Abstract
RTL1000 is a partial human MHC molecule coupled to a human myelin peptide. We previously demonstrated that RTL1000 was protective against experimental ischemic stroke in HLA-DR2 transgenic (DR2-Tg) mice. Since thrombolysis with recombinant tissue plasminogen activator (t-PA) is a standard therapy for stroke, we determined if RTL1000 efficacy is altered when combined with t-PA in experimental stroke. Male DR2-Tg mice underwent 60 min of intraluminal middle cerebral artery occlusion (MCAO). t-PA or vehicle was infused intravenously followed by either a single or 4 daily subcutaneous injections of RTL1000 or vehicle. Infarct size was measured by 2, 3, 5-triphenyltetrazolium chloride staining at 24h or 96 h of reperfusion. Our data showed that t-PA alone reduced infarct size when measured at 24 h but not at 96 h after MCAO. RTL1000 alone reduced infarct size both at 24 and 96h after MCAO. Combining RTL1000 with t-PA did not alter its ability to reduce infarct size at either 24 or 96 h after MCAO and provides additional protection in t-PA treated mice at 24 h after ischemic stroke. Taken together, RTL1000 treatment alone improves outcome and provides additional protection in t-PA treated mice in experimental ischemic stroke.
Keywords: Ischemic stroke, Immunotherapy, Recombinant T-cell receptor Ligand, tissue plasminogen activator, HLA-DR2 transgenic mice
Introduction
Stroke has become the second leading cause of death worldwide from 2011 by World Health Organization [1]. Currently, thrombolytic treatment with recombinant tissue plasminogen activator (t-PA) is the only FDA approved therapy for acute ischemic stroke. However, the use of this drug is limited due to the therapeutic window of 4.5 h, and the rate of t-PA use remains less than 4% [2]. Therefore, effective therapy for ischemic stroke remains elusive and the development of new strategies are much needed.
We have previously shown that stroke induces the activation of peripheral immune system during first 24 h, followed by immunosuppression characterized by pronounced atrophy of spleen and thymus 96 h after stroke [3–5]. Peripheral inflammatory cells enter the brain and contribute to ischemic brain injury [6–9]. Recombinant T-cell receptor ligands (RTLs), a class of partial major histocompatibility complex (MHC) class II molecules comprised of covalently linked α1 and β1 chains that are tethered to antigenic peptides, can inhibit brain specific T-cell activation with other portions of the immune system remaining intact, minimizing brain damage while not exacerbating immunosuppression [10–14].
We have previously demonstrated that RTL551, a mouse partial MHC construct coupled to mouse myelin oligodendroglial glycoprotein (MOG) peptide, reduces infarct size 96 h after stroke and improves sensorimotor outcome without compromising the immune system in C57BL/6 mice [13–14]. Our investigation showed RTL551 specifically targets myelin-specific T cells and profoundly changes their functional properties from proinflammatory to anti-inflammatory cells, inhibiting the accumulation of inflammatory cells in brain and partially preserving spleen cell numbers that are typically ablated after MCAO. In order to translate this result from animal experiments into human clinical trials, we tested the effect of RTL1000, a human MHC construct (from the HLA-DR2 allele) covalently linked to a human MOG peptide (hMOG-35-55), against experimental ischemic stroke in human MHC class II expressing HLA-DRB1*1502 (DR2-Tg) mice [15]. Our data revealed that RTL1000 reduces infarct size at 96 h after stroke in DR2-Tg mice when administered within a 6-h time window. We have also confirmed that RTL1000 improves long-term neurobehavioral functional recovery after stroke.
The Stroke Therapy Academic Industry Roundtable (STAIR) criteria suggested the use of the so called “cocktail” approach that targets multiple pathways with multiple neuroprotective therapies since cerebral ischemia involves a cascade of injury pathways [16–17]. Furthermore, with the increasing use of thrombolysis and likely benefit of neuroprotection when used in combination with t-PA, it is also suggested by STAIR that examination of interaction with t-PA should be performed when developing a new therapeutic strategy for acute ischemic stroke. The latest STAIR VIII Consortium update addressed clinical trial design where t-PA is combined with neurothrombectomy therapy [18]. Therefore, in the current study, we evaluated the combined effect of RTL1000 plus t-PA in the treatment of ischemic stroke.
Materials & Methods
Ethics Statement
All animal experiments were conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and protocols were approved by the Animal Care and Use Committees at Oregon Health & Science University and the Portland Veteran Affairs Medical Center.
Animals and experimental groups
Experiments were carried out on 90 male DR2-Tg mice (produced at the Portland VA Medical Center with foundation breeders provided by Dr. Chella David [15]), aged 8 to 12 weeks and weighing 20.1 to 27.7 grams. Mice were randomly assigned to groups and the surgeon was blinded to treatment groups.
RTL1000 production and purification
RTL molecules consist of the α1 and β1 domains of MHC II molecule expressing as a single polypeptide with or without antigenic amino terminal extensions [10, 19]. RTL1000 is comprised of covalently linked α1 and β1 domains of HLA-DR2 with a human MOG-35-55 (hMOG-35-55) peptide (MEVGWYRPPFSRVVHLYRNGK) extension [20]. RTL1000 was constructed de novo or by sequential site-directed mutagenesis of previous constructs. Protein purification was performed with a 30 to 40 mg yield of purified protein per liter of bacterial cell culture.
Treatment with RTL1000 and t-PA
In the 96 h reperfusion groups, mice were treated with 100 μl (1 μg/μl) of RTL1000 or vehicle (5% dextrose in Tris-HCl, pH 8.5) by 4 subcutaneous injections at 4, 24, 48, and 72 h after MCAO. In the 24 h reperfusion groups, mice were given 1 injection of 400 μl (1 μg/μl) of RTL1000 or vehicle at 4 h after MCAO. t-PA (10 mg/kg) or vehicle (Sterile water for injection) was infused intravenously (I.V.) though the jugular vein over 30 min starting at 15 min into MCAO.
Reversible Middle Cerebral Artery Occlusion
Male DR2-Tg mice underwent 60 min of intraluminal reversible middle cerebral artery occlusion (MCAO) as described previously with slight modifications [21]. Mice were anesthetized with isoflurane (5% for induction; 1% for maintenance) through a mask connected with a vaporizer (Isotec 4; Cyprane, England). Rectal temperature was monitored and maintained at 36.5 ± 0.5°C with a warm water pad and a heating lamp. Cortical blood flow was monitored by Laser-Doppler flowmetry (LDF; Model DRT4, Moor Instruments Ltd., Oxford, England). The right common carotid artery was exposed and the external carotid artery was ligated and cauterized. A 6-0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip was inserted into the internal carotid artery via the external carotid artery stump. The successful occlusion of middle cerebral artery was confirmed by sustained reduction in LDF. The filament was withdrawn to allow for reperfusion at 60 min of occlusion and mice were then allowed to recover from anesthesia and survived for 24 or 96 h after MCAO. Animals were excluded if LDF did not drop below 30% of baseline during MCAO or due to subarachnoid hemorrhage (SAH).
Determination of Infarct Volume
Animals were euthanized and brains were harvested at 24 or 96 h after MCAO. Brains were stained with 1.2% 2, 3, 5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA). Four slices of 2-mm-thick coronal sections were incubated in 1.2% TTC for 15 min at 37°C, and then fixed in 10% formalin overnight. Brain slices were photographed and evaluated by SigmaScan Pro 5.0 (Jandel, San G, Rarael, CA, USA). Infarct size was expressed as a percentage of the contralateral structure (cortex, striatum or hemisphere). To account for the effect of edema, infarct volume was calculated by subtracting the ipsilateral non-infarct region from the total contralateral structure volume, and dividing the difference by the contralateral volume [22].
Statistical Analysis
Data are presented as mean ± SEM. Differences of infarct volume among groups in cortex, striatum and hemisphere were analyzed respectively by one-way ANOVA with the Holm-Sidak method. Statistical analyses were performed using SigmaStat 3 statistical software (Systat Software, Inc., Chicago, IL, USA). Statistical significance was set at pitalic>0.05.
Results
Mortality and exclusions
Overall mortality was 7% (6 out of 90 mice, with no mortality in the 24 h reperfusion groups and a mortality ranging from 8.3% to 25% in the different 96 h reperfusion groups). One animal was excluded because LDF was greater than 30% of baseline during MCAO. There were no significant differences in LDF before, during or 5 min after MCAO, and no differences in age and weight among groups.
The effect of combined RTL1000 and t-PA at 96 h after MCAO
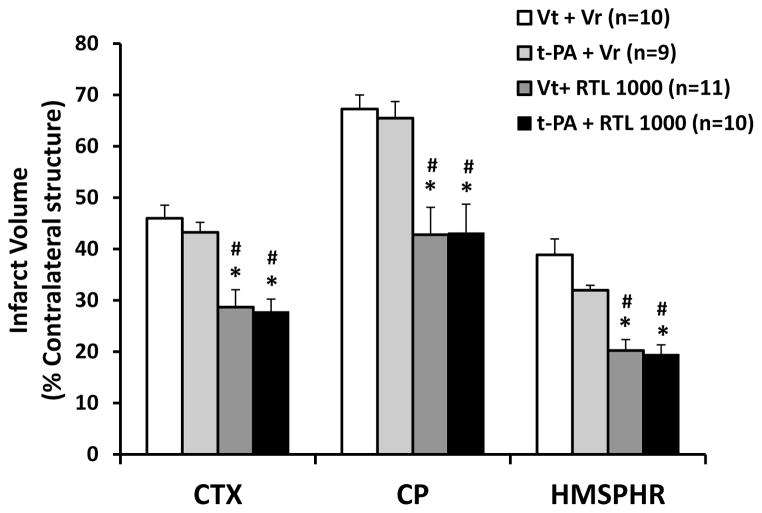
We tested the combined effect of RTL1000 and t-PA on infarct size measured 96 h after stroke. Consistent with our previous results, RTL1000 alone reduced infarct size 96 h after MCAO (Figure 1A and B). Infarct sizes were 28.7% ± 3.4%, 42.8% ± 5.3% and 20.2% ± 2.1% in cortex, striatum and hemisphere in RTL1000 treated mice compared to 46.0% ± 2.5%, 67.3% ± 2.7% and 38.9% ± 3.1% in vehicle treated mice, respectively (pbold>0.01). Unexpectedly, there were no differences between t-PA and vehicle treated mice at 96 h after MCAO (infract sizes in the t-PA group were 43.2% ± 2.0%, 65.5% ± 3.2% and 32.0% ± 0.9% in cortex, striatum and hemisphere; p>0.05). When mice were treated with t-PA plus RTL1000, infarct sizes were 27.7% ± 2.5%, 43.1% ± 5.6% and 19.4% ± 1.9% in cortex, striatum and hemisphere, respectively, which were smaller than corresponding regions in vehicle-treated mice (p<0.01), but not different from mice which were treated with RTL1000 alone.
Figure 1.

Effect of combined RTL1000 plus t-PA on infarct size 96 h after MCAO. Young adult male mice were subjected to transient 60-min MCAO, and were treated with either t-PA, t-PA vehicle (Vt), RTL1000 or RTL1000 vehicle (Vr). t-PA (10 mg/kg) or Vt was infused through jugular vein over 30 min period during MCAO. RTL1000 (100 μg) or Vr (100 μl) was administered subcutaneously (s.c.) at 4, 24, 48 and 72 h after MCAO. Brains were harvested 96 h after MCAO and brain slices were stained with TTC (A), and infarct volumes were measured as percentage of contralateral structure (B) (cortex, CTX; caudate-putamen, CP; and total hemisphere, HMSPHR). * indicates p<0.01 compared with Vt + Vr, and # indicates p<0.01 compared with t-PA + Vr group by one-way ANOVA
The effect of combined RTL1000 and t-PA at 24 h after MCAO
Since t-PA alone has no effect on infarct size measured at 96 h after MCAO, we wondered if it had an effect on infarct size at 24 h after stroke as previously reported [23, 24], and if the combined administration of RTL1000 and t-PA had an additive effect on infarct size at 24 h after MCAO. Indeed, our data confirmed the infarct size reduction by t-PA compared to vehicle (Figure 2A and B). The infarct volumes were 29.4% ± 3.0%, 59.2% ± 2.9% and 19.4 ± 2.3% in cortex, striatum and hemisphere in t-PA treated animals compared to 42.2% ± 2.6%, 69.5% ± 4.2% and 31.3% ± 2.4% in vehicle treated mice. RTL1000 alone also significantly reduced infarct size 24 h after ischemia compared with vehicle treatment, with infarct volumes being 20.2% ± 1.8%, 45.9% ± 3.0% and 13.3% ± 1.3% in cortex, striatum and hemisphere in RTL1000 treated mice (p<0.01). The infarct volumes of combined RTL1000 and t-PA treated mice were 19.9% ± 3.7%, 47.7% ± 4.6% and 13.2% ± 2.1% in cortex, striatum and hemisphere, which were smaller than that of vehicle treated mice (p<0.01). There was also a significant difference between mice treated with combined RTL1000 plus t-PA and with t-PA alone in striatum (p<0.05), but not in cortex or total hemisphere. There were no differences between mice treated with combined RTL1000 plus t-PA and mice treated with RTL1000 alone.
Figure 2.
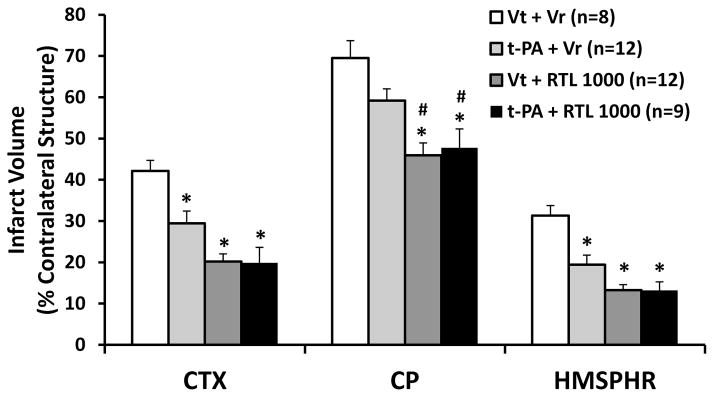
Effect of combined RTL1000 plus t-PA on infarct size 24 h after MCAO. Young adult male mice were subjected to transient 60-min MCAO, and were treated with either t-PA, t-PA vehicle (Vt), RTL1000 or RTL1000 vehicle (Vr). t-PA (10 mg/kg) or Vt was infused through jugular vein over 30 min period during MCAO. RTL 1000 (400 μg) or Vr (400 μl) was administered subcutaneously (s.c.) at 4 h after MCAO. Brains were harvested 96 h after MCAO and brain slices were stained with TTC (A), and infarct volumes were measured as percentage of contralateral structure (B) (cortex, CTX; caudate-putamen, CP; and total hemisphere, HMSPHR). * indicates p< 0.05 compared with Vt + Vr, and # indicates p<0.05 compared with t-PA + Vr by one-way ANOVA
Discussion
We evaluated the efficacy of RTL1000 in the presence of t-PA in experimental stroke. Our data show that the combined administration of RTL1000 with t-PA does not attenuate the protective effect of RTL1000. We also report the novel finding that t-PA reduces infarct size at 24 h, but not 96 after MCAO. RTL1000 on the other hand was protective both at 24 and 96 h after stroke. Recanalization therapy with t-PA is the most effective strategy to improve outcome in acute ischemic stroke patients, and the only FDA approved therapy for stroke. Several reports revealed that t-PA administration during MCAO reduces infarct size in rodents [23, 24]. The mechanism of protection by t-PA in the mechanical intraluminal filament occlusion model is not clear. Previous studies suggested the development of secondary microthrombosis, which may contribute to the expansion of cerebral ischemic lesions after MCAO. In support of this idea, inhibition of coagulation factors such as factor XII and glycoprotein (Gp) Ibα [25, 26], reduced infarct size after MCAO. Secondary microthrombosis presumably occurs within minutes after reperfusion in the MCAO models, and leads to delayed hypoperfusion and exacerbation of ischemic lesion. Furthermore, we previously reported that experimental stroke induces a massive peripheral immune response, which contributes to and exacerbates ischemic brain injury [3, 14]. These and other findings led to the idea that ischemic stroke is a thrombo-inflammatory disorder [27]. However, not all experimental stroke models showed microthombosis. Mechanisms other than microthrombosis may also contribute to microvascular impairment after stroke, including arteriolar vasoconstriction, endothelial cell swelling and inflammatory cell adhesions [28].
Progressive microvascular thrombosis and benefit by t-PA have been documented in the reversible intraluminal MCAO model [23]. Our data confirm the benefit obtained by t-PA when infarct size was measured at 24 h, but not 96 h. The lack of protection at 96 h, despite efficacy at 24 h, which has not previously been reported, suggests that either the thrombus reforms after 24 h, or that t-PA merely delays the infarction but does not necessarily provide long-lasting protection. The half-life of t-PA in human and mouse plasma is in minutes, so it is conceivable that t-PA effect was short-lived and only sufficient to impact 24-h infarct [29–30]. Recanalization of large vessels by t-PA, without effective microvascular reperfusion, has been observed clinically [31] and may have also contributed to the differential effect of t-PA at 24 vs. 96 h infarction. Specifically, microvascular injury could be a slow process that develops after the first day of ischemia, such that it is not affected mechanistically by t-PA and not coinciding with its action temporally.
In the current study, we confirm our previous findings that RTL1000 protect against ischemic stroke in DR2-Tg male mice [14]. The protection was observed both at 24 h, when peripheral immune system is activated and at 96 h after MCAO, during the immune-suppressive phase. The addition of t-PA did not enhance the efficacy of RTL1000 either at 24 or 96 h after MCAO. On the other hand, combined administration of RTL1000 with t-PA resulted in further infarct size decrease 24 h after MCAO, compared with t-PA alone. Therefore, our data suggest that the anti-inflammatory treatment with RTL1000 is superior to thrombolytic therapy because it provides long-term protection, and because it protects against both vascular injury and downstream parenchymal inflammatory mechanisms of ischemic injury. Interestingly, and in support of vascular mechanism of protection, we recently reported that RTL1000 interacts with human platelets and decreases aggregation [32]. In addition to serving as T cell receptor ligand, RTL is also a platelet ligand. Specifically, in our previous study [32], we showed that RTL suppresses collagen-induced platelet aggregation in vitro, and inhibits occlusive thrombus formation on collagen-coated surfaces under physiologically relevant pressure gradients. Therefore, in addition to the anti-inflammatory mechanism of protection, RTL1000 may also prevent platelet re-aggregation after thrombolysis by t-PA. This may explain the additional benefit from the combined administration of RTL1000 with t-PA compared to t-PA alone. The apparent lack of additional protection by RTL1000 when t-PA is added may be related to the “floor” effect; i.e., a maximal protection that cannot be further augmented by t-PA.
Altogether, RTL1000 treatment alone improves outcome and provides additional protection in t-PA treated mice in experimental ischemic stroke. These properties make RTL1000 an attractive safe and efficacious choice in the so called “cocktail” stroke therapy, and in stroke patients admitted beyond the therapeutic time window of t-PA.
Acknowledgments
This work was supported by NIH Grants #NS076013 (STTR) and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Footnotes
Conflict Of Interest
Dr. Offner and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
Wenbin Zhu declares that he has no conflict of interest. Nicole L. Libal declares that she has no conflict of interest. Amanda Casper declares that she has no conflict of interest. Sheetal Bodhankar declares that she has no conflict of interest. Nabil J. Alkayed declares that he has no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human subjects.
References
- 1.Fact sheet N°310. The top 10 causes of death. [Accessed July 2013];Media center of World Health Organization website. http://who.int/mediacentre/factsheets/fs310/en/index.html.
- 2.Wang Y, Zhang Z, Chow N, et al. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 4.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 7.Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- 8.Gee JM, Kalil A, Shea C, et al. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38(2 Suppl):783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- 9.Nilupul Perera M, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Burrows GG, Chang JW, Bachinger HP, et al. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Mooney JL, Meza-Romero R, et al. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbark AA, Rich C, Mooney J, et al. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35-55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 13.Dziennis S, Mader S, Akiyoshi K, et al. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–133. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian S, Zhang B, Kosaka Y, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Zanelli E, Khare SD, et al. Human leukocyte antigen-DRB1*1502 (DR2Dw12) transgene reduces incidence and severity of arthritis in mice. Hum Immunol. 1996;50:54–60. doi: 10.1016/0198-8859(96)00123-1. [DOI] [PubMed] [Google Scholar]
- 16.Stroke therapy academic industry roundtable. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 17.Albers GW, Goldstein LB, Hess DC, et al. Stroke Treatment Academic Industry Roundable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Jovin TG, Smith WS, et al. Stroke treatment academic industry roundtable: research priorities in the assessment of neurothrombectomy devices. Stroke. 2013;44:3596–3601. doi: 10.1161/STROKEAHA.113.002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huan JY, Meza-Romero R, Mooney JL, et al. Rationally designed mutations convert complexes of human recombinant T cell receptor ligands into monomers that retain biological activity. J Chem Technol Biotechnol. 2005;80:2–12. doi: 10.1002/jctb.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JW, Mechling DE, Bächinger HP, et al. Design, engineering and production of human recombinant T cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276:24170–24176. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Wang L, Zhang L, et al. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–769. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Davis CM, Edin ML, et al. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLos One. 2013;8(4):e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilic E, Hermann DM, Hossmann KA. Recombinant tissue plasminogen activator reduces infarct size after reversible thread occlusion of middle cerebral artery in mice. Neuroreport. 1999;10:107–111. doi: 10.1097/00001756-199901180-00021. [DOI] [PubMed] [Google Scholar]
- 24.Beerny-Lang MA, Hurst S, Tucker EI, et al. Thrombin mutant W215A/E217A treatment improves neurological outcome and reduces cerebral infarct size in a mouse model of ischemic stroke. Stroke. 2011;42:1736–1741. doi: 10.1161/STROKEAHA.110.603811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham M, Kleinschnitz C, Helluy X, et al. Enhanced cortical reperfusion protects coagulation factor XII-deficient mice from ischemic stroke as revealed by high-field MRI. Neuroimage. 2010;49:2907–2914. doi: 10.1016/j.neuroimage.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Momi S, Tantucci M, Van Roy M, et al. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood. 2013;121:5088–5097. doi: 10.1182/blood-2012-11-464545. [DOI] [PubMed] [Google Scholar]
- 27.Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: a thrombo-inflammatory disease? J Physiol (Lond) 2011;589:4115–4123. doi: 10.1113/jphysiol.2011.212886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauberti M, Martinez de Lizarrondo S, Orset C, et al. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J Thromb Haemost. 2014;12:409–414. doi: 10.1111/jth.12487. [DOI] [PubMed] [Google Scholar]
- 29.Chandler WL, Alessi MC, Aillaud MF, et al. Clearance of tissue plasminogen activator (t-PA) and t-PA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated t-PA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96:761–768. doi: 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 30.Narita M1, Bu G, Herz J, et al. Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo. J Clin Invest. 1995;96:1164–1168. doi: 10.1172/JCI118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebeskind DS. Recanalization and reperfusion in acute ischemic stroke. F1000 Med Rep. 2010;2 doi: 10.3410/M2-71. pii: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itakura A, Aslan JE, Sinha S, et al. Characterization of human platelet binding of recombinant T cell receptor ligand. J Neuroinflammation. 2010;7:75. doi: 10.1186/1742-2094-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]