrome. As barriers to this process may vary between CRC and EC patients, it is thus important to assess the factors impacting genetic counseling and testing in patients who present with endometrial cancer as well. This study is one of few that have focused on barriers to the identification of endometrial cancer patients with Lynch syndrome. Our data suggest that compliance with follow up genetic counseling and testing is less than 100%, which is consistent with previous literature (9-11). The uptake of genetic counseling and testing was much higher in the prospective cohort, in which re-contact was done in real time, than in the retrospective cohort, in which re-contact was sometimes years after the initial diagnosis. This is consistent with one of the significant predictors of attendance to genetic counseling within the retrospective cohort – time since diagnosis. Some of the barriers to genetic counseling we identified have been noted in previous studies, including lack of insurance coverage for the genetic counseling visit, perceived lack of relevance, and adjusting to a cancer diagnosis (10, 22). These barriers may be addressed by addressing health literacy issues, misconceptions, and emphasizing the clinical utility of genetics (22). Additionally, our study identified unique barriers not previously reported, such as being unable or unwilling to return to our institution, age at diagnosis and at re-contact, and time to re-contact. A study of 245 EC patients suggested that having a genetic counselor contact the patient directly to schedule an appointment and explain the tumor study results may be one reason for the higher rate of genetic counseling uptake in their study (76%) (11). Incorporating this service delivery model along with eliminating other barriers to genetic counseling (such as insurance coverage) may improve the uptake of genetic counseling, and therefore the identification of individuals with Lynch syndrome. Other genetic counseling service delivery models, including telemedicine, may also help eliminate this barrier. Additionally, it is important to note that length of time between tumor testing and follow-up genetic counseling may be a significant barrier in EC patients, which suggests that timely completion of tumor testing and genetic counseling referral is important.
Once endometrial cancer patients are seen for genetic counseling, the uptake of germline genetic testing is high (15/19 in the retrospective cohort, and 10/13 in the prospective cohort). This suggests that one of the most significant barriers to the identification of EC patients with Lynch syndrome is actually getting patients to genetic counseling. Once they are seen, the most common reason for declining genetic testing is lack of insurance coverage. Current insurance coverage guidelines for Lynch syndrome testing vary for CRC vs. EC patients, particularly for patients with tumor studies suggestive of Lynch syndrome, which suggests a possible area for improvement.
The other challenge identified in our study relates to the results of germline genetic testing. In these two unselected cohorts of endometrial cancer patients, 60% of patients with tumor studies suggestive of Lynch syndrome did not have an identifiable germline MMR mutation. This is consistent with other similar studies in unselected populations (11-13, 23). This may reflect limitations in current genetic testing technology or the possibility of a sporadic mechanism for the tumor study results. However, the positive predictive value (PPV) of tumor studies for germline mutation status has not been as thoroughly investigated in other studies assessing the efficacy of universal MSI/IHC in endometrial cancer. In highly selected patient populations, such as those in the Colon Cancer Family Registry who were ascertained on the basis of young age at diagnosis or family history, a higher PPV is noted than in the unselected population-based cohorts (13). In this study of 1061 unselected MSI-high colorectal cancer patients, approximately 12% had a detectable germline MMR mutation, as compared to 70% in the highly selected patient cohort. It is well established that screening tests perform more poorly when applied to a population-based cohort versus a high-risk cohort, in which the prior probability of having the disease is lower. If one extends this concept to tumor screening for Lynch syndrome, when one moves to a more universal tumor testing approach, the PPV of the screening test (MSI and/or IHC) declines. Management of patients with mismatch repair deficient (MMRD) tumors (defined by microsatellite instability or IHC loss of a MMR protein) but who lack a germline MMR mutation (MMRD+/germline-) poses a clinical conundrum, as published guidelines do not address this cohort. This is a relatively under-recognized limitation of universal tumor testing that has not been adequately addressed in recent Lynch syndrome literature. Rodriguez-Soler et al. recently published a study estimating colorectal cancer risk in a Spanish cohort of CRC patients (24). They demonstrated that those with MMRD tumors but no detectable germline MMR mutation had lower risks of second primary colorectal cancer and non-colorectal Lynch syndrome-related cancers than individuals with a germline mutation (Lynch syndrome), but higher than those with sporadic CRC. This data suggests the possibility that individuals with MMRD+/germline- tumors represent a mixed group of people who truly have Lynch syndrome but with undetectable mutations and those who do not have Lynch syndrome but rather a sporadic cause for their mismatch repair deficient tumor. The finding of biallelic somatic mutations also suggests the possibility of a sporadic cause for some MMRD tumors (25). Alternatively, Lynch syndrome may be a heterogeneous syndrome, with some families having extensive histories of related cancers and other families having less impressive cancer histories, similar to those seen in families with MSH6 and PMS2 mutations.
In a health-care environment with limited resources, when deciding how to implement a screening strategy for Lynch syndrome, one needs to consider how to spend these resources - family history assessment, genetic counseling, and/or universal tumor testing. Increased genetic counseling resources may be necessary in order to improve genetic counseling uptake and limit the time gap between tumor study results and follow up genetic testing. Advocating for improved insurance coverage for germline genetic testing in endometrial cancer patients with abnormal tumor studies may also help reduce the number of patients declining testing due to lack of insurance coverage. It is also important to factor in the cost, both financial and emotional, associated with failure to detect a germline mutation in patients with mismatch repair deficient tumors. Most of the mutation-positive (Lynch syndrome) cases in the current study occurred in individuals who met our previously established clinical screening criteria, which suggests an alternative approach to identifying endometrial cancer patients with Lynch syndrome (16). A limitation of a universal tumor testing approach compared to a selective approach is a lower germline mutation yield, with its associated lack of clinical management guidelines. A limitation of a selective approach is the potential to miss patients with Lynch syndrome. When implementing a Lynch syndrome screening protocol for endometrial cancer patients, it is important to consider these factors.
One of the strengths of our study is the incorporation of clinical populations with the inherent challenges that cannot be appreciated in a purely research-based cohort. Thus, this study accounts for real-life health care encounters, which adds a different perspective to the existing body of literature on how to optimally identify endometrial cancer patients with Lynch syndrome. An additional strength of our study is the inclusion of two separate patient cohorts, thereby increasing the number of patient encounters and sample size. Limitations include the retrospective design of those in cohort 1; small sample size of patients with Lynch syndrome, which limits our ability to detect significant demographic differences in those who were seen for genetic counseling versus those who were not; inability to control for time from diagnosis to re-contact in the retrospective cohort; and inability to re-contact all patients for genetic counseling and testing. We are continuing to attempt to re-contact patients, as well as attempting to eliminate the barriers to genetic counseling and testing. The optimal strategy to identifying endometrial cancer patients with Lynch syndrome remains a moving target; however, this study highlights some of the unique clinical issues that should be considered when deciding on an approach.
Conclusions
This study highlights the real-world clinical implications of a universal tumor testing program to identify endometrial cancer patients with Lynch syndrome. There are several barriers to this process, including genetic counseling and testing uptake, and uninformative genetic test results. It is important to consider these limitations and barriers when implementing an approach to screening endometrial cancer patients for Lynch syndrome. Additional studies are necessary to determine the most efficacious, cost-effective approach to identifying endometrial cancer patients with Lynch syndrome.
Research Highlights.
-
-
Genetic counseling follow up after Lynch syndrome tumor testing is important
-
-
Barriers include perceived lack of relevance, age, location, and insurance coverage
-
-
Uninformative germline test results after tumor testing pose a management challenge
Acknowledgments
This research is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672 and the National Institutes of Health SPORE in Uterine Cancer NIH 2P50 CA098258-08.
Footnotes
All authors declare that they have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer research. 2006 Aug 1;66(15):7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 2.Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstetrics and gynecology. 2005 Mar;105(3):569–74. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 3.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Diseases of the colon and rectum. 1991 May;34(5):424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999 Jun;116(6):1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic oncology. 2007 Nov;107(2):159–62. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Ryan P, Mulligan AM, Aronson M, Ferguson SE, Bapat B, Semotiuk K, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012 Feb 1;118(3):681–8. doi: 10.1002/cncr.26323. [DOI] [PubMed] [Google Scholar]
- 7.Plaschke J, Engel C, Kruger S, Holinski-Feder E, Pagenstecher C, Mangold E, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Nov 15;22(22):4486–94. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008 Aug;135(2):419–28. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecologic oncology. 2009 Sep;114(3):486–90. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Backes FJ, Mitchell E, Hampel H, Cohn DE. Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecologic oncology. 2011 Dec;123(3):532–6. doi: 10.1016/j.ygyno.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecologic oncology. 2013 Jul;130(1):121–6. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sanchez-Heras AB, et al. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PloS one. 2013;8(11):e79737. doi: 10.1371/journal.pone.0079737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, Lindor N, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008 Nov;17(11):3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross DS, Rahm AK, Kauffman TL, Webster J, Le AQ, Spencer Feigelson H, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genetics in medicine : official journal of the American College of Medical Genetics. 2013 Dec;15(12):933–40. doi: 10.1038/gim.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South CD, Yearsley M, Martin E, Arnold M, Frankel W, Hampel H. Immunohistochemistry staining for the mismatch repair proteins in the clinical care of patients with colorectal cancer. Genetics in medicine : official journal of the American College of Medical Genetics. 2009 Nov;11(11):812–7. doi: 10.1097/GIM.0b013e3181b99b75. [DOI] [PubMed] [Google Scholar]
- 16.Daniels MS, Urbauer DL, Zangeneh A, Batte BA, Dempsey KM, Lu KH. Outcomes of screening endometrial cancer patients for Lynch syndrome by patient-administered checklist. Gynecologic oncology. 2013 Dec;131(3):619–23. doi: 10.1016/j.ygyno.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Dec 20;26(36):5965–71. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esplen MJ, Madlensky L, Aronson M, Rothenmund H, Gallinger S, Butler K, et al. Colorectal cancer survivors undergoing genetic testing for hereditary non-polyposis colorectal cancer: motivational factors and psychosocial functioning. Clinical genetics. 2007 Nov;72(5):394–401. doi: 10.1111/j.1399-0004.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 19.Vernon SW, Gritz ER, Peterson SK, Amos CI, Perz CA, Baile WF, et al. Correlates of psychologic distress in colorectal cancer patients undergoing genetic testing for hereditary colon cancer. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1997 Jan;16(1):73–86. doi: 10.1037//0278-6133.16.1.73. [DOI] [PubMed] [Google Scholar]
- 20.Pujol P, Lyonnet DS, Frebourg T, Blin J, Picot MC, Lasset C, et al. Lack of referral for genetic counseling and testing in BRCA1/2 and Lynch syndromes: a nationwide study based on 240,134 consultations and 134,652 genetic tests. Breast cancer research and treatment. 2013 Aug;141(1):135–44. doi: 10.1007/s10549-013-2669-9. [DOI] [PubMed] [Google Scholar]
- 21.Dekker N, van Dorst EB, van der Luijt RB, van Gijn ME, van Tuil M, Offerhaus JA, et al. Acceptance of genetic counseling and testing in a hospital-based series of patients with gynecological cancer. Journal of genetic counseling. 2013 Jun;22(3):345–57. doi: 10.1007/s10897-012-9553-3. [DOI] [PubMed] [Google Scholar]
- 22.Tomiak E, Samson A, Spector N, Mackey M, Gilpin C, Smith E, et al. Reflex testing for Lynch syndrome: If we build it, will they come? Lessons learned from the uptake of clinical genetics services by individuals with newly diagnosed colorectal cancer (CRC) Familial cancer. 2014 Mar;13(1):75–82. doi: 10.1007/s10689-013-9677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Jan 10;32(2):90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barbera VM, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013 May;144(5):926–32. e1. doi: 10.1053/j.gastro.2013.01.044. quiz e13-4. [DOI] [PubMed] [Google Scholar]
- 25.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, Goossens M, Ouchene H, Hendriks-Cornelissen SJ, et al. Somatic Mutations in MLH1 and MSH2 Are a Frequent Cause of Mismatch-Repair Deficiency in Lynch Syndrome-Like Tumors. Gastroenterology. 2014 Mar;146(3):643–6. e8. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]


