Abstract
Purpose
To compare the health care costs of women with unilateral breast cancer who underwent contralateral prophylactic mastectomy (CPM) with those of women who did not.
Methods
We conducted a retrospective study of 904 women treated for stage I–III breast cancer with or without CPM. Women were matched according to age, year at diagnosis, stage, and receipt of chemotherapy. We included healthcare costs starting from the date of surgery to 24 months. We identified whether care was immediate or delayed (CPM within 6 months or 6–24 months after initial surgery, respectively). Costs were converted to approximate Medicare reimbursement values and adjusted for inflation. Multivariable regression analysis was performed to evaluate the effect of CPM on total breast cancer care costs adjusting for patient characteristics and accounting for matched pairs.
Results
The mean difference between the CPM and no-CPM matched groups was $3,573 (standard error [SE]=$455) for professional costs, $4,176 (SE=$1,724) for technical costs, and $7,749 (SE=$2,069) for total costs. For immediate and delayed CPM, the mean difference for total costs was $6,528 (SE =$2,243) and $16,744 (SE=$5,017), respectively. In multivariable analysis, the CPM group had a statistically significant increase of 16.9% in mean total costs compared to the no-CPM group (P<0.0001). HER-2/neu-positive status, receipt of radiation, and reconstruction were associated with increases in total costs.
Conclusions
CPM significantly increases short-term healthcare costs for women with unilateral breast cancer. These patient-level cost results can be used for future studies that evaluate the influence of costs of CPM on decision making.
Keywords: contralateral prophylactic mastectomy (CPM), cost analysis
INTRODUCTION
Over the past decade, women with unilateral breast cancer have increasingly undergone contralateral prophylactic mastectomy (CPM) despite the lack of clear evidence that the procedure provides a survival benefit.1 The annual population-based rates of CPM among surgically treated patients with invasive breast cancer and ductal carcinoma in situ are estimated at 3.4% and 4.1%, respectively.2,3 However, the reported CPM frequency is much higher in academic medical centers, ranging from 14% to 28%,4–6 and the rate of CPM has increased 4-fold over the past 10 years in some institutions.7 These higher-than-average rates of CPM in multidisciplinary cancer care centers may reflect higher rates of breast magnetic resonance imaging utilization, genetic testing of high-risk patients, access to plastic surgery expertise for breast reconstruction, and an increase in patient and/or physician preference for more aggressive breast cancer management.8,9
Cost is considered a major factor in treatment decision-making. It has been demonstrated that bilateral prophylactic mastectomy is more cost-effective in cancer-free BRCA1 or BRCA2 gene mutation carriers than breast cancer surveillance.10–12 However, data are limited on the economic value of CPM in women with unilateral breast cancer. Zendejas et al. conducted a cost-effectiveness analysis of CPM versus routine surveillance among women with unilateral breast cancer.13 For patients without BRCA1 or BRCA2 mutation, CPM was cost-effective in patients younger than 70 and under the assumption that the utility for the disease-free state with CPM was equal to or greater than that for the disease-free state with surveillance. Inpatient and outpatient costs were obtained from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database14 and from 2007 rates for Medicare reimbursement. These costs were point estimates that did not include patient-level variation.
The majority of the costs associated with CPM are direct medical care costs (e.g., surgical procedure cost). Frost et al reported that 27% of women had at least one unanticipated reoperation after CPM,15 and Barton et al (2005) and Crosby et al (2011) reported that 27% to 66% of women who underwent CPM had at least one complication.16,17 This means that at least 1/3 of patients might not have experienced a surgical complication if they had not chosen CPM. The costs of unanticipated operations and treatment complications associated with CPM further increase the direct medical care costs of the procedure.
The objective of our study was to compare the direct, short-term health care costs of women with unilateral breast cancer who underwent contralateral prophylactic mastectomy (CPM) with those who did not, matched by demographic and tumor characteristics at a single institution, and to evaluate the contribution of CPM to the total cost of breast cancer care relative to other specific clinical components.
MATERIALS AND METHODS
Patient Selection
We searched the prospective Breast Cancer Management System database of The University of Texas MD Anderson Cancer Center to identify women with clinical stage I to III primary unilateral invasive breast cancer who underwent a mastectomy between June 1997 and August 2009. Patients who underwent bilateral mastectomies for metachronous bilateral breast cancer (contralateral breast cancer within 6 months of diagnosis of the primary breast cancer), and patients with contralateral invasive or ductal carcinoma in situ incidentally discovered at the time of CPM were excluded. We collected data on patient demographics (age, sex, race, comorbidity, and method of payment [private insurance, indigent care, government (“Medicare or Medicaid”), or self-pay]), breast tumor characteristics (histology, tumor size, lymph node metastasis status, stage, nuclear grade, and estrogen receptor [ER], progesterone receptor [PR], and HER-2/neu status), and treatment type (chemotherapy, endocrine therapy, radiation therapy and surgery).18
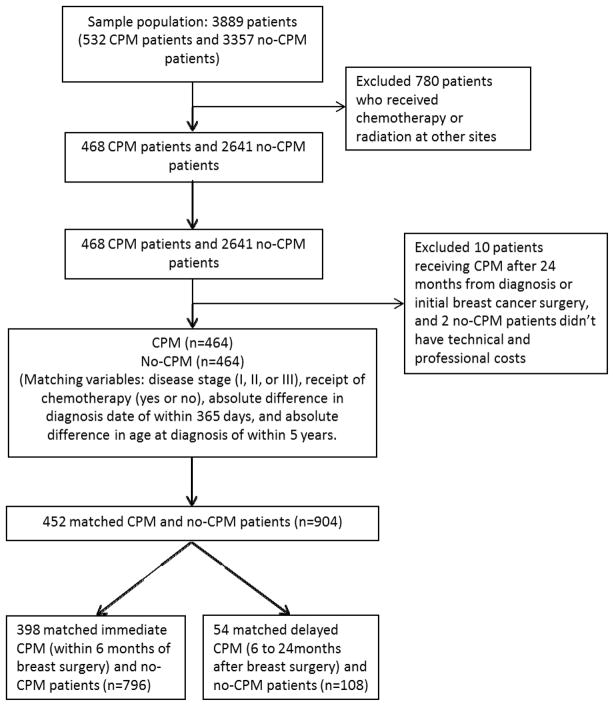
Patient selection details are summarized in Figure I. We identified 3889 patients (532 in the CPM and 3357 in the no-CPM groups) who met our eligibility criteria. We excluded patients who received chemotherapy or radiation therapy outside of MD Anderson (n=780). Thus, 468 patients in the CPM group and 2641 patients in the no-CPM group were eligible for matching.
Figure I.
Flow Chart for Patient Selection
We matched each patient in the CPM group with a patient in the no-CPM group at a 1:1 ratio on the basis of age at diagnosis (within 5 years), year of diagnosis (within 1 year), disease stage (I, II, or III), and receipt of chemotherapy at MD Anderson (yes or no), resulting in 464 matched pairs. Post-matching, we excluded 10 patients in the CPM group who underwent CPM >24 months after the date of primary breast cancer surgery and 2 no-CPM patients who had no professional or technical charge records. In the final study analysis, there were 452 CPM and no-CPM matched pairs. We further divided our 452 matched case-control pairs into two subgroups – 398 matched pairs that included patients who had CPM within 6 months from initial surgery (immediate CPM) and 54 matched pairs that included patients who had CPM 6–24 months after initial surgery (delayed CPM).
Our dataset did not have a variable to identify the patients who underwent breast reconstruction after mastectomy. We identified 544 of 904 patients using the Current Procedural Technology (CPT) codes for breast reconstruction (codes 15777, 19324, 19325, 19340, 19342, 19350, 19357, 19361, 19364, 19366, 19367, 19368, 19369, 19370, 19371, 19380, and 19396).
This research was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Defining Costs
We defined the cost as the amount reimbursed by Medicare. Medicare payments may be considered a reasonable proxy for the opportunity cost of goods and services.19,20
For both the CPM and no-CPM groups, we totaled costs of healthcare reimbursed by Medicare for 24 months starting from the date of primary breast surgery. Total cost of care was the sum of professional and technical costs. Professional component included clinician activities (e.g., interpretation of services). Technical component included billing for equipment, supplies, technicians, and facilities.
Cost Estimation
Resources utilized and their respective charges from the MD Anderson billing system were transformed to costs.
For physician reimbursement, we identified proxy costs from the Medicare database using the formula for the Medicare physician payment schedule. The Medicare physician payment schedule includes relative value units (RVUs) that correspond to services, geographical practice conversion indices (GPCIs) that correspond to payment localities, and constant conversion factors for each year. The conversion factor converts the number of RVUs for each resource used in the Medicare physician payment schedule into a dollar amount. To identify the proxy costs, we matched CPT codes for resource use from the professional components of the MD Anderson billing system with CPT codes and corresponding physician payment schedules from the Medicare database. GPCIs for Houston were applied to give Houston-specific physician reimbursement amount.
For hospital reimbursement (i.e., inpatient hospitalization, outpatient visits, and pharmacy), we applied urban Texas-specific cost-to-charge ratios to the hospital component of the dataset to obtain an estimate of the costs. The cost-to-charge ratios were identified from the Federal Register,21 and charges were identified from the technical component of the MD Anderson billing system. The cost-to-charge ratio particular to the region of interest (i.e., urban Texas) adjusted for geographical variability in costs.
All costs were converted to 2010 US dollars by multiplying the costs by the appropriate ratio of the consumer price indices for medical care.22
Statistical Analysis
Descriptive statistics, i.e., the number of non-missing observations (n), mean, and standard error (SE) were used to summarize continuous variables (e.g., cost). Frequencies and percentages were reported for categorical variables. The chi-square or Fisher’s exact test was used to evaluate the association between categorical patient characteristics variables and CPM status. Paired Wilcoxon’s signed rank test was used to compare the distributions of cost differences between the matched CPM and no-CPM groups. Multivariable generalized estimating equation regression models were used to fit the cost data and to determine the effect of CPM on total costs after adjusting for other covariates and accounting for matched pairs. Because medical cost data are usually highly skewed, we performed the logarithmic transformation on the original cost data to satisfy the underlying assumptions of the generalized estimating equation models. All tests were two sided. P values less than 0.05 were considered statistically significant. All analyses were conducted using SAS (version 9.3, Cary, NC) statistical software.
RESULTS
Patient Characteristics
Selected patient and clinical characteristics of the CPM (n=452) and no-CPM (n=452) matched groups are presented in Table I. The average time from date of surgery to CPM was 1.43 months (range, 0–21 months), and the average time from diagnosis to CPM was 5.59 months (range, 0–22 months). Compared to the no-CPM group, the CPM patients were more likely to be white (P<0.0001) and have private insurance (P=0.005). There was no significant difference between the CPM and no-CPM group for receipt of radiation or endocrine therapy. Patients in the no-CPM group were more likely to be HER-2/neu-positive (P = 0.0027). Women who underwent CPM were more likely to receive reconstruction than those who did not (71% versus 49%) (P≤0.0001).
Table I.
Patient Characteristics by CPM Status
| Characteristic | No-CPM (n = 452), number (%) | CPM (n = 452), number (%) | P Value* |
|---|---|---|---|
| Year of diagnosis | 0.7926 | ||
| 1997–2001 | 103 (22.8%) | 98 (21.7%) | |
| 2002–2005 | 164 (36.3%) | 159 (35.2%) | |
| 2006–2009 | 185 (40.9%) | 195 (43.1%) | |
| Age | 0.4384 | ||
| <50 | 238 (52.7%) | 257 (56.9%) | |
| 50–59 | 133 (29.4%) | 123 (27.2%) | |
| ≥60 | 81 (17.9%) | 72 (15.9%) | |
| Race | <0.0001 | ||
| Black | 57 (12.6%) | 26 (5.8%) | |
| Other | 110 (24.3%) | 49 (10.8%) | |
| White | 285 (63.1%) | 377 (83.4%) | |
| Method of payment | 0.0054 | ||
| Government Insurance | 54 (11.9%) | 48 (10.6%) | |
| Indigent Fund | 14 (3.1%) | 5 (1.1%) | |
| Private Insurance | 316 (69.9%) | 343 (75.9%) | |
| Self-pay | 23 (5.1%) | 7 (1.5%) | |
| Unknown | 45 (10%) | 49 (10.8%) | |
| Charlson Score | 0.3389 | ||
| 0 | 370 (81.9%) | 378 (83.6%) | |
| 1 | 46 (10.2%) | 49 (10.8%) | |
| 2+ | 36 (8%) | 25 (5.5%) | |
| Stage | 1.0000 | ||
| I | 140 (31.0%) | 140 (31.0%) | |
| II | 230 (50.9%) | 230 (50.9%) | |
| III | 82 (18.1%) | 82 (18.1%) | |
| ER/PR status | 0.8432 | ||
| Negative | 93 (20.7%) | 90 (20.2%) | |
| Positive | 356 (79.3%) | 356 (79.8%) | |
| HER-2/neu status | 0.0027 | ||
| Negative | 338 (79.3%) | 369 (87.0%) | |
| Positive | 88 (20.7%) | 55 (13.0%) | |
| Nuclear Grade | 0.3841 | ||
| I | 35 (7.9%) | 25 (5.6%) | |
| II | 192 (43.3%) | 200 (44.6%) | |
| III | 216 (48.8%) | 223 (49.8%) | |
| Reconstruction | <0.0001 | ||
| No | 230 (50.9%) | 130 (28.8%) | |
| Yes | 222 (49.1%) | 322 (71.2%) | |
| Radiation | 0.1676 | ||
| No | 276 (61.1%) | 296 (65.5%) | |
| Yes | 176 (38.9%) | 156 (34.5%) | |
| Chemotherapy | 1.0000 | ||
| No | 92 (20.4%) | 92 (20.4%) | |
| Yes | 360 (79.6%) | 360 (79.6%) | |
| Endocrine therapy | 0.5446 | ||
| No | 122 (27.0%) | 114 (25.2%) | |
| Yes | 330 (73.0%) | 338 (74.8%) |
P values were based on Chi-square or Fisher’s exact test.
For our subgroup analysis based on the timing of CPM, in the immediate CPM subgroup there was a significant difference in the proportion of HER-2/neu-positive breast cancers (P=0.0042) and method of payment (P=0.0032) between the immediate CPM and no-CPM patients. There was a significant difference in the proportion of the patients who had reconstruction in the immediate CPM and no-CPM matched group (P<0.0001) and the delayed CPM and no-CPM matched group (P<0.0001) (Table II; only significant variables presented.)
Table II.
Comparison of Significant Patient Characteristics for Immediate and Delayed CPM Matched Pairs
| Characteristics | Immediate CPM and no-CPM | Delayed CPM and no-CPM | ||||
|---|---|---|---|---|---|---|
| No-CPM (n = 398), number (%) | CPM (n = 398), number (%) | P Value* | No-CPM (n = 54), number (%) | CPM (n = 54), number (%) | P Value* | |
| Race | <0.0001 | 0.0209 | ||||
| Black | 52 (13.1%) | 22 (5.5%) | 5 (9.3%) | 4 (7.4%) | ||
| Other | 93 (23.4%) | 43 (10.8%) | 17 (31.5%) | 6 (11.1%) | ||
| White | 253 (63.6%) | 333 (83.7%) | 32 (59.3%) | 44 (81.5%) | ||
| Method of payment | 0.0032 | 0.7111 | ||||
| Government Insurance | 50 (12.6%) | 44 (11.1%) | 4 (7.4%) | 4 (7.4%) | ||
| Indigent Fund | 12 (3.0%) | 5 (1.3%) | 2 (3.7%) | 0 (0%) | ||
| Private Insurance | 275 (69.1%) | 299 (75.1%) | 41 (75.9%) | 44 (81.5%) | ||
| Self-pay | 22 (5.5%) | 5 (1.3%) | 1 (1.9%) | 2 (3.7%) | ||
| Unknown | 39 (9.8%) | 45 (11.3%) | 6 (11.1%) | 4 (7.4%) | ||
| HER-2/neu status | 0.0042 | 0.3619 | ||||
| Negative | 300 (80.0%) | 328 (87.7%) | 38 (74.5%) | 41 (82.0%) | ||
| Positive | 75 (20.0%) | 46 (12.3%) | 13 (25.5%) | 9 (18.0%) | ||
| Reconstruction | <0.0001 | <0.0001 | ||||
| No | 201 (50.5%) | 123 (30.9%) | 29 (53.7%) | 7 (13.0%) | ||
| Yes | 197 (49.5%) | 275 (69.1%) | 25 (46.3%) | 47 (87.0%) | ||
P values were based on Chi-square or Fisher’s exact test.
Cost Comparison of CPM Versus No CPM
In every comparison, CPM increased the total costs. The mean differences in costs between the CPM and no-CPM matched patients were $3,573 (SE=$455) for professional costs, $4,176 (SE=$1,724) for technical costs, and $7,749 (SE=$2,068) for total costs (Table III) estimated for the entire duration of 0–24 months. Breaking down the cost differences by immediate and delayed receipt of CPM, we found that the differences in professional, technical, and total costs were $3,346 (SE=$480), $3,182 (SE=$1,877), and $6,528 (SE=$2,243), respectively; between the immediate CPM and matched no-CPM patients and $5,245 (SE=$1,406), $11,499 (SE=$4,011), and $16,744 (SE=$5,017), respectively, for the delayed CPM and matched no-CPM patients. The differences in costs were statistically significant (P<0.05) for all three cost components for the overall sample as well as the subgroups (i.e., 0–24 months, ≤6 months, and 6–24 months).
Table III.
Cost Components by CPM Status
| Characteristic | CPM = No | CPM = Yes | Difference | P Value* | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | ||
| Cost difference between CPM and No-CPM (452 pairs) | |||||||
| Professional costs | $15,250 | $373 | $18,823 | $340 | $3,573 | $455 | <0.001 |
| Technical costs | $45,318 | $1,423 | $49,494 | $1,398 | $4,176 | $1,724 | 0.001 |
| Total costs | $60,568 | $1,713 | $68,317 | $1,650 | $7,749 | $2,069 | <0.001 |
| Cost difference between immediate CPM and No-CPM (398 pairs) | |||||||
| Professional costs | $14,937 | $395 | $18,283 | $350 | $3,346 | $480 | <0.001 |
| Technical costs | $44,330 | $1,520 | $47,513 | $1,504 | $3,182 | $1,877 | 0.033 |
| Total costs | $59,268 | $1,823 | $65,796 | $1,767 | $6,528 | $2,243 | 0.001 |
| Cost difference between delayed CPM and No-CPM (54 pairs) | |||||||
| Professional costs | $17,555 | $1,086 | $22,801 | $1,079 | $5,245 | $1,406 | <0.001 |
| Technical costs | $52,596 | $3,953 | $64,095 | $3,112 | $11,499 | $4,011 | <0.001 |
| Total costs | $70,152 | $4,858 | $86,896 | $3,752 | $16,744 | $5,017 | <0.001 |
P values were based on a paired non-parametric (Wilcoxon signed rank) test. P-values are for testing the differences to be zero between CPM and no CPM
Women who had reconstruction had higher total costs compared to women who did not. The mean difference in the total costs between women who had reconstruction versus those who did not was higher among the delayed CPM and matched no-CPM patients (n=$23,593) than the immediate CPM and matched no-CPM patients ($9,285).
In multivariable analysis, the mean total costs were 16.9% higher for women who underwent CPM than women who did not (95% CI=9.9–24.3; P<0.0001) (Table IV). The mean total cost of breast cancer care was higher for patients diagnosed from 2002 to 2005 (P=0.0008) and from 2006 to 2009 (P=0.0730) than for women diagnosed from 1997 to 2001. In addition, HER-2/neu-positive status, receipt of radiation, and reconstruction were associated with 30.8% (95% CI=18.2–44.7), 57.2% (95% CI=43.9–71.8, and 76.3% (95% CI=59.8–94.5) increases in total cost, respectively. Mean total cost was 19% lower among patients with hormone receptor-positive tumors versus hormone receptor-negative tumors (95% CI=11.6–25.8, P<0.001). In multivariable analysis for the immediate and delayed CPM subgroups, receipt of CPM increased the mean total costs by 18.9% and 12.8%, respectively.
Table IV.
Predictors of Total Breast Cancer Care Costs
| Characteristic | CPM and no-CPM (n=452) | Immediate CPM and no-CPM (n=398) | Delayed CPM and no-CPM (n=54) | |||
|---|---|---|---|---|---|---|
| Coefficient* (95% CI) | P Value | Coefficient* (95% CI) | P Value | Coefficient* (95% CI) | P Value | |
| Surgery | ||||||
| No CPM | Reference | Reference | Reference | |||
| CPM | 1.169 (1.099, 1.243) | <0.0001 | 1.189 (1.109, 1.274) | <0.0001 | 1.128 (1.025, 1.242) | 0.0139 |
| Year of Diagnosis | ||||||
| 1997–2001 | Reference | Reference | Reference | |||
| 2002–2005 | 1.263 (1.102, 1.447) | 0.0008 | 1.271 (1.097, 1.473) | 0.0014 | 1.271 (1.007, 1.605) | 0.0438 |
| 2006–2009 | 1.132 (0.989, 1.296) | 0.0730 | 1.132 (0.980, 1.308) | 0.0926 | 1.164 (0.908, 1.492) | 0.2294 |
| Charlson Score | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 1.048 (0.935, 1.175) | 0.4161 | 1.063 (0.943, 1.199) | 0.3140 | 0.838 (0.617, 1.139) | 0.2599 |
| 2+ | 1.230 (1.036, 1.459) | 0.0180 | 1.267 (1.060, 1.515) | 0.0093 | 0.814 (0.685, 0.967) | 0.0192 |
| Method of payment | ||||||
| Private Insurance | Reference | Reference | Reference | |||
| Government Insurance | 1.152 (1.036, 1.281) | 0.0088 | 1.147 (1.026, 1.283) | 0.0161 | 1.436 (1.139, 1.809) | 0.0022 |
| Indigent Fund | 1.115 (0.849, 1.466) | 0.4325 | 1.241 (1.011, 1.522) | 0.0386 | 0.150 (0.129, 0.174) | <0.0001 |
| Self-pay | 0.904 (0.722, 1.131) | 0.3761 | 0.943 (0.744, 1.195) | 0.6274 | 0.773 (0.600, 0.995) | 0.0456 |
| Unknown | 1.172 (0.965, 1.423) | 0.1087 | 1.146 (0.928, 1.415) | 0.2063 | 1.318 (1.050, 1.654) | 0.0174 |
| Number of Nodes Positive | ||||||
| 1 unit increase | 1.0228 (1.013, 1.033) | <0.0001 | 1.026 (1.014, 1.039) | <0.0001 | 1.002 (0.993, 1.012) | 0.6350 |
| Tumor Size | ||||||
| 1 unit increase | 1.0576 (1.035, 1.081) | <0.0001 | 1.064 (1.037, 1.092) | <0.0001 | 1.007 (0.984, 1.030) | 0.5661 |
| ER/PR status | ||||||
| Negative | Reference | Reference | Reference | |||
| Positive | 0.810 (0.742, 0.884) | <0.0001 | 0.804 (0.729, 0.887) | <0.0001 | 0.899 (0.777, 1.040) | 0.1527 |
| HER-2/neu status | ||||||
| Negative | Reference | Reference | Reference | |||
| Positive | 1.308 (1.182, 1.447) | <0.0001 | 1.345 (1.200, 1.506) | <0.0001 | 1.046 (0.909, 1.203) | 0.5314 |
| Radiation | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 1.572 (1.439, 1.718) | <0.0001 | 1.577 (1.429, 1.739) | <0.0001 | 1.398 (1.193, 1.637) | <0.0001 |
| Reconstruction | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 1.763 (1.598, 1.945) | <0.0001 | 1.814 (1.628, 2.022) | <0.0001 | 1.270 (1.110, 1.452) | 0.0005 |
Coefficients were obtained from exponentiating estimates from multivariable regression of log-transformed total costs.
DISCUSSION
In conclusion, the increase in short-term healthcare costs for women receiving CPM was $7,749. Of the total costs, the technical costs contributed 53.9%, and the professional costs contributed 46.1%. In addition to the costs of the surgical procedure, breast reconstruction, chemotherapy, and radiation therapy comprised the majority of the costs of care. The costs of care in the delayed CPM subgroup were approximately 2.5-times greater than the costs of the immediate CPM subgroup.
Our finding that immediate CPM costs were less compared to delayed CPM may be related to several factors. In the immediate CPM setting, the patient is already anesthetized for the primary surgery and her post-surgical care and management of complications are part of the index mastectomy time period. Patients selected for immediate CPM were also less likely to undergo reconstruction than patients selected for delayed CPM and the mean difference in the total cost of care was significantly lower among patients who underwent reconstruction in the immediate CPM subgroup compared to women who underwent reconstruction in the delayed CPM subgroup. Khoo et al. showed that mastectomy with immediate reconstruction is significantly less expensive than mastectomy followed by delayed reconstruction and should be considered to improve cost-effectiveness of breast surgery.23 However, a similar approach to reducing costs associated with CPM will need to take into consideration other factors that influence the timing of CPM with or without reconstruction, such as the availability of genetic testing, patient psychosocial factors, age at onset of the index breast cancer and the need for post-mastectomy radiation in selected patients.5,24,25 As expected, treatment with trastuzumab-based chemotherapy for HER2neu-positive tumors and receipt of radiation significantly contributed to total costs.
The costs reported in our study account for patient-level variation in the components of resource use (e.g., laboratory tests, length of stay, and medications) at the hospital. This approach has been recognized as the most precise approach for reporting hospital costs.26 We then converted the hospital-specific charges to more generalized cost estimates using cost estimation formulas. These procedures are becoming more commonplace in US studies.27–29 This method of converting charges to costs is a reasonable compromise between accuracy and convenience. However, when the costs estimated in the current study are compared across studies that adopted similar methodologies, the differences in observed costs would be partially due to the types of cost-to-charge adjustments used. A direct cost accounting per patient at the national level would have given more precise estimates.
Our study has limitations. The costs were not directly calculated and the study was conducted at a single site; thus, hospital-level variation in reporting costs is not incorporated. In addition, we did not use a lifetime horizon in the determination of costs; rather, the costs were estimated from the date of surgery up to 24 months. Therefore the costs associated with long-term events e.g. treatment of breast cancer recurrence and annual screening mammography were not considered. Our total costs of care include the costs associated with complications and we did not measure separately the costs of complications between the CPM and no-CPM groups. Several clinical factors (e.g., body mass index) and reconstruction characteristics (e.g., implant volume) are predictive of overall complications and are difficult to capture using CPT codes.17 Despite these limitations, this is the first study to estimate the increased short-term health care costs associated with CPM that are representative of widely generalizable national estimates. Knowledge of the cost of cancer drugs30 and Medicare reimbursement information for medical imaging31 can have a significant influence on physicians’ recommendations for treatment. Our results can be used for studies evaluating the influence of cost on physician decision making regarding CPM. Given the growing cost of cancer care, evaluating the cost of CPM is important especially in light of its uncertain breast cancer survival benefit but potential positive psychological impact.
SYNOPSIS.
We compared the health care costs of women with unilateral breast cancer who underwent contralateral prophylactic mastectomy (CPM) with those who did not. The difference between groups was $7,749; CPM, HER-2/neu-positive status, radiation, and reconstruction were associated with increases in total breast cancer care costs.
Acknowledgments
The authors wish to thank Robert Prater for research data support and Zach Bohannan and Dawn Chalaire for editorial contributions that enhanced the quality of the manuscript.
Financial support for this study was provided by grant number 5 R21 CA149803 (A.M.B., P.A.P.) from the National Cancer Institute and National institute of Health Cancer Center Support Grant P30CA016672 for shared resources. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;(11):CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007 Nov 20;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009 Mar 20;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 4.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009 Oct;16(10):2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 5.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila) 2010 Aug;3(8):1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones NB, Wilson J, Kotur L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol. 2009 Oct;16(10):2691–2696. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 7.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011 Jun 1;29(16):2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 8.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16(7):935–941. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz SJ, Morrow M. Contralateral Prophylactic Mastectomy for Breast Cancer: Addressing Peace of Mind. JAMA. 2013 Aug 1;310(8):793–794. doi: 10.1001/jama.2013.101055. [DOI] [PubMed] [Google Scholar]
- 10.Anderson K, Jacobson JS, Heitjan DF, et al. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006 Mar 21;144(6):397–406. doi: 10.7326/0003-4819-144-6-200603210-00006. [DOI] [PubMed] [Google Scholar]
- 11.Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837–847. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrag D, Kuntz KM, Garber JE, Weeks JC. Benefit of prophylactic mastectomy for women with BRCA1 or BRCA2 mutations. JAMA. 2000 Jun 21;283(23):3070–3072. [PubMed] [Google Scholar]
- 13.Zendejas B, Moriarty JP, O’Byrne J, Degnim AC, Farley DR, Boughey JC. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. J Clin Oncol. 2011 Aug 1;29(22):2993–3000. doi: 10.1200/JCO.2011.35.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; 2000–2001. http://www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 15.Frost MH, Slezak JM, Tran NV, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. 2005 Nov 1;23(31):7849–7856. doi: 10.1200/JCO.2005.09.233. [DOI] [PubMed] [Google Scholar]
- 16.Barton MB, West CN, Liu IL, et al. Complications following bilateral prophylactic mastectomy. J Natl Cancer Inst Monogr. 2005;(35):61–66. doi: 10.1093/jncimonographs/lgi039. [DOI] [PubMed] [Google Scholar]
- 17.Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg. 2011 Nov;128(5):1025–1033. doi: 10.1097/PRS.0b013e31822b6682. [DOI] [PubMed] [Google Scholar]
- 18.Brewster AM, Bedrosian I, Parker PA, et al. Association between contralateral prophylactic mastectomy and breast cancer outcomes by hormone receptor status. Cancer. 2012 Nov 15;118(22):5637–5643. doi: 10.1002/cncr.27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhardt JH, Sunshine JH. Core-needle and surgical breast biopsy: comparison of three methods of assessing cost. Radiology. 1999 Jul;212(1):181–188. doi: 10.1148/radiology.212.1.r99jl46181. [DOI] [PubMed] [Google Scholar]
- 20.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982 Jan;96(1):102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 21.Federal Register. Statewide average operating cost-to-charge ratios for urban and rural hospitals. Centers for Medicare & Medicaid Services, Department of Health and Human Services; 1997–2010. [Google Scholar]
- 22.Bureau of Labor Statistics. [Accessed Fall, 2013];Consumer Price Index - All Urban Consumers. 2010
- 23.Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg. 1998 Apr;101(4):964–968. doi: 10.1097/00006534-199804040-00011. discussion 969–970. [DOI] [PubMed] [Google Scholar]
- 24.van Geel AN. Prophylactic mastectomy: the Rotterdam experience. Breast. 2003 Dec;12(6):357–361. doi: 10.1016/s0960-9776(03)00136-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee MC, Bhati RS, von Rottenthaler EE, et al. Therapy choices and quality of life in young breast cancer survivors: a short-term follow-up. Am J Surg. 2013 Nov;206(5):625–631. doi: 10.1016/j.amjsurg.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drummond MF, Sculpher MJ, Torrance GW, O’brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford University Press; 2005. [Google Scholar]
- 27.Elting LS, Shih YC, Stiff PJ, et al. Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant. 2007 Jul;13(7):806–813. doi: 10.1016/j.bbmt.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Nigrovic LE, Chiang VW. Cost analysis of enteroviral polymerase chain reaction in infants with fever and cerebrospinal fluid pleocytosis. Arch Pediatr Adolesc Med. 2000 Aug;154(8):817–821. doi: 10.1001/archpedi.154.8.817. [DOI] [PubMed] [Google Scholar]
- 29.Zupancic JA, Richardson DK, O’Brien BJ, Eichenwald EC, Weinstein MC. Cost-effectiveness analysis of predischarge monitoring for apnea of prematurity. Pediatrics. 2003 Jan;111(1):146–152. doi: 10.1542/peds.111.1.146. [DOI] [PubMed] [Google Scholar]
- 30.Neumann PJ, Palmer JA, Nadler E, Fang C, Ubel P. Cancer therapy costs influence treatment: a national survey of oncologists. Health Aff (Millwood) 2010 Jan-Feb;29(1):196–202. doi: 10.1377/hlthaff.2009.0077. [DOI] [PubMed] [Google Scholar]
- 31.Gimbel RW, Fontelo P, Stephens MB, et al. Radiation exposure and cost influence physician medical image decision making: a randomized controlled trial. Med Care. 2013 Jul;51(7):628–632. doi: 10.1097/MLR.0b013e3182928fd5. [DOI] [PubMed] [Google Scholar]