Abstract
Tim-3 is a member of the T cell immunoglobulin and mucin domain (Tim) family of proteins, which are expressed by several cell types in the immune system, including CD4 and CD8 T cells activated under certain conditions. These molecules are generally thought to act as receptors for multiple ligands and thus to function by engaging intracellular signaling pathways in a ligand-dependent manner. In recent years, the function of the Tim-3 protein has been studied in some detail, particularly with respect to its role in the regulation of CD4 and CD8 T cell function. Here, we review the structural features of Tim-3, known ligands for this molecule and the links established between Tim-3 and signal transduction pathways. In addition, we review the current literature regarding the role of Tim-3 in the regulation of effector responses by CD4 and CD8 T cells. Overall, findings published thus far strongly support the conclusion that Tim-3 functions to inhibit T cell responses, particularly under conditions involving chronic stimulation. Conversely, some reports have provided evidence that Tim-3 can stimulate T cells under conditions involving acute stimulation, suggesting that the role of Tim-3 may vary depending on context. Further study of Tim-3 is likely to advance our understanding of how CD4 and CD8 T cell responses are regulated and could uncover novel approaches for manipulating T cell function for therapeutic benefit.
Keywords: T cells, Tim protein family, Surface receptor, Signal transduction, Immune system regulation
Introduction
The Tim-3 protein is a member of the T cell immunoglobulin and mucin domain (Tim) family, which encompasses a group of type I transmembrane proteins expressed by both innate and adaptive cell types within the immune system [1-4]. All Tim proteins are expressed on the cell surface and have been shown to function as receptors for soluble or cell-associated ligands. Additionally, certain Tim proteins are produced in soluble forms or can be shed from the cell surface due to cleavage by membrane-associated proteases, suggesting these molecules may also serve as cell-free ligands for other proteins [5-9]. Loci encoding the Tim proteins are found in close proximity to each other within mammalian genomes and genetic studies have uncovered associations between polymorphisms in Tim genes and the development of autoimmune and allergic diseases in humans [10-12]. These findings, together with a growing body of published data from experimental studies, suggest that the Tim proteins have key roles in the immune system. Thus, defining the function of Tim proteins in detail could expand our understanding of how the immune system is regulated and provide new insights regarding mechanisms that contribute to protective and pathologic immune responses.
In humans and other mammals, the Tim family consists of 3 proteins (Tim-1, -3 and -4). An exception to this is mice, which express an additional family member called Tim-2 that bears a relatively high degree of similarity (~75%) to Tim-1, suggesting functional overlap. A hallmark feature of the Tim proteins is an extracellular domain consisting of an N-terminal immunoglobulin variable region-like (IgV) domain encompassing roughly 100 amino acids and a serine/threonine rich mucin-like region of varying length that contains target sites for O- and N-linked glycosylation (see Fig. 1). In addition, all Tim proteins possess a single transmembrane domain and a C-terminal cytoplasmic tail containing tyrosine residues, suggesting these molecules activate intracellular signaling pathways. Consistent with this possibility, biochemical studies have shown that tyrosines within the cytoplasmic tails of Tim-1 and Tim-3 can be phosphorylated and can interact with proteins involved in signal transduction [13-20]. These and other studies [21-25] suggest that Tim proteins influence intracellular signaling pathways that regulate immune cell function. However, despite these insights, the mechanisms by which the Tim proteins regulate the function of particular immune cell types are not well understood.
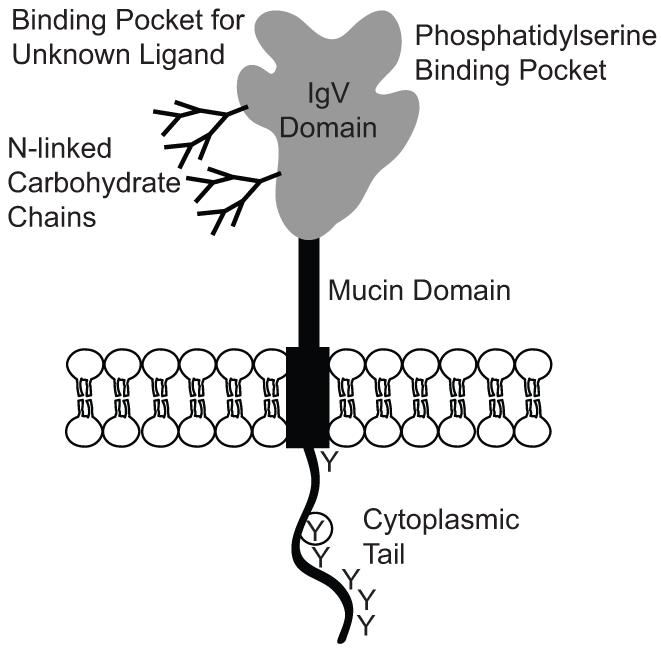
Fig 1.
Structure of the membrane-bound form of Tim-3. Figure shows a schematic diagram representing key structural features of the Tim-3 protein. The extracellular IgV and mucin domains are noted; O- and N-linked glycosylation sites in the latter domain are not shown. For the IgV domain, ligand-binding pockets and sites for N-linked glycosylation are represented. Glycosylation of the IgV domain has been shown to be necessary for recognition of Tim-3 by Galectin-9 [33]. The cytoplasmic tail is noted, with “Y” representing tyrosine residues that can be phosphorylated by intracellular kinases. The circled Y represents a tyrosine within a sequence with homology to the consensus target site for tyrosine kinases.
Work in our laboratory is focused on determining the role of the Tim family member Tim-3 in the regulation of T cell responses, which is the main subject of this review article. For more information regarding other Tim family members, or the role of Tim-3 in innate immune responses, the reader is referred to other recently published review articles [1-4, 26].
Structural features of the Tim-3 gene and protein
The gene encoding Tim-3 is known as Havcr2, which denotes hepatitis A virus (HAV) cellular receptor 2. Rather than signifying a role for Tim-3 in HAV infection, this name stems from the homology between Tim-3 and Tim-1, which was initially discovered as a receptor for HAV [27]. Havcr2 contains 7 exons that encode the membrane-bound form of Tim-3; exon 1 codes for the signal peptide sequence, exon 2 for the IgV domain, exons 3-5 for the mucin domain, and exons 6 and 7 for the cytoplasmic tail [28]. In addition to the membrane-bound form of Tim-3, Havcr2 can express a soluble form of Tim-3, which is encoded by exons 1, 2, 6, and 7 [6]. The soluble form of Tim-3 can inhibit T cell-mediated immune responses [7, 6], suggesting that Tim-3 does not function exclusively as a membrane-bound receptor. However, the majority of work performed thus far has focused on determining the function of the membrane-bound form of Tim-3, which is depicted in figure 1.
The IgV domain of Tim-3, as well as that within other Tim family members, functions to mediate interactions with extracellular ligands. Crystallographic studies showed that a group of phylogenetically conserved residues positioned at the apex of the IgV domains of Tim-1, -3 and -4 form a pocket that can recognize phosphatidylserine, a molecule displayed on the surface of apoptotic cells [29-32]. As discussed below, this specificity has been shown to have functional relevance. Interestingly, crystallographic analysis also revealed that the Tim-3 IgV domain forms a distinct cleft structure not typically found in IgV domains [29]. Further, this domain can recognize a ligand of unknown identity that is widely expressed on leukocytes [29]. Additionally, the IgV domain of Tim-3 is subject to O- and N-linked glycosylation, which is important for recognition of Tim-3 by the carbohydrate-binding protein Galectin-9 [33, 34]. As outlined in more detail below, interaction between Tim-3 and Galectin-9 appears to have a critical role in the regulation of T cell responses.
The cytoplasmic tails of mouse and human Tim-3 are 66 and 77 amino acids in length, respectively, which contrasts with the somewhat shorter tails (41-49 amino acids) in Tim-1 and Tim-4. The cytoplasmic tails of human and mouse Tim-3 each contain 6 tyrosines surrounded by stretches of highly conserved amino acids. Moreover, a single tyrosine found roughly in the center of the cytoplasmic tail is embedded within a region bearing strong homology to the consensus target site for nonreceptor tyrosine kinases. Studies involving ectopic expression of wild-type and mutant forms of Tim-3 in cell lines have demonstrated that several of the tyrosine residues in the cytoplasmic tail can be recognized as substrates by intracellular phosphokinases [15, 16, 25, 19]. These findings support the conclusion that Tim-3 interfaces with signal transduction pathways. However, as described below, understanding the events that lead to Tim-3 phosphorylation and the consequences of this modification has proven challenging.
Ligands for Tim-3
To date, the IgV domain of Tim-3 has been shown to interact with phosphatidylserine displayed on the surface of apoptotic cells, the alarmin protein HMGB1 (High-Mobility Group Box 1) and Galectin-9, a widely expressed soluble protein with specificity for carbohydrate chains containing β-galactoside sugars. Binding to phosphatidylserine by Tim-3 can mediate the uptake of apoptotic cells by Tim-3-expressing phagocytes [35, 32]. The importance, if any, of such interactions in the regulation of T cell responses by Tim-3 remains unclear. Interaction between Tim-3 and HMGB1 has been reported to suppress the activation of dendritic cells associated with tumors [36]. Interestingly, the binding of Tim-3 to HMGB1 interferes with the trafficking of nucleic acids into endosomes, thus decreasing stimulation of endosomal Toll-like receptors and other nucleic acid-sensing pathways. Interaction between HMGB1 and Tim-3 expressed on T cells has not been reported; thus whether such contacts regulate T cell responses remains unknown.
A key report by Zhu et al. [33] was the first to show that Galectin-9 is a ligand for Tim-3 and that interactions between these molecules has impact on T cells. This study showed that Tim-3-Galectin-9 interaction requires recognition of carbohydrate chains attached to the Tim-3 IgV domain by the two CRD (carbohydrate recognition domains) within Galectin-9. Moreover, addition of Galectin-9 to cultured Tim-3+ Th-1-type CD4 T (Th1) cells induced apoptosis and necrosis, while injection of Galectin-9 into mice blunted immune responses driven by antigen-specific Th1 cells. A more recent study showed that Galectin-9 can also induce apoptosis of Tim-3+ CD8 T cells in vitro [37]. Based on these and other findings, a prevailing hypothesis is that the binding of Galectin-9 to Tim-3 results in the suppression of T cell responses, which supports the notion that Tim-3 functions is an inhibitory receptor for T cells. Of note, a subsequent study showed that Galectin-9 can influence T cell function in a Tim-3-independent manner [38] while another provided evidence refuting a role for Galectin-9-Tim-3 interactions in regulating T cell responses [39]. These findings suggest that further study of both Tim-3 and Galectin-9 is required to better understand the relationship between these molecules. Finally, it should be noted that reports by Wilker et al. [40] and Cao et al. [29] suggest that additional, as yet unknown, ligands for Tim-3 may exist, suggesting that Tim-3 can function as a receptor for other molecules in addition to phosphatidylserine, HMGB1 and Galectin-9.
Connections between Tim-3 and intracellular signal pathways
As described above, the primary structure of Tim-3 suggests a role in the regulation of intracellular signaling pathways. While published data support this conclusion, the results are somewhat conflicting, with some suggesting an inhibitory role for Tim-3 and others suggesting a stimulatory role. Regarding a negative effect, Zhu et al. [33], showed that Galectin-9 induced intracellular calcium flux in Tim-3+ Th1 cells that subsequently underwent cell death. A later study showed that interaction between Galectin-9 and Tim-3 induces the release of the protein Bat3 from cytoplasmic tail of Tim-3, allowing for activation of cell death pathways [25]. Studies by Lee et al. [41] demonstrated that Jurkat T cells expressing Tim-3 from a retroviral vector, as well as Tim-3+ human primary CD4 T cells, produced less IL-2 in response to pharmacologic agents that mimic TCR stimulation. These effects were linked to impaired activation of the transcription factor NFAT and reduced expression of the transcription factors c-Fos and c-Jun. More recent work employing human primary CD8 T cells showed that Tim-3 associates with the immune synapse formed between CD8 T cells and target cells and that Tim-3 colocalizes with phosphatases that can suppress T cell receptor (TCR) signaling [19]. Additionally, several reports have provided evidence that Tim-3 can inhibit to innate cell activation [36, 42-45], although for the most part the mechanisms underlying these effects remain unclear. Together, these data support the conclusion that engagement of Tim-3 results in the mobilization of pathways that suppress immune cell function.
In contrast to findings outlined above, an early study by Anderson et al. [22] showed that treatment of cloned Th1 cells or the dendritic cell (DC) line D2SC1 with Tim-3 antibodies could induce tyrosine phosphorylation of several proteins and also activate Erk and IkBα degradation. In the case of the D2SC1 cells, these effects correlated with increased TNFα secretion. Additionally, studies by Lee et al. [16] showed that ectopic expression of Tim-3 on Jurkat T cells could augment NFAT and NF-kB activation as induced by T cell receptor and CD28 engagement. Together, these data provide evidence that Tim-3 can have a positive effect on signal transduction pathways and thus, at least under some circumstances, can function to promote responses by immune cells.
Regulation of CD4 T cell responses by Tim-3
Two key papers published in 2001 and 2002 were the first to suggest a role for Tim-3 in regulating responses by CD4 T cells. Using a positional cloning approach, McIntire et al. [46] identified a locus that cosegregated with decreased airway hyperreactivity and reduced Th2 cytokine production by CD4 T cells in response to antigen challenge. More refined analysis demonstrated that the relevant locus contained the Tim genes and provided evidence that polymorphisms in Tim-1 and Tim-3 can influence allergic disease development. In the second paper, Monney et al. [28] generated a panel of monoclonal antibodies using Th1 cells as immunogen. Screening of the panel identified two Th1-specific antibodies, both of which recognized Tim-3. Further analysis established that Tim-3 is not expressed by naïve CD4 T cells but is induced following TCR-induced activation and polarization toward the Th1 phenotype. This report also showed that Tim-3 was transiently expressed by T cells in mice subjected to experimental autoimmune encephalitis (EAE) challenge and that injection of anti-Tim-3 antibodies accelerated EAE development as well as its severity. These results are consistent with the idea that injection of anti-Tim-3 antibodies disrupts a pathway that restrains Th1-dependent responses. Of note, Lee et al. [47] reported that Tim-3 KO mice subjected EAE challenge did not show the same dramatic effects on disease caused by injecting Tim-3 antibodies, although changes were observed that suggest Tim-3 suppresses EAE and Th1-driven responses.
A number of reports following that by Monney et al. showed injecting mice with Tim-3-specific antibodies or Tim-3-Fc fusion proteins can increase Th1-dependent immune responses. Collectively, these studies employed several different immune system challenges, including mouse models for autoimmunity and autoinflammation [6, 48-54], allotransplantation [55, 48, 56], tolerance induction [6, 48] and allergy [57, 58]. Thus, a substantial body of data has accumulated to support the conclusion that Tim-3 exerts a suppressive effect on Th1 cells. A complication inherent to these studies is that Tim-3 is expressed by multiple immune cell types, including dendritic cells, macrophages, natural killer cells and regulatory T cells [28, 35, 22, 55, 59, 60, 45]. Thus, the effects caused by injection of Tim-3 antibodies or Tim-3-Fc fusion proteins could reflect changes in the activity of several cell types and pathways.
As mentioned previously, Galectin-9 can function as a ligand for Tim-3 and induces the death of Tim-3+ Th1 cells in vitro [33]. Given this finding, a number of studies sought to determine whether Galectin-9 regulates Th1 responses in vivo; these included analysis of how Galectin-9 injection into mice affects the development of autoimmune disease [33, 61, 52], allograft rejection [62-64], responses to infection [65] as well as other experimentally-induced conditions [66-70]. Overall, the results from these studies suggest that ligation of Tim-3 by Galectin-9 leads to suppression of Th1-dependent immune responses. Interestingly, some of these reports provided evidence that injecting Galectin-9 has several distinct effects on the immune system, including the expansion of regulatory T (Treg) cells [61, 71, 65] and myeloid suppressor cells [67, 68] in addition to reductions in Th1 cell populations. Therefore, as with injection of Tim-3 antibodies or Tim-3-Fc fusion proteins, injecting Galectin-9 may affect several cell types and pathways, with the net effect being inhibition of responses driven by Th1 cells. Additionally, Galectin-9 can bind protein disulfide isomerases displayed on the cell surface [72], raising the possibility that Galectin-9 regulates immune system function through Tim-3-independent mechanisms.
Recent studies have investigated how Tim-3 influences CD4 T cell responses to microbial infections. Exploration of this aspect of Tim-3 biology has lagged behind, although some insights have been gained. Sehrawat et al. [65] were the first to examine whether Tim-3 (and Galectin-9) regulate CD4 T cell responses to microbial infection. Using a mouse model for herpes simplex virus (HSV) infection of the eye, this study showed that the majority of T cells within the cornea and trigeminal ganglia of HSV-infected mice express Tim-3. Further, injecting Tim-3 antibodies exacerbated HSV-induced ocular lesion formation, while injecting Galectin-9 inhibited this process. Together, these findings suggest that Tim-3 functions to restrain pathologic immune responses to HSV infection. Similarly, a study involving mice infected with Schistosoma japonicum showed that injection of Tim-3-Fc fusion protein increased the frequency of CD4 T cells expressing IFNγ, suggesting that Tim-3 suppresses Th1 responses elicited by the parasite [73].
How Tim-3 influences CD4 T cell responses to infections in humans has also been examined. Here, the majority of published studies focused on analyzing samples from patients suffering from chronic infections. In a landmark study published in 2008, Jones et al. [74] showed that the frequencies of Tim-3+ CD4 T cells were higher in peripheral blood lymphocytes (PBL) from HIV-1-infected patients relative to controls and that increases in the frequencies of Tim-3+ CD4 T cells correlated with disease progression. Similar findings were reported by a subsequent study [75]. Strikingly, Jones et al. also showed that Tim-3+ CD4 T cells in PBL from HIV-infected patients had defective responses to TCR-mediated stimulation in vitro and that addition of a soluble form of Tim-3 or Tim-3-specific antibodies could restore functionality to these cells. Similarly, a study by Golden-Mason et al. [76] showed that CD4 T cells in PBL from patients suffering from chronic hepatitis C virus (HCV) infection expressed elevated levels of Tim-3 and displayed signs of functional impairment that could be reversed by the addition Tim-3-specific antibodies. A later study reproduced some of these findings and also provided data suggesting that Tim-3 regulates Treg function in the context of chronic HCV infection [77]. Together, these findings were the first to associate Tim-3 expression by CD4 T cells with a dysfunctional, or exhausted, state characterized by impaired functional responses to TCR stimulation [78].
Similar to some of the results described above, Qiu et al. [79] showed that the frequency of Tim-3+ CD4 T cells was increased in PBL from patients actively infected with Mycobacterium tuberculosis (Mtb). Surprisingly, however, this study also showed that Tim-3 expression marks CD4 T cells with augmented, rather than impaired, effector responses to TCR stimulation or exposure to Mtb-infected macrophages. Moreover, the addition of Tim-3 antibodies to PBL from Mtb patients increased TCR-induced responses by Tim-3+ CD4 T cells, while siRNA-mediated knockdown of Tim-3, or the addition of a soluble form of Tim-3, resulted in decreased cytokine production by these cells. Overall, these findings suggest that Tim-3 can promote CD4 T cell responses mounted against Mtb infection. This conclusion contrasts with that from studies of how Tim-3 affects CD4 T cell responses to other chronic infections, which, as described above, established an association between Tim-3 and T cell exhaustion. Considered together, these findings raise the possibility that Tim-3 function is influenced by context and that Tim-3 may inhibit or promote CD4 T cell responses depending upon the microbe involved and the characteristics of the immune response elicited by the infection.
Regulation of CD8 T cell responses by Tim-3
As described above, substantial effort has been directed toward understanding how Tim-3 regulates effector responses by Th1-type CD4 T cells. Yet, in more recent years, defining how Tim-3 affects CD8 T cell function has emerged as a major focus of investigation. Studies employing mice have demonstrated that Tim-3 is essentially absent on naïve CD8 T cells but is expressed by CD8 T cells that become activated due to microbial infection [80, 37, 81-85]. In addition, a number of studies involving mice or human samples have shown that chronic infections result in high levels of Tim-3 expression by activated CD8 T cells [74, 76, 86, 87, 81, 88, 79, 89-92]. Lastly, data from mouse cancer models or analysis of patient samples revealed that CD8 T cells infiltrating tumors express Tim-3 [93-98]. Overall, these findings suggest that pathways which promote CD8 T cell responses control Tim-3 expression and that Tim-3 expression is a general feature of activated CD8 T cells. This contrasts with CD4 T cells, which seem to largely restrict Tim-3 expression to Th1 cells and some fraction of Tregs.
To date, few studies have addressed the role of Tim-3 in regulating CD8 T cell responses to acute infection. From analysis of mice infected with HSV via footpad injection, Sehrawat et al. [37] showed that HSV-specific CD8 T cells express Tim-3 and that infusion of α-lactose to block Galectin-9-mediated interactions enhanced CD8 T cell responses to HSV challenge. Conversely, injecting Galectin-9 inhibited HSV-specific CD8 T cell responses. This study also showed that HSV-specific CD8 T cell responses were greater in Galectin-9 KO mice relative to controls. Similarly, Sharma et al. [83] showed that CD8 T cell responses to influenza A virus (IAV) were greater in Galectin-9 KO mice. This study also reported that injection of a Tim-3-Fc fusion protein could augment IAV-specific CD8 T cell responses. In another study involving IAV, Cho et al. [82], showed that CD8 T cell responses to IAV infection were attenuated in mice expressing a form of Tim-3 lacking the cytoplasmic tail. The authors attributed this effect to enhanced inhibitory function by the mutant form of Tim-3, although other explanations are plausible, including a dominant-negative effect on normal Tim-3 function. Taken together, these findings suggest that Tim-3 functions to restrain CD8 T cell responses to acute infection.
In our studies, we sought to determine whether Tim-3 KO mice had altered responses to acute infection by Listeria monocytogenes (LM) [85]. Surprisingly, we found that both primary and secondary CD8 T cell responses to LM infection were impaired, rather than augmented, by the absence of Tim-3. To determine whether these defects reflected a direct effect on CD8 T cells, samples containing an equal number of wild-type and Tim-3 KO CD8 T cells were injected into wild-type hosts, which were then infected with LM. These experiments showed that both primary and secondary responses by Tim-3 KO CD8 T cells were blunted relative those mounted by wild-type cells in the same host. Considered together, our findings indicate that Tim-3 promotes CD8 T cell responses to acute LM infection via a cell-intrinsic mechanism. Similar to some observations regarding the molecular function of Tim-3 and the influence of Tim-3 on CD4 T cell responses, our results seem contradictory to those obtained from the studies involving HSV or IAV infection outlined above. Here again, one possible explanation for the conflicting outcomes is that role of Tim-3 in regulating CD8 T cells is influenced by context. Thus, Tim-3 may either inhibit or promote CD8 T cell responses depending upon the microbial infection involved and the characteristics of the resulting immune response.
In contrast to how Tim-3 influences acute responses by CD8 T cells, the role of Tim-3 in regulating CD8 T cell responses to chronic infections has been more intensively investigated. As with CD4 T cells, exploration of this area was sparked by findings published by Jones et al. [74]. This study showed that the frequencies of activated CD8 T cells expressing Tim-3 were significantly higher in patients infected with HIV-1 and that these cells are functionally impaired based on in vitro analysis. The functional impairment of Tim-3+ CD8 T cells was correlated with reduced activation of Stat5 in response to IL-2 and impaired activation of p38 and ERK in response to pharmacologic agents that mimic TCR stimulation. Moreover, TCR-induced responses by Tim-3+ CD8 T cells were augmented by the addition of a soluble form of Tim-3 or Tim-3-specific antibodies, suggesting that Tim-3 enforces functional impairment. Following this report, several studies showed that the frequencies of Tim-3+ CD8 T cells were elevated in PBL from patients chronically infected with HBV or HCV and that these cells were in a dysfunctional state, with some of these studies providing evidence that impairment of CD8 T cell function was at least partly dependent on Tim-3 [76, 87, 86, 89, 99, 92].
Studies involving mouse models for chronic infections produced results similar to those described above. Jin et al. [81] showed that mice chronically infected with the clone 13 strain of lymphocytic choriomeningitis virus (LCMV) generate Tim-3+ CD8 T cells that are exhausted but which regain function following injection of a Tim-3 fusion protein together with antibodies that block engagement of the inhibitory receptor PD-1. Somewhat similar results were obtained from two studies of the Friend Virus mouse model for chronic infection, although the outcome of combined PD-1 and Tim-3 blockade varied depending on the strategy used [100, 101]. Finally, studies involving either mouse models or human samples have shown that Tim-3+ CD8 T cells localized to sites of tumor growth display an exhausted phenotype and that blockade of ligand interactions by Tim-3 alone or in combination with blockade of PD-1 can restore functionality to these cells [93, 94, 102, 95]. Considered together, these findings support the conclusion that, in settings involving chronic stimulation, Tim-3 expression serves as a marker for CD8 T cells that have entered a dysfunctional, or exhausted, state [78]. Moreover, these findings also indicate that Tim-3 is involved in enforcing CD8 T cell exhaustion, as illustrated by the restoration of function to CD8 T cells when reagents that block Tim-3-ligand interactions are employed.
An exception to the paradigm described above is illustrated by findings reported by Qiu et al. [79]. This study showed that Tim-3+ CD8 T cells from Mtb-infected patients have augmented, rather than impaired cytotoxic responses and cytokine production capacity relative to Tim-3− CD8 T cells. These effects correlated with data showing that TCR-mediated stimulation caused increased activation of Stat5, Stat3, p38 and ERK in Tim-3+ CD8 T cells relative to their Tim-3− counterparts. Further, siRNA-mediated knockdown of Tim-3 resulted in decreased effector responses by Tim-3+ CD8 T cells, while the addition of anti-Tim-3 antibodies promoted these responses. Of note, however, studies by Wang et al. [103] provided some evidence that Tim-3+ CD8 T cells from Mtb-infected patients had impaired, rather than augmented, functionality. With this caveat in mind, the studies by Qiu et al. at least suggest that Tim-3 may not always mark exhausted CD8 T cells in contexts involving chronic stimulation.
Conclusions and future perspectives
Roughly a dozen years have passed since the publication of initial papers [46, 28] indicating that Tim-3 has a role in the immune system. These reports suggested that Tim-3 regulates CD4 T cell responses and identified Tim-3 as a potential therapeutic target for modulating CD4 T cell function. However, with further characterization of Tim-3, it has become clear that several cell types within the immune system express Tim-3, including activated CD8 T cells. It has also become clear that Tim-3 regulates both the innate and adaptive arms of the immune system. Adding further complication, multiple ligands exist for Tim-3 and there is evidence that Tim-3 stimulates as well as inhibits immune cell function. Lastly, although the majority of in vivo studies suggested that Tim-3 functions to suppress immune responses, others have challenged this conclusion, putting forth the possibility that the function of Tim-3 is influenced by context-dependent factors. Thus, the landscape of existing information regarding Tim-3 presents a complex picture and our understanding of Tim-3’s role in the immune system is far from complete. What has been learned thus far suggests that Tim-3 still holds promise as a therapeutic target for manipulating immune responses. Nonetheless, fulfilling this promise hinges on establishing a more detailed picture of how Tim-3 functions at the molecular, cellular and systemic level.
With regard to the role of Tim-3 in T cell responses, it seems necessary to further characterize the relationships between Tim-3 and its ligands and more precisely determine how these affect T cell function. A substantial body of data suggests that interaction between Galectin-9 and Tim-3 exerts an inhibitory effect on responses by CD4 and CD8 T cells. Although in vitro experiments showed that Galectin-9 can directly regulate T cells, in vivo studies indicated that Galectin-9 has a more complex role in the immune system and likely affects that activity of several cell types. Therefore, it would be informative to determine the impact of Galectin-9 in settings where Tim-3 expression is absent or is restricted to specific cell types. This approach could identify the immune cell subsets and pathways that are affected by Galectin-9 and perhaps uncover strategies for manipulating Tim-3 and Galectin-9 dependent pathways. In a similar vein, examining how injecting anti-Tim-3 antibodies affects immune responses when Tim-3 expression is restricted to specific cell types could provide insights regarding how this treatment modulates immune responses.
Another key challenge for future studies will be to better define how Tim-3 functions at the molecular level. Much of the information gained in this area comes from characterizing Tim-3 function in transformed cell lines. Although this approach has proven valuable, future studies should examine the impact of Tim-3 on signaling pathways and its relationship with known functional partners in primary immune cells. Goals for these efforts would include determining mechanisms by which Tim-3 functions to inhibit, or activate, immune cell function and determining the relevance of particular pathways to the regulation of specific immune cell types. A prominent challenge in this area is elucidating the molecular mechanisms by which Tim-3 contributes to the maintenance and reversal of CD8 T cell exhaustion. This aspect of Tim-3 biology is especially intriguing given that antibodies specific for the inhibitory receptors CTLA-4 and PD-1 have efficacy as treatments for certain forms of cancer [104]. Thus, gaining more insight in how Tim-3 suppresses CD8 T cell function could elucidate novel strategies for treating tumors or chronic infections.
Acknowledgments
This work was supported by the following grants from the National Institutes: T32 AI007485 (to J.V.G.); R01AI054821 and R01AI093737 (to J.D.C.).
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Kane LP. T cell Ig and mucin domain proteins and immunity. J Immunol. 2010;184(6):2743–9. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–89. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 4.Han G, Chen G, Shen B, Li Y. Tim-3: An Activation Marker and Activation Limiter of Innate Immune Cells. Front Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277(42):39739–48. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 6.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–10. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 7.Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176(3):1411–20. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 8.Moller-Hackbarth K, Dewitz C, Schweigert O, Trad A, Garbers C, Rose-John S, et al. A Disintegrin and Metalloprotease (ADAM) 10 and ADAM17 Are Major Sheddases of T Cell Immunoglobulin and Mucin Domain 3 (Tim-3) J Biol Chem. 2013;288(48):34529–44. doi: 10.1074/jbc.M113.488478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweigert O, Dewitz C, Moller-Hackbarth K, Trad A, Garbers C, Rose-John S, et al. Soluble T cell immunoglobulin and mucin domain (TIM)-1 and -4 generated by A Disintegrin And Metalloprotease (ADAM)-10 and -17 bind to phosphatidylserine. Biochim Biophys Acta. 2014;1843(2):275–87. doi: 10.1016/j.bbamcr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11(8):362–9. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AC, Anderson DE. TIM-3 in autoimmunity. Curr Opin Immunol. 2006;18(6):665–9. doi: 10.1016/j.coi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Oh JM, Hwang JW, Ahn JK, Bae EK, Won J, et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis. Scand J Rheumatol. 2011;40(5):334–40. doi: 10.3109/03009742.2010.547871. [DOI] [PubMed] [Google Scholar]
- 13.de Souza AJ, Oriss TB, Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102(47):17113–8. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol. 2008;180(10):6518–26. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351(2):571–6. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, et al. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol. 2011;31(19):3963–74. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtiss ML, Hostager BS, Stepniak E, Singh M, Manhica N, Knisz J, et al. Fyn binds to and phosphorylates T cell immunoglobulin and mucin domain-1 (Tim-1) Mol Immunol. 2011;48(12-13):1424–31. doi: 10.1016/j.molimm.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J Immunol. 2007;178(7):4342–50. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 19.Clayton KL, Haaland MS, Douglas-Vail MB, Mujib S, Chew GM, Ndhlovu LC, et al. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J Immunol. 2014;192(2):782–91. doi: 10.4049/jimmunol.1302663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180(7):4706–13. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6(5):447–54. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–3. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 23.Mariat C, Degauque N, Balasubramanian S, Kenny J, DeKruyff RH, Umetsu DT, et al. Tim-1 signaling substitutes for conventional signal 1 and requires costimulation to induce T cell proliferation. J Immunol. 2009;182(3):1379–85. doi: 10.4049/jimmunol.182.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HS, Kim HS, Lee CW, Chung DH. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol. 2010;184(8):4095–106. doi: 10.4049/jimmunol.0901991. [DOI] [PubMed] [Google Scholar]
- 25.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18(9):1394–400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229(1):259–70. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone SM. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15(16):4282–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 29.Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26(3):311–21. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27(6):941–51. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26(3):299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918–30. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230(1):144–59. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–30. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 36.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13(9):832–42. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6(5):e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su EW, Bi S, Kane LP. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology. 2011;21(10):1258–65. doi: 10.1093/glycob/cwq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leitner J, Rieger A, Pickl WF, Zlabinger G, Grabmeier-Pfistershammer K, Steinberger P. TIM-3 does not act as a receptor for galectin-9. PLoS Pathog. 2013;9(3):e1003253. doi: 10.1371/journal.ppat.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int Immunol. 2007;19(6):763–73. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Woo MY, Chwae YJ, Kwon MH, Kim K, Park S. Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription. Immunobiology. 2012;217(10):986–95. doi: 10.1016/j.imbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178(11):6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, et al. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol. 2013;190(5):2068–79. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, et al. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukocyte Biol. 2012;91(2):189–96. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52(3):322–9. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 46.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2(12):1109–16. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 47.Lee SY, Goverman JM. The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells. J Immunol. 2013;190(10):4991–9. doi: 10.4049/jimmunol.1300083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4(11):1093–101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Chen G, Li Y, Wang R, Wang L, Lin Z, et al. Involvement of T cell Ig Mucin-3 (Tim-3) in the negative regulation of inflammatory bowel disease. Clin Immunol. 2010;134(2):169–77. doi: 10.1016/j.clim.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Schroll A, Eller K, Huber JM, Theurl IM, Wolf AM, Weiss G, et al. Tim3 is upregulated and protective in nephrotoxic serum nephritis. Am J Pathol. 2010;176(4):1716–24. doi: 10.2353/ajpath.2010.090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi F, Guo X, Jiang X, Zhou P, Xiao Y, Zhou T, et al. Dysregulated Tim-3 expression and its correlation with imbalanced CD4 helper T cell function in ulcerative colitis. Clin Immunol. 2012;145(3):230–40. doi: 10.1016/j.clim.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Kanzaki M, Wada J, Sugiyama K, Nakatsuka A, Teshigawara S, Murakami K, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. 2012;153(2):612–20. doi: 10.1210/en.2011-1579. [DOI] [PubMed] [Google Scholar]
- 53.Foks AC, Ran IA, Wasserman L, Frodermann V, Ter Borg MN, de Jager SC, et al. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(11):2558–65. doi: 10.1161/ATVBAHA.113.301879. [DOI] [PubMed] [Google Scholar]
- 54.Kaneyama T, Tomiki H, Tsugane S, Inaba Y, Ichikawa M, Akiba H, et al. The TIM-3 pathway ameliorates Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Int Immunol. 2014 doi: 10.1093/intimm/dxt056. doi:10.1093/intimm/dxt056. [DOI] [PubMed] [Google Scholar]
- 55.Boenisch O, D’Addio F, Watanabe T, Elyaman W, Magee CN, Yeung MY, et al. TIM-3: a novel regulatory molecule of alloimmune activation. J Immunol. 2010;185(10):5806–19. doi: 10.4049/jimmunol.0903435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veenstra RG, Taylor PA, Zhou Q, Panoskaltsis-Mortari A, Hirashima M, Flynn R, et al. Contrasting acute graft-versus-host disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 2012;120(3):682–90. doi: 10.1182/blood-2011-10-387977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Akiba H, et al. Antibodies to T-cell Ig and mucin domain-containing proteins (Tim)-1 and -3 suppress the induction and progression of murine allergic conjunctivitis. Biochem Biophys Res Commun. 2007;353(1):211–6. doi: 10.1016/j.bbrc.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Kearley J, McMillan SJ, Lloyd CM. Th2-driven, allergen-induced airway inflammation is reduced after treatment with anti-Tim-3 antibody in vivo. J Exp Med. 2007;204(6):1289–94. doi: 10.1084/jem.20062093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, et al. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J Clin Invest. 2012;122(7):2395–404. doi: 10.1172/JCI45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7(2):e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127(1):78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Wang F, He W, Yuan J, Wu K, Zhou H, Zhang W, et al. Activation of Tim-3-Galectin-9 pathway improves survival of fully allogeneic skin grafts. Transpl Immunol. 2008;19(1):12–19. doi: 10.1016/j.trim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Chou FC, Kuo CC, Wang YL, Lin MH, Yen BL, Chang DM, et al. Overexpression of Galectin-9 in Islets Prolongs Grafts Survival via Downregulation of Th1 Responses. Cell Transpl. 2012;22(11):2135–45. doi: 10.3727/096368912X657891. [DOI] [PubMed] [Google Scholar]
- 64.Sakai K, Kawata E, Ashihara E, Nakagawa Y, Yamauchi A, Yao H, et al. Galectin-9 ameliorates acute GVH disease through the induction of T-cell apoptosis. Eur J Immunol. 2011;41(1):67–75. doi: 10.1002/eji.200939931. [DOI] [PubMed] [Google Scholar]
- 65.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182(5):3191–201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niwa H, Satoh T, Matsushima Y, Hosoya K, Saeki K, Niki T, et al. Stable form of galectin-9, a Tim-3 ligand, inhibits contact hypersensitivity and psoriatic reactions: a potent therapeutic tool for Th1- and/or Th17-mediated skin inflammation. Clin Immunol. 2009;132(2):184–94. doi: 10.1016/j.clim.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185(3):1383–92. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kojima K, Arikawa T, Saita N, Goto E, Tsumura S, Tanaka R, et al. Galectin-9 attenuates acute lung injury by expanding CD14-plasmacytoid dendritic cell-like macrophages. Am J Resp Crit Care. 2011;184(3):328–39. doi: 10.1164/rccm.201010-1566OC. [DOI] [PubMed] [Google Scholar]
- 69.Wang F, Xu J, Liao Y, Wang Y, Liu C, Zhu X, et al. Tim-3 ligand galectin-9 reduces IL-17 level and accelerates Klebsiella pneumoniae infection. Cell Immunol. 2011;269(1):22–8. doi: 10.1016/j.cellimm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD4(+)CD25(low/int) effector T-Cell apoptosis and increasing regulatory T cell number. PLoS One. 2012;7(10):e48379. doi: 10.1371/journal.pone.0048379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5(3):e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bi S, Hong PW, Lee B, Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci U S A. 2011;108(26):10650–5. doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi Y, Song XR, Shen JL, Xu YH, Shen Q, Luo QL, et al. Tim-2 up-regulation and galectin-9-Tim-3 pathway activation in Th2-biased response in Schistosoma japonicum infection in mice. Immunol Lett. 2012;144(1-2):60–6. doi: 10.1016/j.imlet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205(12):2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, et al. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185(5):3007–18. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83(18):9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, et al. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189(2):755–66. doi: 10.4049/jimmunol.1200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 79.Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, et al. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 2012;8(11):e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, et al. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6(1):35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho JL, Roche MI, Sandall B, Brass AL, Seed B, Xavier RJ, et al. Enhanced Tim3 activity improves survival after influenza infection. J Immunol. 2012;189(6):2879–89. doi: 10.4049/jimmunol.1102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma S, Sundararajan A, Suryawanshi A, Kumar N, Parga T, Kuchroo VK, et al. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc Natl Acad Sci U S A. 2011;108(47):19001–6. doi: 10.1073/pnas.1107087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cyktor JC, Carruthers B, Beamer GL, Turner J. Clonal expansions of CD8+ T cells with IL-10 secreting capacity occur during chronic Mycobacterium tuberculosis infection. PLoS One. 2013;8(3):e58612. doi: 10.1371/journal.pone.0058612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorman JV, Starbeck-Miller G, Pham NL, Traver GL, Rothman PB, Harty JT, et al. Tim-3 Directly Enhances CD8 T Cell Responses to Acute Listeria monocytogenes Infection. J Immunol. 2014;192(7):3133–42. doi: 10.4049/jimmunol.1302290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue FY, et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol. 2010;40(9):2493–505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 87.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–57. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virology J. 2011;8:113. doi: 10.1186/1743-422X-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7(10):e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tandon R, Giret MT, Sengupta D, York VA, Wiznia AA, Rosenberg MG, et al. Age-related expansion of Tim-3 expressing T cells in vertically HIV-1 infected children. PLoS One. 2012;7(9):e45733. doi: 10.1371/journal.pone.0045733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013;9(6):e1003422. doi: 10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumida K, Shimoda S, Iwasaka S, Hisamoto S, Kawanaka H, Akahoshi T, et al. Characteristics of Splenic CD8 T Cell Exhaustion in Patients with Hepatitis C. Clin Exp Immunol. 2013;174(1):172–8. doi: 10.1111/cei.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7(2):e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–51. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 98.Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, et al. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19(19):5351–60. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, et al. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42(5):1180–91. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 100.Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, et al. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J Immunol. 2010;184(9):4696–707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- 101.Dietze KK, Zelinskyy G, Liu J, Kretzmer F, Schimmer S, Dittmer U. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8(+) T cells and efficiently reduces chronic retroviral loads. PLoS Pathog. 2013;9(12):e1003798. doi: 10.1371/journal.ppat.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Cao Z, Jiang J, Li Y, Dong M, Ostrowski M, et al. Elevated expression of Tim-3 on CD8 T cells correlates with disease severity of pulmonary tuberculosis. J Infection. 2011;62(4):292–300. doi: 10.1016/j.jinf.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukocyte Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]