Abstract
Objective
To examine the association between heart rate (HR) responses during rest, exercise and post exercise with incident hypertension (HTN) in men.
Patients and methods
A total of 10,418 healthy normotensive men, without an abnormal electrocardiogram or history of heart attack, stroke, cancer, or diabetes, performed a maximal exercise test and were followed for incidence of HTN. HR reserve was defined as the maximal HR minus resting HR. HR recovery was defined as HR 5 minutes post exercise test.
Results
During a mean follow-up of 6 years, there were 2831 cases of HTN. Compared with men who had lower HR Reserve, the risk of incident HTN was significantly lower for men with higher HR Reserve (Hazard Ratio (HazR): 0.84; 95% confidence interval (CI): 0.74–0.95 for the highest quartile versus lowest quartile of HR Reserve) when adjusted for age, baseline examination year, smoking, heavy drinking, body mass index, resting blood pressure, cholesterol, glucose and cardiorespiratory fitness. Compared with men who had higher HR Recovery, the risk of incident HTN was significantly lower for men with lower HR Recovery (HazR: 0.90; 95% CI: 0.80–0.99 for quartile 3 versus highest quartile) after adjusting for the above confounders. However, the overall linear trend for HR Recovery is not significant (P=0.26).
Conclusion
The risk of HTN decreased in men with higher HR Reserve. Therefore, HR Reserve may be considered as a useful exercise parameter for predicting the risk of HTN in men.
Keywords: Cardiorespiratory fitness, chronic disease, epidemiology, high blood pressure
Introduction
Hypertension (HTN) is the most prevalent risk factor for cardiovascular (CV) disease (CVD) among US adults, and affects nearly one-third of the population aged 18 and older.1,2 The prevalence of HTN has increased by almost one-fourth over the past 15 years among adults in the US, increasing from 22.2% in 1995 to 27.8% in 2007.3,4 Risk of stroke, coronary heart disease (CHD), congestive heart failure and renal failure is also increased in people with HTN.5 The estimated direct cost of HTN for 2007 was $43.5 billion, a major burden on the US health care system.6 The World Health Organization report on global health risk indicated that high blood pressure is the leading risk factor for cause of deaths worldwide.7
Considerable data has established the importance of heart rate (HR) characteristics and future CVD. Resting heart rate (RHR) has consistently been found to be a predictor of HTN, CHD, and other measures of CVD morbidity and mortality.8–13 Some studies have shown that maximal HR is a strong predictor of CVD and all-cause mortality.14,15 Very few studies, however, have examined the relationships between HR reserve and CVD mortality. We earlier reported that HR Reserve predicts CVD mortality in young men that is independent of cardiorespiratory fitness (CRF).13,15 Other studies have shown that HR Recovery is also a predictor of CVD and all cause morbidity and mortality.16,17 HR Reserve and HR Recovery are parameters influenced by CRF, and CRF also depends on various factors such as CV, lung, and muscle fitness. This distinction may be important since HTN is a CVD risk factor that is less related to pulmonary function and muscle fitness. Therefore, we hypothesize that HR Reserve and HR Recovery might be significant predictors of incident HTN. The purpose of this study was to analyze the association of HR Reserve and HR Recovery with incident HTN in a group of men who enrolled in the Aerobics Center Longitudinal Study (ACLS), a prospective epidemiological investigation.
METHODS
Study participants
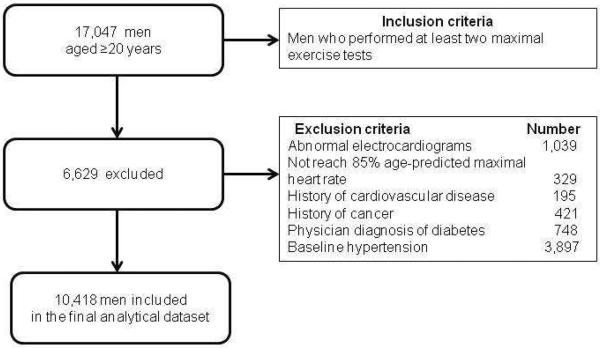
Participants received a comprehensive health examination at the Cooper Clinic in Dallas, TX. The Cooper Institute institutional Review Board reviewed and approved the study annually and details of the study have been published previously.18,19 Participants come to the Cooper Clinic for preventive medical examinations and counseling regarding exercise, diet, and other lifestyle factors associated with an increased risk of chronic disease. The present study consists of 10,418 men who completed at least two examinations including a maximal exercise test on a treadmill during 1974–2003. Study participants were predominantly non-Hispanic white (97%), well educated, and from the middle and upper socioeconomic strata. Although the sample came from middle and upper socioeconomic strata, they were similar to other well-characterized population-based cohorts in terms of blood pressure, cholesterol level, body weight, and CRF.20 At baseline, all participants included in the analysis were free of known CVD, cancer, or diabetes; had a normal resting or exercise electrocardiogram (ECG); and achieved an 85% age-predicted maximal HR (220 - age) during the maximal exercise treadmill test. In addition, all participants had no known HTN at baseline. The current analysis only included men because the number of women with incident HTN in this population was too small for a meaningful statistical analysis. Figure 1 shows the flow diagram of the study population.
Figure 1.
Participant flow diagram
Measurements
The baseline clinical examination was conducted after participants gave their informed written consent and followed an overnight fast of at least 12 h. The examination consisted of resting blood pressure, blood chemistry analyses, personal and family health history, anthropometry, and a maximal exercise test on a treadmill. Previous reports have described the clinical examination in detail.18,21,22 Briefly, height and weight were measured on a standard balance beam scale and stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Participants provided self-report of weekly alcohol consumption. Heavy drinking is defined as ≥14drinks/week. Serum samples were analyzed for lipids and glucose using standardized automated bioassays at the Cooper Clinic Laboratory, which participated in and met quality control criteria of the Centers for Disease Control and Prevention Lipid Standardization Program.
Resting blood pressure was measured in the seated position, and was recorded as the first and fifth Korotkoff sounds by auscultatory methods after at least 5 minutes of rest. A standard sphygmomanometer was used and two readings separated by 2 minutes were averaged. If the first two readings differed by >5 mm Hg, additional readings were obtained and averaged. The maximal exercise test was conducted following a modified Balke protocol.23 Participants began walking at 88 m/min with no elevation. At the end of the first minute, grade was increased to 2% and thereafter increased 1% per minute until the 25th minute. After 25 min the grade remained constant while the speed increased each subsequent min by 5.4m/min. Participants were encouraged to give a maximal effort during the test. Heart rates were obtained from the exercise ECG. The highest value recorded from the ECG during exercise test was defined as the maximal HR. All men in the present analyses were able to complete the test to at least 85% of their age-predicted maximal HR (220 minus age in years). Each age group exceeded the age-predicted average maximal HR, which indicates that the exercise test can be considered a maximal performance. HR Recovery was the HR 5 minutes after the exercise test. Participants continued the test to the limits of volitional fatigue.24 RHR was determined with the participants recumbent after 5-min rest before the test and was obtained from the resting ECG. CRF was estimated using maximal metabolic equivalents (METs) attained during the test (1 MET = resting metabolic rate (RMR), defined as an oxygen uptake of 3.5 mL·kg−1·min−1) assessment. Men who had exercise test treadmill time in the upper 80% of the average maximal treadmill time, were kept in high CRF group, rest were in low CRF group.
Ascertainment of HTN
The incidence of HTN was ascertained from clinical examination after their baseline examination, and HTN was defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90, or physician diagnosed HTN.25 The follow-up time for each participant was determined from the baseline examination to the first follow-up event of HTN or the last follow-up observation through 2006 in men who did not develop HTN. The mean number of follow-up visits by those who were diagnosed with HTN was approximately 6. Overall visits by the population were approximately 4.
Statistical analyses
All analyses were performed using SAS statistical software, (version 9.2; SAS Institute, Cary, NC). HR Reserve and HR Recovery were analyzed categorically where categories were defined according to the quartiles of the observed distribution. Differences in baseline covariates were tested using chi-square tests for categorical variables and analysis of variance tests for continuous variables. Cox proportional hazards regression analysis was used to estimate hazard ratios (HazRs) and 95% confidence intervals (CIs) of incident HTN according to quartiles of HR Reserve and HR Recovery categories. Multivariable-adjusted models included controls for baseline measures: age (in years), examination year, BMI (kg/m2), smoking habit (smokers or non-smokers), alcohol intake (≥14 drinks/day or not), resting systolic and diastolic blood pressure (mmHg), blood glucose, total cholesterol and maximal treadmill time. Inspection of empirical cumulative hazards plots grouped by exposure suggested that proportional hazards assumption was justified. All P values were 2-sided alternative hypotheses. P values <0.05 indicated statistically significant comparisons.
Results
The baseline characteristics of the study sample by their HR Reserve and HR Recovery quartiles are presented in Tables 1 and 2 respectively. HR Reserve was calculated based on the difference between maximal HR during the exercise test and Resting HR. The quartiles of HR Reserve scores were, quartile 1 (<112 beats/min), quartile 2 (112–122 beats/min), quartile 3 (123–131 beats/min) and quartile 4 (≥132 beats/min). We identified quartiles of HR Recovery as quartile 1 (≤97 beats/min), quartile 2 (98–106 beats/min), quartile 3 (107–115 beats/min), and quartile 4 (≥116 beats/min). The mean (SD) values of HR Reserve in quartile 1, 2, 3, 4 were 103±8, 118±3, 127±2, 134±6 respectively and for HR Recovery, they were 90±7, 102±3, 111±3, 125±9 respectively. Participants with lower HR Reserve and higher HR Recovery tended to have higher BMI, lower CRF, and worse CV profiles than those with higher HR Reserve and lower HR Recovery. During the mean follow-up of 6 years, 2831 cases of HTN developed.
Table 1.
Baseline characteristics of study participants (N=10,418) by heart rate reserve quartiles, Aerobics Center Longitudinal Study (mean±SD)
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Age(years) | 48.0±8.8 | 43.6±7.9 | 41.5±7.7 | 37.9±7.3 |
| Fasting glucose level (mg/dl) | 98.9±8.9 | 97.9±9.1 | 97±8.7 | 95.9±8.5 |
| Total Cholesterol level (mg/dl) | 210.9±38.3 | 207.6±37.4 | 203.7±37.8 | 196.1±35.5 |
| Resting Systolic Blood Pressure (mm Hg) | 118±10 | 117±9 | 116±9 | 115±9 |
| Resting diastolic blood Pressure (mm Hg) | 78±6 | 78±7 | 77±7 | 76±7 |
| Resting pulse (beats/min) | 65±10 | 61±9 | 57±8 | 52±8 |
| Maximum pulse (beats/min) | 168±11 | 178±9 | 183±8 | 191±9 |
| Body mass index (kg/m2) | 26.6±3.1 | 25.7±2.7 | 25.2±2.6 | 24.7±2.5 |
| Maximal Treadmill Time (minutes) | 16±4 | 18.7±4 | 20.2±4 | 21.9±4 |
| Heart rate reserve (beats/min) | 103±8 | 118±3 | 127±2 | 134±6 |
| Current Smoker | 21.5 | 17.6 | 14.5 | 12.3 |
| Heavy drinkers | 17.4 | 16.1 | 16.8 | 14.9 |
P-value for all variables is <0.001 except heavy drinkers which was 0.04
Table 2.
Baseline characteristics of study participants (N=10,418) by heart rate recovery quartiles, Aerobics Center Longitudinal Study (mean±SD)
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Age(years) | 44.0±9.3 | 42.6±8.7 | 42.6±8.4 | 41.3±8.2 |
| Fasting glucose level (mg/dl) | 96.8±8.9 | 97.4±9.0 | 97.5±9.2 | 97.7±8.9 |
| Total Cholesterol level (mg/dl) | 203.8±37.4 | 204±37.8 | 206.2±38.6 | 203.0±36.5 |
| Resting Systolic Blood Pressure (mm Hg) | 115±9 | 115±9 | 116±9 | 117±9 |
| Resting diastolic blood Pressure (mm Hg) | 76±6 | 77±7 | 77±6 | 78±6 |
| Resting pulse (beats/min) | 52±8 | 57±8 | 60±9 | 64±10 |
| Maximum pulse (beats/min) | 172±11 | 179±10 | 183±11 | 189±10 |
| Body mass index (kg/m2) | 25.2±2.6 | 25.4±2.7 | 25.7±3.0 | 25.6±2.9 |
| Maximal Treadmill Time (minutes) | 20.3±4.9 | 19.5±5 | 18.9±4.4 | 18.7±4.0 |
| Heart rate recovery (beats/min) | 89.7±6.6 | 102±3 | 111±3 | 125±9 |
| Current Smoker | 16.9 | 16.5 | 16.4 | 14.9 |
| Heavy drinkers | 18.9 | 17.4 | 16.7 | 11.7 |
P-value for all variables is <0.001 except glucose which was 0.005
Table 3 shows that participants who had a greater HR Reserve were less likely to develop HTN. When adjusted for age and baseline examination year, BMI, smoking, heavy drinking, resting systolic and diastolic blood pressure, total cholesterol, and fasting glucose, there was a significantly inverse association between higher levels of HR Reserve and incident HTN (P for linear trend=0.002). Association of HR Reserve and HTN was similar in both younger and older men, with HazR of 0.77 and 0.78, respectively in quartile 4 (Data not shown). Table 4 shows a direct trend between HR Recovery and incidence of HTN. After adjusting for the above confounders, HR Recovery quartile 3 had a 10% lower risk of incident HTN, however, the linear trend is not significant across the quartiles (P for linear trend=0.26).
TABLE 3.
Multivariable-adjusted hazard ratio and 95% confidence interval of incident hypertension by heart rate (HR) reserve quartiles, Aerobics center Longitudinal Study.
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR Reserve Quartile 1 | 1 | 1 | 1 |
| HR Reserve Quartile 2 | 0.89 (0.80 – 0.99) | 0.93 (0.86 – 1.10) | 0.96 (0.86 – 1.10) |
| HR Reserve Quartile 3 | 0.78 (0.70 – 0.88) | 0.89 (0.80 – 0.99) | 0.90 (0.80 – 1.01) |
| HR Reserve Quartile 4 | 0.67 (0.60 – 0.76) | 0.82 (0.73 – 0.93) | 0.84 (0.74 – 0.95) |
| P for linear trend | <0.001 | <0.001 | 0.002 |
Model 1 adjusted for age and baseline exam year.
Model 2: adjusted for confounders in Model 1 and smoking, heavy alcohol drinking, body mass index, resting systolic and diastolic blood pressure, total cholesterol, and blood glucose.
Model 3: adjusted further for confounders in model 2 and cardiorespiratory fitness.
TABLE 4.
Multivariable-adjusted hazard ratio and 95% confidence interval of incident hypertension by heart rate (HR) recovery quartiles, Aerobics center Longitudinal Study.
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR Recovery Quartile 1 | 0.8 (0.71 – 0.89) | 0.91 (0.81 – 1.02) | 0.93 (0.83 – 1.04) |
| HR Recovery Quartile 2 | 0.81 (0.72 – 0.90) | 0.90 (0.80 – 1.01) | 0.91 (0.82 – 1.02) |
| HR Recovery Quartile 3 | 0.88 (0.79 – 0.98) | 0.89 (0.80 – 0.99) | 0.90 (0.80 – 0.99) |
| HR Recovery Quartile 4 | 1 | 1 | 1 |
| P for linear trend | <0.001 | 0.12 | 0.26 |
Model 1 adjusted for age and baseline exam year.
Model 2: adjusted for confounders in Model 1 and smoking, heavy alcohol drinking, body mass index, resting systolic and diastolic blood pressure, total cholesterol, and blood glucose.
Model 3: adjusted further for confounders in model 2 and cardiorespiratory fitness.
Mean maximal HR in the group that did not develop HTN was 181 (133–300) whereas, in the group that developed HTN it was 180 (138– 224). This shows that, max HR achieved by the group who did not develop HTN was higher than the group who developed HTN. We created 2 groups based on the year of the initial examination 1974–1988 and 1989–2003. Similar inverse associations were found between HR Reserve and incident HTN across the two groups. However, no significant associations were observed for HR Recovery.
Discussion
Principle findings
In this large prospective study of men, we found that a higher HR Reserve was significantly associated with a lower risk of incident HTN. The inverse association between HR Reserve and incident HTN persisted even after adjusting for multiple confounders, including age and CRF. Compared with the lowest quartile of HR Reserve, the highest quartile of HR Reserve had approximately 16 % lower risk of developing HTN in the fully adjusted model. Men in the lower quartile of HR Recovery had approximately 10% lower risk of developing HTN in the fully adjusted model. However, the overall linear trend across quartiles of HR Recovery was not significant. The current study demonstrates that HR Reserve, but not HR Recovery is associated with higher incidence of HTN independent of other major predictors, including CRF.
Comparison with previous reports
Previous studies have found that lower HR at peak exercise, high Resting HR, and high HR Recovery are independent risk factors for the development of CHD and CVD morbidity and mortality.8–12,14–17 These studies used only one parameter, either Resting HR or maximal HR. Our study analyzed associations of HTN with HR Reserve which was calculated by using Resting HR and maximal HR. A 13 year longitudinal study concluded that HR Reserve, independent of CRF, was inversely associated with CVD mortality among men and can be an important exercise parameter to predict CVD mortality in younger men (20–39), whereas CRF and other established risk factors are better predictors of CVD and all-cause mortality in older men (30–59).13 This prior study focused on a broad but important outcome, CVD mortality. Our study examined effect of HR Reserve and HR Recovery on incident HTN and found significant associations between HR Reserve and incident HTN in both younger and older men. The results suggest that HR Reserve might be a reliable indicator for predicting HTN in all age groups.
Mechanisms underlying the observed associations
Elevated Resting HR may be a result of increased sympathetic activity, reduced vagal activity or both, which explains the association of Resting HR and HTN to some extent.26,27 Several underlying mechanisms have been suggested as being responsible for the association between elevated HR and HTN. A high Resting HR may intensify the pulsatile nature of the arterial blood flow which may favor injury to the endothelium and consequent initiation or exacerbation of the atherosclerosis.28,29 Inability to increase HR properly during exercise is a phenomenon called chronotropic incompetence30, which suggests that impaired baroreflex sensitivity modulates both the parasympathetic influence in early exercise and sympathetic effects in the later phase on HR response to exercise.31 This phenomenon, however, does not apply to our data because the subjects who did not reach 85% of the expected maximum HR were excluded from the study. Although subjects who developed HTN later did not have chronotropic incompetence, most of them were nonetheless unable to increase their HR at peak exercise to the levels achieved by people who did not develop HTN, a finding that indicates an impairment in the ability to increase sympathetic activity to its maximum extent. It has been suggested that low HR Recovery is due to autonomic dysfunction. Defective auto regulation of the cerebral blood flow may lead to change in the balance of autonomic nervous system in the cerebral vasculature, which may be associated with elevated blood pressure.32
Strength and limitations
Strengths of the current study includes the relatively large sample size, a mean follow-up of 6 years, objectively measured resting HR, maximum HR during the exercise test and HR Recovery 5 minutes following exercise test. The findings were adjusted for several potentially important confounders. In addition, this study includes an extensive baseline medical examination, which is important since undetected subclinical disease at baseline is a concern in prospective studies. The following limitations should be considered when interpreting our data. First, the majority of participants were well-educated white men, limiting the generalizability of the findings. Second, all of the participants were measured at the baseline visit, and HR Reserve and HR Recovery might have changed during the follow-up period. The lack of pharmacotherapy information in our database limits evaluation of the specific medications that may affect the results of the exercise treadmill test. Additionally, we defined HR Recovery based as the HR at 5 minutes post-exercise. There is evidence that heart rate at 1 or 2 min of recovery is sensitive to detection of the association of HR Recovery and CVD events.10,33 HR Recovery at 1 or 2 minutes are not present in our data-base, so we used HR Recovery at 5 minutes. We were not able to determine the relative strengths of prediction at 1, 2, and 5 min HR Recovery values. A previous study shows that HR Recovery at 5 minutes was independent predictor of CVD and all-cause mortality.34 Although there is little additional data on HR Recovery at 5 minutes for predicting major CVD events, our data demonstrates the potential usefulness of such an assessment, at least regarding the development of subsequent HTN.
Finally, our study only included men who were able to achieve 85% of the age-predicted peak HR, so one could presume that those with more pronounced chronotropic insufficiency may have an even higher risk of HTN, as well as other CVD.
Conclusion and perspectives
HR Reserve was inversely associated with future development of HTN in a cohort of men who were normotensive and healthy at baseline. In terms of therapeutic or preventive implications, these observations might help explain known benefits of physical activity and exercise training, which may have beneficial effects on HR Reserve. We believe that clinicians should counsel their patients to be physically active to improve HR Reserve, which may lead to reductions in the subsequent risk of HTN. Future studies are also warranted to further examine the association between HR recovery and incident HTN.
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data entry and data management.
Funding Sources This work was supported by National Institutes of Health grants AG06945, HL62508 and DK088195. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of health.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CRF
cardiorespiratory fitness
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HazR
hazard ratio
- HTN
hypertension
- HR
heart rate
- RHR
resting heart rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None
Reference
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics—2007 Update:A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.CDC . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: 1995. [Google Scholar]
- 4.CDC . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: 2007. [Google Scholar]
- 5.Ramachandran S, Vasan MD, Martin G, et al. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease. The New England Journal of Medicine. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones D, et al. Heart Disease and Stroke Statistics. 2011 Update : A Report From the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Mortality and burden of disease attributable to selected major risks. World health organization; 2009. [Google Scholar]
- 8.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population:role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am. Heart J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 11.Powel AC, Sutton JR, Wicks JR, Oldridge NB, Jones NL. Reduced heart rate response to exercise in ischemic heart disease: the fallacy of the target heart rate in exercise testing. Med. Sci. Sports. 1979;11:227–233. [PubMed] [Google Scholar]
- 12.Gillum RF. The epidemiology of resting heart rate in a national sample of men and women: associations with hypertension, coronary heart disease, blood pressure, and other cardiovascular risk factors. Am. Heart J. 1988;116(1 Pt 1):163–174. doi: 10.1016/0002-8703(88)90262-1. [DOI] [PubMed] [Google Scholar]
- 13.Cheng YJ, Macera CA, Church TS, Blair SN. Heart rate reserve as a predictor of cardiovascular and all- cause mortality in men. Med. Sci. Sports. 2002;34(12):1873–8. doi: 10.1097/00005768-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kohl HW, Nichaman MZ, Frankowski RF, Blair SN. Maximal exercise hemodynamics and risk of mortality in apparently healthy men and women. Med. Sci. Sports. 1996;28(5):601–609. doi: 10.1097/00005768-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Sandvik L, Erikssen J, Ellestad M, et al. Heart rate increase and maximal heart rate during exercise as predictors of cardiovascular mortality: a 16-year follow-up study of 1960 healthy men. Coron Artery Dis Aug. 1995;6(8):667–679. doi: 10.1097/00019501-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Meibodi AM, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart Rate Recovery After Treadmill Exercise Testing and Risk of Cardiovascular Disease Events (The Framingham Heart Study) Am J Cardiol. 2002;90:848–852. doi: 10.1016/s0002-9149(02)02706-6. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Byun W, Sui X, Lee DC, Cheng YJ, Blair SN. Heart rate recovery after treadmill exercise testing is an independent predictor of stroke incidence in men with metabolic syndrome. J. orcp. 2011;03.007 doi: 10.1016/j.orcp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 19.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness: evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am. J. Epidemiol. 1989;129:1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 21.Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252(4):487–490. 27. [PubMed] [Google Scholar]
- 22.Shuger SL, Sui X, Church TS, Meriwether RA, Blair SN. Body mass index as a predictor of hypertension incidence among initially healthy normotensive women. Am J Hypertens. 2008;21:613–619. doi: 10.1038/ajh.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. U.S. Armed Forces Med. J. 1959;10:675–688. [PubMed] [Google Scholar]
- 24.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward SR, Linnerud AC. A comparative analysis of four protocols for maximum treadmill stress testing. Am. Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 25.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 26.Conway J. Hemodynamic aspects of essential hypertension in humans. Physiol Rev. 1984;64(2):617–660. doi: 10.1152/physrev.1984.64.2.617. [DOI] [PubMed] [Google Scholar]
- 27.Ewing DJ, Campbell IW, Clarke BF. Heart rate changes in diabetes mellitus. Lancet. 1981;1(8213):183–6. doi: 10.1016/s0140-6736(81)90061-1. [DOI] [PubMed] [Google Scholar]
- 28.Gordon D, Guyton JR, Karnovsky MJ. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest. 1981;45(1):14–27. [PubMed] [Google Scholar]
- 29.Perski A, Hamsten A, Lindvall K, Theorell T. Heart rate correlates with severity of coronary atherosclerosis in young postinfarction patients. Am Heart J. 1988;116(5 Pt 1):1369–1373. doi: 10.1016/0002-8703(88)90469-3. [DOI] [PubMed] [Google Scholar]
- 30.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93(8):1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 31.Fukuma N, Oikawa K, Aisu N, et al. Impaired baroreflex as a cause of chronotropic incompetence during exercise via autonomic mechanism in patients with heart disease. Int J Cardiol. 2004;97(3):503–508. doi: 10.1016/j.ijcard.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Deniz F, Katircibasi MT, Pamukcu B, Binici S, Sanisoglu SY. Association of metabolic syndrome with impaired heart rate recovery and low exercise capacity in young male adults. Clin Endocrinol (Oxf) 2006;66:218–223. doi: 10.1111/j.1365-2265.2006.02711.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate rescovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 34.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26(7):2052–2057. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]