Abstract
Background
Rectus sheath block can provide analgesia following umbilical hernia repair. However, conflicting reports on its analgesic effectiveness exist. No study has investigated plasma local anesthetic concentration following ultrasound-guided rectus sheath block (USGRSB) in children.
Objectives
Compare the effectiveness and bupivacaine absorption following USGRSB or wound infiltration (WI) for umbilical hernia repair in children.
Methods
A randomized blinded study comparing WI to USGRSB in 40 children undergoing umbilical hernia repair was performed. Group WI (n=20) received wound infiltration 1mg/kg 0.25% bupivacaine. Group RS (n=20) received USGRSB 0.5mg/kg 0.25% bupivacaine per side in the posterior rectus sheath compartment. Pain scores and rescue analgesia were recorded. Blood samples were drawn at 0, 10, 20, 30, 45 and 60 minutes.
Results
Patients in the WI group had a 2-fold increased risk of requiring morphine (Hazard ratio 2.06, 95% CI 1.01, 4.20, p=0.05). When required, median time to first morphine dose was longer in the USGRSB group (65.5 min vs 47.5 min, p=0.049). Peak plasma bupivacaine concentration was higher following USGRSB than WI (median: 631.9 ng/ml IQR: 553.9 – 784.1 vs 389.7 ng/ml IQR: 250.5-502.7, p= 0.002). Tmax was longer in the USGRSB group (median 45 min IQR: 30 - 60 vs 20 min IQR: 20 – 45, p= 0.006).
Conclusions
USGRSB provides more effective analgesia than WI for umbilical hernia repair. USGRSB with 1mg/kg 0.25% bupivacaine is associated with safe plasma bupivacaine concentration that peaks higher and later than WI. Caution against using larger volumes of higher concentration local anesthetic for USGRSB is advised.
MeSH-compliant keywords: Pharmacokinetics; Drugs, regional; Ultrasound, regional; Pain, child; Age, outpatient; Ambulatory, local anesthetics; Drugs
Background
Rectus sheath block is a technique used to provide postoperative analgesia following umbilical hernia repair in children.(1,2) Since the initial description in 1899, the technique has evolved, most recently to incorporate ultrasound-guidance for placement.(3) Although this technology has arguably improved accuracy - and thus effectiveness,(4) there are data to suggest that ultrasound guidance for tissue plane blocks results in increased local anesthetic absorption and higher plasma concentration.(5-7)
Opinions vary(8) and conflicting data exist regarding the analgesic effectiveness of rectus sheath blocks for patients undergoing umbilical hernia repair.(9,10) Observational data in 30 children who underwent ultrasound-guided rectus sheath block (USGRSB) for umbilical hernia repair demonstrated no need for rescue analgesia in the early postoperative period,(3) while a prospective randomized trial showed decreased perioperative morphine consumption with USGRSB compared to wound infiltration (WI).(10,11) However, data from a small pilot study investigating WI vs rectus sheath block for umbilical hernia repair in children showed no difference in morphine consumption.(9)
Local anesthetic plasma concentration following tissue plane blocks varies widely depending upon block type, volume and concentration used, and patient age.(12) Potentially toxic concentrations with similar tissue-plane techniques have been described in adults,(6,13,14) and recent pharmacokinetic data in children undergoing ilio-inguinal block suggest caution and reduced local anesthetic volumes for ultrasound-guided techniques in children.(5)
The objectives of this study were to 1) assess analgesic effectiveness of USGRSB compared to WI for umbilical hernia repair by comparing morphine consumption in a prospective, blinded fashion, and 2) investigate bupivacaine absorption following USGRSB or WI.
Methods
Following registration at www.clinicaltrials.gov (NCT00836134, SFlack, 02/03/2009), IRB approval and written informed parental consent, a randomized blinded study comparing USGRSB to WI in 40 children undergoing umbilical hernia repair was performed. Power analysis was not performed with the intent to use the morphine consumption data to obtain data for power/sample size calculations in future studies. Exclusion criteria included age <1 or >17 years, bupivacaine or morphine allergy, local infection, coagulopathy, emergency surgery, additional surgical procedures, and patient or parent refusal. Of 129 umbilical hernia repairs performed at our institution during the study period, 32 were excluded because they required multiple surgical procedures. The total number of patients who declined enrollment was not recorded. Computer generated randomization was stratified based on age (1-3 and 4-17 years) and sequences of sealed envelopes were provided by biostatistical services.
All patients received a standard anesthetic including oral premedication with 0.5 mg/kg midazolam, mask induction and maintenance with sevoflurane with or without neuromuscular blockade. A second peripheral intravenous cannula was placed for study blood draws. Group RS (n=20) received an USGRSB using 0.2 ml/kg 0.25% bupivacaine (1 mg/kg) to each side at least 10 minutes prior to incision. Bupivacaine was placed in the posterior compartment of the rectus sheath as demonstrated in Figure 1.(2,15) Group WI (n=20) received fentanyl 2 mcg/kg prior to incision and wound infiltration of 0.4 ml/kg 0.25% bupivacaine (1mg/kg) at the end of surgery in accordance with the surgical group's standard practice. Rescue analgesia for both groups consisted of fentanyl 1mcg/kg, administered if intraoperative heart rate or blood pressure exceeded 20% of baseline. No other intraoperative analgesics or antiemetics were administered.
Figure 1.
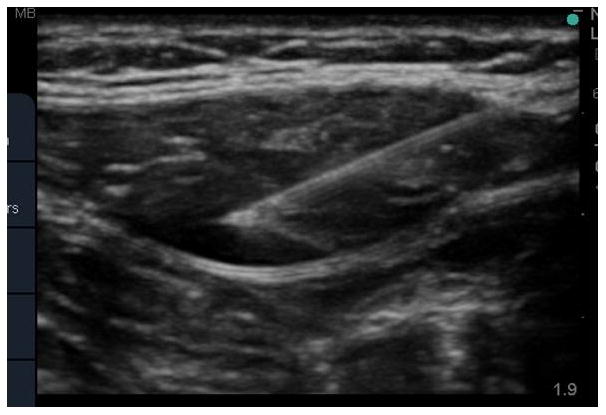
Ultrasound-guided rectus sheath block. The needle tip is visualized in the posterior rectus sheath with tissue plane hydrodissection from local anesthetic spread.
Blood samples (3ml aliquots) were drawn at baseline, and also at 10, 20, 30, 45 and 60 minutes after the USGRSB or wound infiltration in each patient. Rescue analgesia (morphine 30 mcg/kg) was provided as necessary by the recovery room nurse, consistent with our standard practice. Pain scores were assessed by a blinded observer using an age appropriate scale (FLACC age 1-3 years or FACES age 4-17 years).(16) Scoring commenced upon arrival in the recovery room and was performed every 5 minutes until complete emergence from anesthesia, then every 30 minutes until discharge home.
Plasma total bupivacaine concentration analysis was performed according to the Bioanalytical Method for bupivacaine in plasma (FHCRC BAM192, edition 1, 8-10-09). A series 1100 SL mass spectrometer (Agilent Technologies Inc., Santa Clara, CA) was operated in the positive electrospray SIM mode monitoring ions 289 m/z for bupivacaine, 298 m/z for d9-bupivacaine. The LLOQ was 0.89 ng/mL with a precision of 2.36%. The assay bias (accuracy) across the calibration curve (20 to 1776 ng/mL) was 0.2% (-1.8% to +2%).
Statistical Methods
Analyses included all patients, grouped by randomization assignment (Intention-to-treat). Baseline characteristics were compared using the t-test for continuous variables and the chi-square test for categorical variables. Surgical duration was compared using the Wilcoxon rank-sum test. The distribution of time to first morphine use is displayed using Kaplan-Meier estimates. Time to first morphine use was set to the number of minutes from end of surgery to the administration of the first dose of morphine, or to 96 minutes for patients who did not receive morphine (these patients are censored in the Kaplan-Meier figure at 96 minutes). The hazard ratio for morphine use in the WI group compared to the USGRSB group was estimated using Cox Proportional Hazards Regression with treatment assignment included as a covariate. Pain scores were compared using the Wilcoxon rank-sum test. Peak plasma bupivacaine concentration and time to maximum concentration were compared with the Wilcoxon Rank-Sum Test. Results are presented as medians and inter-quartile range (IQR).
Results
Forty children presenting for umbilical hernia repair were enrolled and randomized (Figure 2. Consort diagram). As demonstrated in Table 1, there was no difference in age, weight, gender, or surgical duration between groups.
Figure 2. Patient allocation and disposition.
Table 1.
Demographic data for rectus sheath and wound infiltration groups.
| Rectus Sheath (N=20) | Infiltration (N=20) | Total (N=40) | P-value | ||
|---|---|---|---|---|---|
| Age (years)1 | Mean ± SD | 5.85 ± 2.16 | 4.95 ± 2.09 | 5.40 ± 2.15 | 0.188 |
| Sex3 | Male | 8 ( 40.0%) | 5 ( 25.0%) | 13 ( 32.5%) | 0.311 |
| Female | 12 ( 60.0%) | 15 ( 75.0%) | 27 ( 67.5%) | ||
| Weight (kg)1 | Mean ± SD | 22.6 ± 8.44 | 21.0 ± 7.76 | 21.8 ± 8.04 | 0.537 |
| Height (cm)1 | Mean ± SD | 117 ± 16.0 | 116 ± 23.5 | 117 ± 19.9 | 0.815 |
| Proc Duration (min)2 | Median | 30.00 | 42.00 | 32.50 | 0.228 |
| 25, 75%tile | 22.00, 44.00 | 27.00, 60.00 | 22.50, 53.00 |
t-test for independent samples
Wilcoxon rank-sum test
chi-square test
Intraoperative HR or BP remained within 20% of baseline in all patients; consequently, rescue analgesia was not required. Figure 3 demonstrates that patients in the WI group had a 2-fold increase in risk of requiring morphine compared to the USGRSB group (Hazard ratio 2.06, 95% CI 1.01, 4.20, p=0.05). Time to first dose of morphine was longer in the USGRSB group (median: 65.5 min, IQR: 45.5-96 min vs median: 47.5 min, IQR: 34.6-63.8 min; p=0.049).
Figure 3.
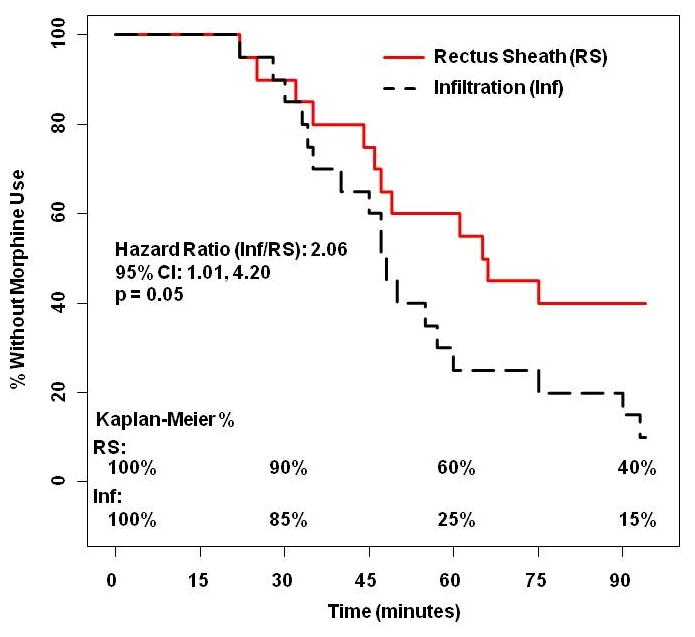
The Kaplan Meier curve demonstrates that patients in the wound infiltration group were twice as likely to require morphine for post-operative pain relief (p<0.05).
The USGRSB group had lower morphine consumption than the WI group (median: 26.35 mcg/kg, IQR: 0-65.04 vs median: 58.54 mcg/kg, IQR: 27.14-115.5; p = 0.042), despite fentanyl 2mcg/kg given to all patients in the WI group. No intraoperative fentanyl was required for any patient in the USGRSB group. There was no difference in the average maximum pain scores between groups. Six patients in each group received antiemetics post-operatively.
As demonstrated in Figure 4, peak plasma bupivacaine concentration was higher following USGRSB than WI (median: 631.9 ng/ml, IQR: 553.9 – 784.1 vs 389.7 ng/ml, IQR: 250.5-502.7; p= 0.002). The time to peak concentration was longer in the USGRSB group (median 45 min, IQR: 30 - 60 vs 20 min, IQR: 20 – 45; p= 0.006).
Figure 4.
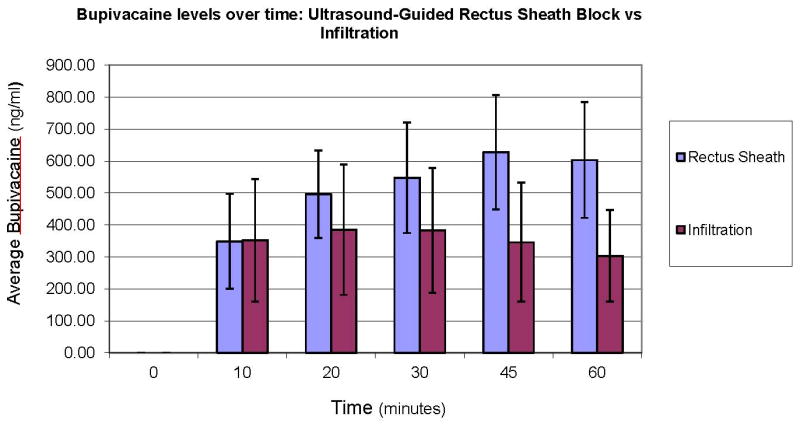
Plasma Bupivacaine concentrations over time. Peak plasma concentrations were higher following USGRSB than WI (median: 631.9 ng/ml IQR: 553.9 – 784.1 vs 389.7 ng/ml IQR: 250.5-502.7, p= 0.002). The time to peak concentration was longer in the USGRSB group (median 45 min IQR: 30 - 60 vs 20 min IQR: 20 – 45, p= 0.006). No measured concentration exceeded 1 mcg/ml.
No measured plasma bupivacaine concentration exceeded 1 mcg/ml. No adverse events or any clinical evidence of toxicity was noted.
Discussion
Rectus sheath blocks can be used to provide analgesia for any midline abdominal procedures including umbilical hernia repair. A number of approaches for rectus sheath blocks have been described and include injecting local anesthetic in the anterior or posterior compartment, or at the aponeurosis of the external oblique, internal oblique and transversus abdominis muscles just lateral to the rectus sheath.(15,17,18) In order to provide uniformity for the bupivacaine absorption study, local anesthetic was uniformly placed in the posterior compartment, as described by Courreges.(15) The total bupivacaine dose for each group was the same.
This procedure is typically a day surgery, and the potential value of optimizing analgesia, minimizing opioid-related side-effects and facilitating timely discharge is an important consideration. Although USGRSB did not eliminate the need for rescue analgesia, the frequency of rescue analgesia was less and median morphine consumption was lower compared to the WI group.
0.2 ml/kg of 0.25% bupivacaine on each side for USGRSB (1 mg/kg total) is associated with safe plasma bupivacaine concentration. No measured plasma concentration exceeded 1mcg/ml, (maximum recorded 972 ng/ml or 0.972 mcg/ml), which is below the reported maximum tolerated plasma bupivacaine concentration for central nervous system (1-2 mcg/ml) or cardiovascular toxicity (3-4mcg/ml).(19,20) Plasma concentration was significantly higher, and peaked later, following USGRSB compared to WI. These results are consistent with existing data on ultrasound-guided tissue plane blocks, and support the contention that significant local anesthetic absorption occurs when local anesthetics are introduced into tissue planes. Greater absorption and higher plasma concentration after tissue plane blocks are presumed to be secondary to increased area of absorption and vascularity of the surrounding muscle tissue.(7,21) Despite its close proximity to the posterior compartment of the rectus sheath, significant absorption through the peritoneum seems unlikely since the posterior rectus sheath is relatively avascular, such that rapid absorption and consequent earlier peak concentration would not be expected. Caution is therefore advised when using higher concentrations or volumes of local anesthetic without epinephrine for tissue plane blocks. The use of epinephrine-containing local anesthetics could effect peak plasma levels after USGRSB, an area for potential future study.
The time to mean peak plasma concentration after USGRSB (45 min) was similar to the pharmacokinetic data published by Wada et al(13) in adults who received rectus sheath blocks, although arterial samples were used in that study. The time to peak concentration is longer than that reported for peak plasma concentration following other tissue plane blocks (20.4 min after ilio-inguinal blocks,(5) 30 min after Transversus Abdominis Plane (TAP) block,(6) and 20 min or 25.3 min following wound infiltration in this and another study respectively).(5) Wada et al suggested that the relatively slower absorption kinetics in the posterior rectus compartment may be due to less vascularity.(13) The relative limitation to local anesthetic spread in this compartment or contact with only one muscle layer may also contribute. A first-pass effect through the liver after peritoneal absorption may be an alternative explanation.
There are several limitations to this study. The WI group received fentanyl 2mcg/kg per study protocol to conform with the practice at our institution at that time. The fentanyl could have influenced the time to first post-operative analgesia in the WI group, but despite this, the time to first analgesia in the USGRSB group was longer. USGRSB was performed prior to the incision while WI was performed at the end of the procedure. The value of pre-emptive analgesia with peripheral nerve blocks and opiates is controversial, but could be relevant when comparing these two groups. In a recent study where the USGRSB and WI were placed at the end of surgery, the USGRSB had lower pain scores and lower opioid and non-opioid consumption compared to WI despite the use of a higher concentration of ropivacaine (0.5%) for WI and all patients received an initial dose of fentanyl and ketorolac.(11)
Although the observers measuring pain scores were blinded, the recovery room nurses administering rescue analgesia were not. This potentially introduced bias and influenced rescue analgesia administration. However, the blinded observers noted no difference in pain scores between the two groups. This finding suggests that analgesia was adequate in both groups, and that the USGRSB group received less morphine because less was required rather than because morphine was avoided in patients who received USGRSB.
An additional limitation of this study is that plasma bupivacaine levels were only measured up to 60 minutes after injection. Given the delay to peak plasma concentration with USGRSB (Tmax 45 min), ideally additional samples at 90, 120 minutes to demonstrate the decline in plasma concentration would have been collected. Wada et al observed a similarly delayed Tmax of 49.6 minutes after USGRSB with 0.25%, 0.5%, and 0.75% ropivacaine in adults.(13) It is reassuring that the mean concentration vs time (measured to 180 minutes) in that study trended down after a similar mean peak concentration. Only total bupivacaine concentration rather than unbound free fraction, the true determinant of LA toxicity, was measured.
Acknowledgments
This study was performed with ethical approval by the IRB, Seattle Children's Hospital. This study was supported, in part, by NIH Grant UL1 RR025014-01 of the University of Washington - Institute of Translational Health Sciences.
Footnotes
This report was previously presented, in part, at the Society for Pediatric Anesthesia Winter meeting 2013. Prof. Adrian Bosenberg is a section editor for Pediatric Anesthesia. This manuscript was handled by Prof. Brian Anderson
References
- 1.Finnerty O, Carney J, McDonnell JG. Trunk blocks for abdominal surgery. Anaesthesia. 2010;65(Suppl 1):76–83. doi: 10.1111/j.1365-2044.2009.06203.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla T, Sawardekar A, Dewhirst E, Jagannathan N, Tobias JD. Ultrasound-guided trunk and core blocks in infants and children. J Anesth. 2013;27:109–23. doi: 10.1007/s00540-012-1476-5. [DOI] [PubMed] [Google Scholar]
- 3.Willschke H, Bosenberg A, Marhofer P, Johnston S, Kettner SC, Wanzel O, Kapral S. Ultrasonography-guided rectus sheath block in paediatric anaesthesia--a new approach to an old technique. Br J Anaesth. 2006;97:244–9. doi: 10.1093/bja/ael143. [DOI] [PubMed] [Google Scholar]
- 4.Willschke H, Bosenberg A, Marhofer P, Johnston S, Kettner S, Eichenberger U, Wanzel O, Kapral S. Ultrasonographic-guided ilioinguinal/iliohypogastric nerve block in pediatric anesthesia: what is the optimal volume? Anesth Analg. 2006;102:1680–4. doi: 10.1213/01.ane.0000217196.34354.5a. [DOI] [PubMed] [Google Scholar]
- 5.Weintraud M, Lundblad M, Kettner SC, Willschke H, Kapral S, Lonnqvist PA, Koppatz K, Turnheim K, Bosenberg A, Marhofer P. Ultrasound versus landmark-based technique for ilioinguinal-iliohypogastric nerve blockade in children: the implications on plasma levels of ropivacaine. Anesth Analg. 2009;108:1488–92. doi: 10.1213/ane.0b013e31819cb1f3. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105:853–6. doi: 10.1093/bja/aeq255. [DOI] [PubMed] [Google Scholar]
- 7.Ala-Kokko TI, Karinen J, Raiha E, Kiviluoma K, Alahuhta S. Pharmacokinetics of 0.75% ropivacaine and 0.5% bupivacaine after ilioinguinal-iliohypogastric nerve block in children. Br J Anaesth. 2002;89:438–41. [PubMed] [Google Scholar]
- 8.Warner BW. Pain Control After Umbilical Hernia Repair: How Difficult Can We Make It?: Comment on “Ultrasonography-Guided Bilateral Rectus Sheath Block vs Local Anesthetic Infiltration After Pediatric Umbilical Hernia Repair”. JAMA Surg. 2013;1 doi: 10.1001/jamasurg.2013.1442. [DOI] [PubMed] [Google Scholar]
- 9.Isaac LA, McEwen J, Hayes JA, Crawford MW. A pilot study of the rectus sheath block for pain control after umbilical hernia repair. Paediatr Anaesth. 2006;16:406–9. doi: 10.1111/j.1460-9592.2005.01785.x. [DOI] [PubMed] [Google Scholar]
- 10.Gurnaney HG, Maxwell LG, Kraemer FW, Goebel T, Nance ML, Ganesh A. Prospective randomized observer-blinded study comparing the analgesic efficacy of ultrasound-guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. Br J Anaesth. 2011;107:790–5. doi: 10.1093/bja/aer263. [DOI] [PubMed] [Google Scholar]
- 11.Dingeman RS, Barus LM, Chung HK, Clendenin DJ, Lee CS, Tracy S, Johnson VM, Dennett KV, Zurakowski D, Chen C. Ultrasonography-Guided Bilateral Rectus Sheath Block vs Local Anesthetic Infiltration After Pediatric Umbilical Hernia Repair: A Prospective Randomized Clinical Trial. JAMA Surg. 2013:1–7. doi: 10.1001/jamasurg.2013.1442. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–75. doi: 10.1016/j.rapm.2004.08.003. discussion 24. [DOI] [PubMed] [Google Scholar]
- 13.Wada M, Kitayama M, Hashimoto H, Kudo T, Kudo M, Takada N, Hirota K. Brief reports: plasma ropivacaine concentrations after ultrasound-guided rectus sheath block in patients undergoing lower abdominal surgery. Anesth Analg. 2012;114:230–2. doi: 10.1213/ANE.0b013e3182367a68. [DOI] [PubMed] [Google Scholar]
- 14.Torup H, Mitchell AU, Breindahl T, Hansen EG, Rosenberg J, Moller AM. Potentially toxic concentrations in blood of total ropivacaine after bilateral transversus abdominis plane blocks; a pharmacokinetic study. Eur J Anaesthesiol. 2012;29:235–8. doi: 10.1097/EJA.0b013e328350b0d5. [DOI] [PubMed] [Google Scholar]
- 15.Courreges P, Poddevin F, Lecoutre D. Para-umbilical block: a new concept for regional anaesthesia in children. Pediatric Anesthesia. 1997;7:211–4. doi: 10.1046/j.1460-9592.1997.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 16.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson S, Thomas V, Lewis I. The rectus sheath block in paediatric anaesthesia: new indications for an old technique? Paediatr Anaesth. 1996;6:463–6. doi: 10.1046/j.1460-9592.1996.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 18.de Jose Maria B, Gotzens V, Mabrok M. Ultrasound-guided umbilical nerve block in children: a brief description of a new approach. Paediatr Anaesth. 2007;17:44–50. doi: 10.1111/j.1460-9592.2006.02025.x. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78:507–14. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 20.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–9. [PubMed] [Google Scholar]
- 21.Tucker GT. Pharmacokinetics of local anaesthetics. Br J Anaesth. 1986;58:717–31. doi: 10.1093/bja/58.7.717. [DOI] [PubMed] [Google Scholar]


