Abstract
The phosphatidylinositol-3-kinase (PI3K) pathway is well known to regulate a wide variety of essential cellular functions, including glucose metabolism, translational regulation of protein synthesis, cell proliferation, apoptosis, and survival. Aberrations in the PI3K pathway are among the most frequently observed in cancer, and include amplifications, rearrangements, mutations, and loss of regulators. As a net result of these anomalies, the PI3K pathway is activated in many malignancies, including in Hodgkin and non-Hodgkin lymphomas, and yields a competitive growth and survival advantage, increased metastatic ability, and resistance to conventional therapy. Numerous inhibitors targeting various nodes in the PI3K pathway are undergoing clinical development, and their current status in lymphoma will be the focus of this review.
Keywords: Akt, Lymphoma, mTor, PI3K, Review, Signalling, Targeted, Therapy
Introduction
The phosphatidylinositol-3-kinase (PI3K) family consists of a number of serine/threonine and lipid kinases, including those that phosphorylate the membrane-bound phosphatidylinositol-3 (PIP3). These enzymes, and the downstream Akt (also referred to as protein kinase B) and mammalian target of rapamycin (mTOR), have a profound role in multiple critical cellular processes, including growth, differentiation, metabolism, survival, and cellular proliferation (Fig. 1).1-3 Recently, many novel inhibitors of various portions of the PI3K pathway have entered clinical trials for patients with lymphomas. Because inhibition of this pathway preliminarily appears to be a promising strategy for other malignancies, there is a high degree of interest regarding the current and future therapeutic relevance of the PI3K pathway and lymphoma.
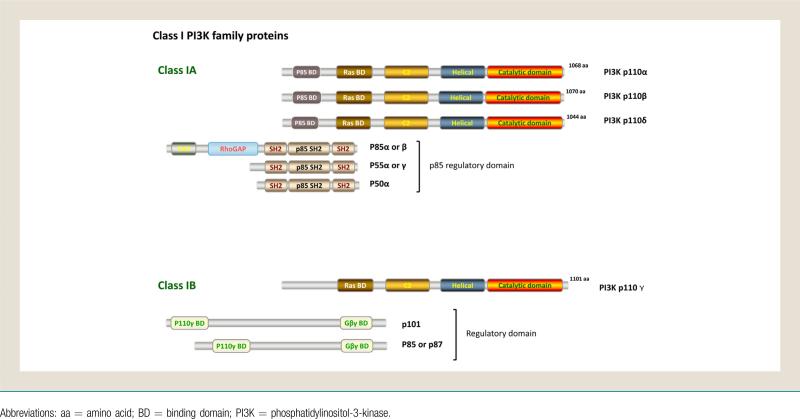
Figure 1.
Class I PI3K Family Proteins
PI3K/Akt/mTOR Pathway Biology
The PI3K enzymes consist of 3 classes with variable primary structure, function, and substrate specificity. Class I PI3Ks, the class most widely implicated as aberrant in cancers, consist of heterodimers of regulatory and catalytic subunits, and are subdivided into 1A and 1B based on their mode of activation (Fig. 1). Class 1A PI3Ks are activated by various cell surface tyrosine kinases, and consist of the catalytic p110 and regulatory p85 subunits. The 3 known isoforms of Class 1A p110 are p110α, p110β, and p110δ, which all contain an amino terminal regulatory interacting region (which interfaces with p85), a Ras binding domain, and a carboxy terminal catalytic domain.3-5 Class 1B PI3Ks consist of the catalytic (p110γ) and regulatory (p101) subunits and are activated by G-protein coupled receptors. The 4 p110 isoforms have variable tissue distribution and different physiologic functions (Table 1).6-11 p110α is expressed ubiquitously, including leukocytes, is encoded by the frequently mutated gene PIK3CA, and is lethal when removed in embryonic mouse models with decreased proliferation.12-14 p110β is also expressed ubiquitously, including leukocytes, and is important in cancer cell motility, insulin signaling, and platelet adhesion. p110β can signal downstream of G-protein coupled receptors, and is also lethal when removed in embryonic mouse models.15-19 p110δ is expressed in leukocytes, thymus, and breast tissue, and is essential for B- and T-cell development and B-cell receptor signaling. Mouse embryonic knockout of p110δ is nonlethal but results in a substantial decrease in B cell number and function.20,21 As a result, p110δ is preferentially targeted in B-cell malignancies. p110γ is expressed in leukocytes, thymus, cardiac, and endothelial tissue, and is involved in physiologic and pathologic immune function. The nonlethal embryonic knockout mouse model has a severe T cell and neutrophil chemotaxis impairment, with essentially normal B cells.11,21-23 If p110δ and p110γ are both knocked out, the T cell and natural killer cell populations are significantly diminished in number and function.10,24
Table 1.
Expression Pattern of PI3K Enzymes
| PI3K Class | Isoform | Tissue Distribution | Mouse -/- Major Phenotype | Function |
|---|---|---|---|---|
| IA | p110α | Leukocytes and ubiquitous | Embryonic lethal | Proliferation, differentiation, survival, migration, chemotaxis, phagocytosis, metabolism |
| p110β | Leukocytes and ubiquitous | Embryonic lethal | ||
| p110δ | Leukocytes, thymus, breast | Impaired B cell development | ||
| IB | p110γ | Leukocytes, thymus, heart, endothelium | Impaired inflammation (+ p110δ-/-: severe T cell and NK cell defect) | Cell migration, chemotaxis, inflammation |
Abbreviations: NK = natural killer; PI3K = phosphatidylinositol-3-kinase.
Class II and class III PI3K are ubiquitously expressed, are essential for normal cellular function, and do not appear to have an oncogenic function. Class II PI3K consists of 3 classes: PI3K-C2α and PI3K-C2β are ubiquitously expressed and PI3K-C2γ is only expressed in hepatocytes. The function of the membrane-bound class II PI3Ks is not yet known, but likely is involved in protein-membrane lipid interactions.25 Class III PI3Ks are ubiquitously expressed and essential for survival, as evidenced by the nonviable mouse embryonic knockout model.26 Class II PI3Ks (α and β isoforms) and class III PI3Ks also play a critical role in the regulation of autophagy.27 Because class II and III PI3K are essential and are not oncogenic, for clarity, “PI3K” will refer to class I for the remainder of this review.
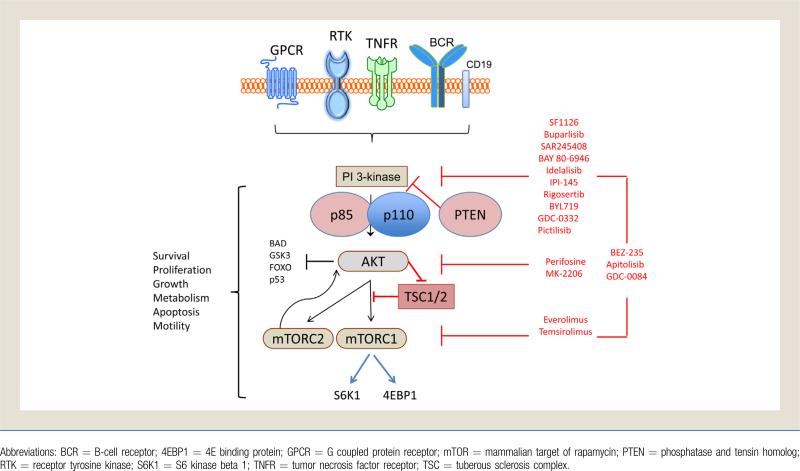
The main product of PI3K is PIP3, the phosphorylated form of membrane-bound phosphoinositides, which initiates a widely active signaling cascade. A main downstream target of PI3K is the serinethreonine kinase Akt, the major oncogenic effector of the PI3K/Akt pathway (Fig. 2). The primary negative regulator of Akt activation is phosphatase and tensin homolog (PTEN), which functions to dephosphorylate PIP3.28 Loss of the tumor suppressor PTEN via somatic mutation or epigenetic silencing is a frequent event in many cancers, but uncommon in lymphoma, and allows for increased PI3K signaling.29 Cell membrane-bound PIP3 allows docking of Akt in proximity to numerous kinase targets, including the mTOR inhibitor tuberous sclerosis complex (TSC)1/2. Additional targets include the oncogenically-relevant MDM2, IKKα, p21, and p27.30 MDM2 promotes cell survival and progression by inhibition of the p53 tumor suppressor, which is reversible by PI3K/Akt inhibition.31 IKKα is a critical regulator of nuclear factor–κB (NFκB) activity, a therapeutically relevant target in many lymphomas, that is activated in part by Akt.32 Activity of Akt can also inhibit the function of the cell cycle inhibitors p21 and p27, thus leading to unchecked growth.33-35
Figure 2.
PI3K/Akt/mTOR Overview and Targets
Akt indirectly activates mTOR, a complicated checkpoint of cellular growth influenced by growth factor signaling, adenosine monophosphate levels, and nutrient and O2 availability.1 mTOR refers to 2 distinct multimolecular complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2). When TSC2 is phosphorylated at Ser939 by activated Akt, it dissociates from TSC1 leading to mTORC1 activation.36 mTORC1, which activates translational repressor eukaryotic translation initiation factor 4EBP1 and S6K1, is sensitive to rapaymycin-like mTOR inhibitors.37,38 mTORC1 activity leads to increased mRNA translation, protein synthesis, and cellular proliferation. mTORC2 is “upstream” from and directly phosphorylates Akt, and is involved in regulation of the cytoskeleton.39,40 Demonstrating the complexity of the cross talk found in this pathway, mTORC1 inhibition can lead to activation of the PI3K pathway due to mTORC2 negative feedback, resulting in phosphorylation of Akt.41 Paradoxically, prolonged mTORC1 inhibition with rapamycin inhibited mTORC2 assembly in approximately 20% of cancer cell lines, and thus resulted in decreased Akt activity.42
PI3K/Akt/mTOR in Lymphoma
Aberrant activation of the PI3K/Akt/mTOR pathway occurs in lymphoma as a result of various anomalies (Fig. 2).5,43 Mutation or gene amplification of the PI3K isoforms can result in increased pathway activity. PIK3CA, the gene encoding p110a, is mutated in < 10% of patients with diffuse large B-cell lymphoma (DLBCL).44,45 The provisional data from the cancer genome atlas (TCGA) DLBCL project found no PIK3 mutations in initial available data, although more cases are being studied.46 In mantle cell lymphoma, mutations of PIK3CA are also rare, but most patients have an increased copy number of PIK3CA, resulting in increased transcription and pathway activation.47 Mantle cell lymphoma also displays increased p110α expression in relapsed disease, which might be more clinically relevant as a therapeutic PI3K pathway biomarker than p110δ.48 Hodgkin lymphoma displays greater expression of p110δ than p110α in preclinical models.49 A large subset of germinal center Becell-like DLBCL is defined by PTEN loss, which in results in increased PI3K/Akt signaling and in vitro PI3K inhibitor sensitivity.50 In many cases, PI3K activation might be induced by aberrant signaling from the microenvironment, such as the CD40 ligand.51
The B-cell receptor (BCR) is a critical signaling pathway for B-cell survival, and is one mechanism of physiologic PI3K pathway activation. BCR-related phosphorylation of the cytoplasmic domain of CD19 provides a docking site for the p85 regulatory subunit of PI3K, which allows for recruitment of the p110 catalytic subunit to the cell membrane.52,53 Bruton tyrosine kinase (BTK), an increasingly therapeutically relevant downstream target of BCR signaling, depends on PIP3, and thus PI3K, for membrane binding and activation.54 Point mutations in the PIP3 binding site of BTK lead to X-linked immunodeficiency and other B-cell deficiencies.
Phosphorylation of Akt represents PI3K pathway activation, and is common in lymphomas. Hodgkin lymphoma commonly demonstrates Akt phosphorylation in cell lines and in 63% of patient biopsies.55 Despite the low rate of PI3KCA mutation in DLBCL, phosphorylation of Akt is common (52%-72% of patient samples) and might be associated with inferior survival.45,56 Mantle cell lymphoma demonstrates variable levels of Akt phosphorylation, although the aggressive blastoid subtype appears to require constitutive Akt activation for survival.57 Peripheral T-cell lymphoma demonstrates phosphorylation of Akt in 49% of cases, which is strongly correlated with inferior clinical outcomes.58
Aberrant activation of the mTOR signaling network is common in multiple subtypes of lymphoma, due to upstream events and/or nutrient availability.59,60 The activity of mTOR often results from the upstream aberrations described, but might also be activated by mTOR-specific biology. In a subset of mantle cell lymphoma, mTOR regulates glycogen synthase kinase (GSK)-3β independently of Akt, and thus controls cyclin D1 regulation.61 Most DLBCL cell lines and patient samples have overexpression of p70S6K, a downstream target of mTOR.62 Increased levels of mTOR activity have been found in most Hodgkin lymphomas, and low levels correlated with improved clinical outcomes.63
Clinical Trials
PI3K Inhibitors
Inhibitors of PI3K might target specific (eg, p110a) or all (pan class I) isoforms. To date, PI3K inhibitors are not specific for mutant isoforms, and thus also affect wild type PI3K and physiologic PI3K activity. Early versions of pan class I PI3K inhibitors, now commonly used as tool compounds for in vitro study (eg, LY294002 or wortmannin), have significant off-target effects or solubility problems, and thus are not clinically viable drugs.64 A recent modification to LY294002 has revived its clinical prospects by binding it to a peptide via a cleavable linker, creating the prodrug SF1126.65 A phase I trial of SF1126 in patients with advanced solid tumors and B-cell malignancies found stable disease in chronic lymphocytic leukemia (CLL) patients (50%; 2/4) and a 40% reduction in lymph node size after 1 cycle in a DLBCL patient.
Newer pan class I PI3K inhibitors, such as buparlisib (BKM120),66 SAR245408,67 and BAY 80-694668 have shown less off-target effects, and generally are well tolerated. A phase I trial evaluating SAR245408 in patients with relapsed lymphomas and CLL found infrequent adverse events including diarrhea, hyper-glycemia, headache, and lymphopenia. Preliminary results from early phase trials show broad activity across non hodgkin lymphoma (NHL) subtypes, with an overall response rate (ORR) of 50% in follicular lymphoma (FL), and small lymphocytic lymphoma (SLL)/CLL (Table 2).69-83 Buparlisib has also been well tolerated, with rash, hyperglycemia, mood alteration, and pruritus reported in < 50% of patients. In a phase I trial in heavily pretreated solid tumor patients, 1 patient achieved a partial response and 16 patients (52%) achieved stable disease.66 Of note, 5 of the 7 patients who continued participation in the trial for > 8 months had documented genomic aberrations in the PI3K pathway, perhaps allowing for future biomarker-based trials. An international phase II trial of buparlisib in relapsed DLBCL, mantle cell lymphoma, and FL has opened and accrual is expected to complete in 2014. In trials of BAY 80-6946, an inhibitor of primarily the PI3K-δ and PI3K-α isoforms, investigators have found toxicities similar to other pan class I PI3K inhibitors and promising response data in patients with relapsed indolent NHL.68,71
Table 2.
Clinical Results of PI3K/Akt/mTOR Pathway-Specific Inhibitors as Single Agents in Unselected Patients With Relapsed Lymphoma
| Response Rate Percentage in Different Histologies | |||||||
|---|---|---|---|---|---|---|---|
| Agent | Target | DLBCL | FL | MCL | SLL/CLL | T-Cell | HL |
| SF1126 65 | PI3K-class I | 0 | - | - | 0 | - | - |
| Buparlisib 66 | PI3K-class I | a | a | a | - | - | - |
| SAR24 54 08 67,69,70 | PI3K-class I | 25 | 50 | - | 50 | - | 0 |
| BAY 80-6946 68,71 | PI3K-class I | 11 | 40 | 83 | 67 | 50 | - |
| Idelalisib 72-74 | PI3K-δ | 0 | 45 | 40 | 64 | - | 15 |
| IPI-145 75-77 | PI3K-γδ | 0 | - | 67 | 74 | 33 | |
| Rigosertib 78 | PI3K-αβ | 0 | 0 | - | - | - | 0 |
| Everolimus 79-81 | mTORC1 | 30 | 38 | 32 | 18 | - | 42 |
| Temsirolimus 82,83 | mTORC1 | 28 | 54 | 38 | 11 | - | - |
| Apitolisib | PI3K/mTOR | a | a | a | a | a | - |
| GDC-0084 | PI3K/mTOR | b | b | b | b | b | b |
| BEZ235 | PI3K/mTOR | b | b | b | b | b | b |
| GDC-0332 | PI3K-αγδ | b | b | b | b | b | b |
| Pictilisib | PI3K-αδ | a | a | a | a | a | |
| BYL719 | PI3K-α (WT/mutant) | b | b | b | b | b | b |
| MK-2206 | Akt | a | a | a | a | a | a |
Abbreviations: Akt = protein kinase B; CLL = chronic lymphocytic leukemia; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = hodgkin lymphoma; MCL = mantle cell lymphoma; mTOR = mammalian target of rapamycin; PI3K = phosphatidylinositol-3-kinase; SLL = small lymphocytic lymphoma; WT = wild type.
Trials ongoing without data.
No ongoing trials in lymphoma (as of January 2014).
PI3K inhibitors with activity against specific isoforms include idelalisib (formerly CAL-101 and GS-1101, p110δ) and IPI-145 (p110δ and p110γ) (Table 2).71-84 Idelalisib activity has been examined in a variety of B-cell malignancies. A phase I trial of idelalisib showed minimal toxicities, and ORR of 67% in relapsed indolent lymphomas.72 Based on these data, idelalisib was evaluated in combination with rituximab, bendamustine, or both in patients with relapsed indolent lymphoma.85 No major added toxicities were seen, and the ORRs were 77%, 85%, and 77% for the idelalisib and rituximab, idelalisib and bendamustine, and idelalisib and rituximab and bendamustine treatment groups. The progression-free survival at 20 months for all patients and responders was 66% and 73%, respectively, and randomized phase III trials are planned.86 Preliminary results from a phase I trial of idelalisib with everolimus, bortezomib, or bendamustine and rituximab in relapsed mantle cell lymphoma showed similar mild toxicity and questionable enhanced clinical responses.87 IPI-145 targets p110δ and p110γ, and therefore might have activity in B- and T-cell malignancies. At the 2013 American Society of Clinical Oncology Annual Meeting, an interim update demonstrated the efficacy of IPI-145 in T-cell (ORR, 33%) and B-cell lymphomas (ORR, 52%).77 Preliminary toxicity data appear mild and similar to other PI3K inhibitors, however significant infectious complications have occurred and prophylactic antibiotic, antiviral, and antipneumocystis medications are recommended.84 These infections are likely due to the activity of the drug on the benign immune cells, representing an undesired “on target” effect.
Rigosertib, a multikinase inhibitor, induces reactive oxygen species and directly inhibits PI3K, with preferential activity against the p110α and p110β isoforms. In a phase I trial of rigosertib (formerly ON 01910.Na) in relapsed hematologic malignancies, investigators found mild toxicity and stable disease in 54% (7/13) of evaluable patients, though no clinical responses were observed.78 A phase III trial is under way for myelodysplastic syndrome, and further development in lymphoma would likely require combination therapy or alternate scheduling.
A new generation of PI3K inhibitors that inhibit wild type and mutant class I isoforms are in early phase clinical trials. BYL719, an inhibitor specific for PIK3CA wild type and mutant p110α, has shown favorable safety and interesting efficacy in solid tumors and further trials are planned.88
Akt Inhibitors
Perifosine is a first-generation Akt inhibitor that functions via inhibition of Akt translocation to the cell membrane.89 Combined in a phase II trial with the multikinase inhibitor sorafenib, perifosine had an ORR of 28% in relapsed Hodgkin lymphoma.90 In this trial, the reduction of phosphorylated extracellular signal regulated kinases (p-ERK) and pAKT values at day 60 strongly correlated with response, although it is not clear if basal differences could be used for patient selection as predictive biomarkers. Due to mild efficacy and moderate toxicities, there are currently no ongoing clinical trials evaluating perifosine in patients with lymphoma.
A second-generation Akt inhibitor, MK-2206, functions via allosteric Akt inhibition and has shown strong preclinical activity in a variety of lymphoma cell lines and patient samples.91 In a phase I trial in patients with relapsed solid tumors, investigators found rash and gastrointestinal complaints to be common, but manageable.92 Several clinical trials evaluating MK-2206 in patients with relapsed lymphoma are ongoing.
mTOR Inhibitors
Rapamycin-like inhibitors, often referred to as “rapalogs,” have moderate activity in lymphoma by allosterically inhibiting mTORC1 (Table 2). Temsirolimus has significant activity in relapsed mantle cell lymphoma, alone (ORR of 38%)82 and with rituximab (ORR of 59%).93 Based on these data, temsirolimus has received orphan drug approval for relapsed mantle cell lymphoma in Europe. Temsirolimus has efficacy in other NHL subtypes, including follicular, SLL, and aggressive lymphomas.83
Newer rapalogs, such everolimus, are currently being evaluated in relapsed lymphoma. Everolimus is an oral mTORC1 inhibitor that is approved by the Food and Drug Administration for relapsed renal cell, brain, neuroendocrine, and hormone receptor-positive breast cancers. A phase II trial of everolimus in relapsed aggressive lymphoma showed mild toxicities and an ORR of 30%, including 30% of patients with relapsed DLBCL.79 In heavily pretreated Hodgkin lymphoma, everolimus was well tolerated and resulted in a 42% ORR and 35% stable disease rate.80 When combined with sorafenib in a phase I trial treating relapsed lymphoma and multiple myeloma, everolimus demonstrated modest toxicity and an ORR of 33%, including 5 of 6 Hodgkin lymphoma patients.94 Based on these data and preclinical studies, everolimus was also evaluated in combination with the histone deacetylase inhibitor panobinostat in 2 separate clinical trials in lymphomas and multiple myeloma.93,95,96 Toxicities were mild and the ORR was 33% to 43% in heavily pretreated lymphoma and myeloma patients, with most lymphoma patients achieving some degree of tumor regression.97 Everolimus has also been combined with conventional chemotherapy (CHOP) in T-cell lymphomas in a small phase I trial with acceptable toxicity and impressive efficacy.98 A newer generation of mTOR inhibitors, which are now entering clinical trials, are able to block mTORC1 and mTORC2, and might allow greater efficacy and avoidance of the compensatory phosphorylation of Akt.
Predictive Biomarkers
As our understanding of cancer biology and mechanisms of drug efficacy improve, so does our ability to categorize patients that are more likely to benefit from a particular therapy. Pharmacoprognostic markers are not yet robust enough for patient selection in PI3K/Akt/mTOR pathway clinical trials in lymphoma, but do show potential. In a small clinical trial evaluating the Akt inhibitor perifosine and multikinase inhibitor sorafenib, baseline levels of phosphorylated Akt and ERK in peripheral lymphocytes correlated with therapeutic response.99 In an elegant cell line screen of PI3K inhibitors, Walsh et al identified expression of P21-activated kinase (PAK) expression as a mediator of resistance, although clinical validation is needed.100 RNA interference of PAK1 was able to restore PI3K inhibitor sensitivity, and PI3K and PAK1 inhibitors demonstrated synergy.
Future clinical trials will need to prospectively validate the gene expression and protein phosphorylation patterns associated with the PI3K/Akt/mTOR pathway and clinical responses. Multiple reports have been published that evaluated the protein phosphorylation patterns associated with PI3K pathway inhibition in vitro for solid tumors and leukemia.101-103 Next generation sequencing techniques have shown early promise to identify further oncogenic driving mutations and/or therapeutic vulnerabilities related to the PI3K/Akt/mTOR pathway in solid tumors.104,105 Eventually, these early approaches at therapeutic response predication should allow oncologists to personalize therapy for patients with PI3K/Akt/ mTOR-driven cancers, including lymphomas.106 A challenge that will arise using sequencing and/or proteomics to personalize therapy for Hodgkin lymphoma will be to overcome the relatively low malignant cellularity in biopsy samples. Special techniques will be required to isolate the rare malignant cells to avoid the majority of the data to originate from benign infiltrating immune cells.
Conclusions
The PI3K/Akt/mTOR pathway is known to be important and has been successfully targeted in many cancers, including many lymphomas. Targetable aberrations along the entire course of the pathway have been observed, however, none of the histologically defined lymphoma subtypes appear to be driven primarily by PI3K/ Akt/mTOR. Development of potent inhibitors with specificity for mutant isoforms of PI3K, and rational combination strategies might limit toxicity and improve efficacy. As seen with targeted inhibitors in other cancer patient populations, the efficacy of small molecule inhibitors as single agents for patients with lymphomas might be limited, and long-term disease control might be rare.107 Therefore, it is likely that rationally designed combination clinical trials will be needed to target either multiple “nodes” of the PI3K/Akt/mTOR pathway simultaneously, or to cotarget accessory pathways to overcome resistance mechanisms. The success of future trials will depend on the ability of investigators to define populations with dependence on PI3K/Akt/mTOR aberrations, and to optimally target these aberrations.
Clinical Practice Points.
The PI3K/Akt/mTOR pathway is essential to the growth, differentiation, metabolism, survival, and cellular proliferation of lymphomas.
Activation of the PI3K pathway occurs from multiple genomic aberrations, and no lymphoma subtypes are thought to be primarily “PI3K-driven.”
p110δ is essential for B-cell development and BCR signaling, and is thus primarily targeted in B-cell malignancies.
p110γ is involved in physiologic immune function, and thus “on target” toxicities might include opportunistic infections.
mTOR inhibitors have shown moderate activity across a wide range lymphoma subtypes, but might in turn lead to Akt activation via a feedback loop.
Predictive biomarkers will prove essential to eventually select lymphoma patients most likely to benefit from PI3K pathway inhibition.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 2.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 4.Huang CH, Mandelker D, Schmidt-Kittler O, et al. The structure of a human p110α/p85α complex elucidates the effects of oncogenic PI3Kα mutations. Science. 2007;318:1744–8. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, McMullen JR, Sobkiw CL, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia S, Liu Z, Zhang S, et al. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swat W, Montgrain V, Doggett TA, et al. Essential role of PI3Kδ and PI3Kγ in thymocyte survival. Blood. 2006;107:2415–22. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condliffe AM, Davidson K, Anderson KE, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–40. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 12.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 13.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–8. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 15.Yip SC, El-Sibai M, Hill KM, et al. Overexpression of the p110β but not p110α isoform of PI 3-kinase inhibits motility in breast cancer cells. Cell Motil Cyto-skeleton. 2004;59:180–8. doi: 10.1002/cm.20032. [DOI] [PubMed] [Google Scholar]
- 16.Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson SP, Schoenwaelder SM, Goncalves I, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–14. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 18.Guillermet-Guibert J, Bjorklof K, Salpekar A, et al. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc Natl Acad Sci U S A. 2008;105:8292–7. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169–72. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 20.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297:1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 21.Jou ST, Carpino N, Takahashi Y, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–91. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sujobert P, Bardet V, Cornillet-Lefebvre P, et al. Essential role for the p110δ isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood. 2005;106:1063–6. doi: 10.1182/blood-2004-08-3225. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 24.Kim N, Saudemont A, Webb L, et al. The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood. 2007;110:3202–8. doi: 10.1182/blood-2007-02-075366. [DOI] [PubMed] [Google Scholar]
- 25.Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–40. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Takatoh J, Wang F. The mammalian class 3 PI3K (PIK3C3) is required for early embryogenesis and cell proliferation. PLoS One. 2011;6:e16358. doi: 10.1371/journal.pone.0016358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devereaux K, Dall'Armi C, Alcazar-Roman A, et al. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8:e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 29.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 30.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–60. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 34.Heron-Milhavet L, Franckhauser C, Rana V, et al. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;2:8267–80. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Cai SL, Tee AR, Short JD, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–89. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 39.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 40.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Benson RJ, Hostager BS, Bishop GA. Rapid CD40-mediated rescue from CD95-induced apoptosis requires TNFR-associated factor-6 and PI3K. Eur J Immunol. 2006;36:2535–43. doi: 10.1002/eji.200535483. [DOI] [PubMed] [Google Scholar]
- 44.Abubaker J, Bavi PP, Al-Harbi S, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–70. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 45.Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17:159–65. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 46.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3′-kinase catalytic subunit α gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15:5724–32. doi: 10.1158/1078-0432.CCR-08-3215. [DOI] [PubMed] [Google Scholar]
- 48.Iyengar S, Clear A, Bödör C, et al. P110α-mediated constitutive PI3K signaling limits the efficacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–84. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meadows SA, Vega F, Kashishian A, et al. PI3Kδ inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood. 2012;119:1897–900. doi: 10.1182/blood-2011-10-386763. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer M, Grau M, Lenze D, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:12420–5. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi MY, Kipps TJ. Inhibitors of B-cell receptor signaling for patients with B-cell malignancies. Cancer J. 2012;18:404–10. doi: 10.1097/PPO.0b013e31826c5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inabe K, Kurosaki T. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood. 2002;99:584–9. doi: 10.1182/blood.v99.2.584. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki H, Terauchi Y, Fujiwara M, et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science. 1999;283:390–2. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 55.Georgakis GV, Li Y, Rassidakis GZ, Medeiros LJ, Mills GB, Younes A. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. Br J Haematol. 2006;132:503–11. doi: 10.1111/j.1365-2141.2005.05881.x. [DOI] [PubMed] [Google Scholar]
- 56.Uddin S, Hussain AR, Siraj AK, et al. Role of phosphatidylinositol 3’-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–86. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 57.Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–76. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai Q, Deng H, Xie D, Lin T, Lin T. Phosphorylated AKT protein is overex-pressed in human peripheral T-cell lymphomas and predicts decreased patient survival. Clin Lymphoma Myeloma Leuk. 2012;12:106–12. doi: 10.1016/j.clml.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Argyriou P, Economopoulou P, Papageorgiou S. The role of mTOR inhibitors for the treatment of B-cell lymphomas. Adv Hematol. 2012;2012:435342. doi: 10.1155/2012/435342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wlodarski P, Kasprzycka M, Liu X, et al. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer Res. 2005;65:7800–8. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- 61.Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111:5142–51. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 62.Zhao MY, Auerbach A, D'Costa AM, et al. Phospho-p70S6K/p85S6K and cdc2/cdk1 are novel targets for diffuse large B-cell lymphoma combination therapy. Clin Cancer Res. 2009;15:1708–20. doi: 10.1158/1078-0432.CCR-08-1543. [DOI] [PubMed] [Google Scholar]
- 63.Mark A, Hajdu M, Varadi Z, et al. Characteristic mTOR activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease—a combined tissue microarray, in vitro and in vivo study. BMC Cancer. 2013;13:250. doi: 10.1186/1471-2407-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Mahadevan D, Chiorean EG, Harris WB, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012;48:3319–27. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 67.Brown JR, Davids MS, Rodon J, et al. Phase I trial of SAR245408 (S08), a panphosphatidylinositol 3 kinase (PI3K) inhibitor, in patients with chronic lymphocytic leukemia (CLL) and lymphoma. ASH Annual Meeting Abstracts. 2011:118. Abstract 2683. [Google Scholar]
- 68.Patnaik A, Ramanathan RK, Appleman LJ, et al. Phase I study of intravenous PI3K inhibitor Bay 80-6946: preliminary activity in patients with relapsed non-hodgkin lymphoma (NHL) treated in an MTD expansion cohort. ASH Annual Meeting Abstracts. 2012:120. Abstract 3704. [Google Scholar]
- 69.Brown JR, Davids MS, Rodon J, et al. Update on the safety and efficacy of the pan class I PI3K inhibitor SAR245408 (XL147) in chronic lymphocytic leukemia and non-Hodgkin lymphoma patients. Blood. 2013;122:4170. [Google Scholar]
- 70.Hamadani M, Arnason J, Karlin L, et al. SAR245409 monotherapy in relapsed/refractory follicular lymphoma: preliminary results from the phase II ARD12130 study. ASH Annual Meeting Abstracts. 2013:122. Abstract 86. [Google Scholar]
- 71.Morschhauser F, Bron D, Bouabdallah K, et al. Preliminary results of a phase II study of single agent bay 80-6946, a novel PI3K inhibitor, in patients with relapsed/refractory, indolent or aggressive lymphoma. ASH Annual Meeting Abstracts. 2013;122:87. [Google Scholar]
- 72.Kahl B, Furman R, Flinn I, et al. Final report of a phase I study of idelalisib, a selective inhibitor of PI3Kδ, in patients with relapsed or refractory indolent non-Hodgkin lymphoma (abstract). Hematol Oncol. 2013;31(suppl 1):195. [Google Scholar]
- 73.Kahl B, Byrd JC, Flinn IW, et al. Clinical safety and activity in a phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110δ, in patients with relapsed or refractory non-Hodgkin lymphoma. ASH Annual Meeting Abstracts. 2010:116. Abstract 1777. [Google Scholar]
- 74.Leonard J, Kahl B, Furman R, et al. Final report of a phase I study of idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase P110δ, in patients with relapsed or refractory mantle cell lymphoma (abstract). Hematol Oncol. 2013;31(suppl 1):194. [Google Scholar]
- 75.Kahl B, Patel M, Younes A, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,-g, in patients with relapsed/refractory B-cell lymphoma (abstract). Hematol Oncol. 2013;31(suppl 1):118. [Google Scholar]
- 76.Flinn I, Patel M, Horwitz S, et al. Preliminary safety and efficacy of IPI-145: a potent inhibitor of phosphoinositide-3-kinase-δ,-γ, in patients with relapsed/refractory CLL/SLL. Hematol Oncol. 2013;31(suppl 1):145. [Google Scholar]
- 77.Horwitz S, Flinn I, Patel M, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ, in patients with relapsed/refractory lymphoma. J Clin Oncol. 2013 (abstract 8518) [Google Scholar]
- 78.Roschewski M, Farooqui M, Aue G, et al. Phase I study of On 01910.Na (rigosertib), a multikinase PI3K inhibitor in relapsed/refractory B-cell malignancies. ASH Annual Meeting Abstracts. 2012:120. Abstract 1803. [Google Scholar]
- 79.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–7. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnston PB, Pinter-Brown L, Rogerio J, Warsi G, Chau Q, Ramchandren R. Everolimus for relapsed/refractory classical Hodgkin lymphoma: multicenter, open-label, single-arm, phase II study. ASH Annual Meeting Abstracts. 2012:120. Abstract 2740. [Google Scholar]
- 81.Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116:2201–7. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–56. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 83.Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin lymphoma subtypes: the University of Chicago Phase II Consortium. J Clin Oncol. 2010;28:4740–6. doi: 10.1200/JCO.2010.29.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flinn IW, Horwitz SM, Patel M, et al. Clinical safety and activity in a phase I trial of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ, in patients with advanced hematologic malignancies. ASH Annual Meeting Abstracts. 2012:120. Abstract 3663. [Google Scholar]
- 85.Fowler NH, de Vos S, Schreeder MT, et al. Combinations of the phosphatidylinositol 3-kinase-delta (PI3Kδ) inhibitor Gs-1101 (CAL-101) with rituximab and/or bendamustine are tolerable and highly active in previously treated, indolent non-Hodgkin lymphoma: results from a phase I study. ASH Annual Meeting Abstracts. 2012:120. Abstract 3645. [Google Scholar]
- 86.Leonard J, Wagner-Johnston N, Coutre S, et al. Tolerability and activity of combinations of the PI3Kδ inhibitor idelalisib (GS-1101) with rituximab and/or bendamustine in patients with previously treated, indolent non-Hodgkin lymphoma (iNHL): updated results from a phase I study (abstract). J Clin Oncol. 2013 [Google Scholar]
- 87.Wagner-Johnston N, De Vos S, Leonard J, et al. Preliminary results of PI3Kδ inhibitor idelalisib (GS-1101) treatment in combination with everolimus, bortezomib, or bendamustine/rituximab in patients with previously treated mantle cell lymphoma (MCL) (abstract). J Clin Oncol. 2013 [Google Scholar]
- 88.Geva-Zatorsky N, Dekel E, Cohen AA, Danon T, Cohen L, Alon U. Protein dynamics in drug combinations: a linear superposition of individual-drug responses. Cell. 2010;140:643–51. doi: 10.1016/j.cell.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–103. [PubMed] [Google Scholar]
- 90.Guidetti A, Viviani S, Marchiano A, et al. Dual targeted therapy with the AKT inhibitor perifosine and the multikinase inhibitor sorafenib in patients with relapsed/refractory lymphomas: final results of a phase II trial. ASH Annual Meeting Abstracts. 2012:120. Abstract 3679. [Google Scholar]
- 91.Buglio D, Lemoine M, Estrella J, et al. The allosteric AKT inhibitor MK-2206 demonstrates potent antiproliferative activity in lymphoma cells and synergizes with the HDAC inhibitor vorinostat. ASH Annual Meeting Abstracts. 2011:118. Abstract 3729. [Google Scholar]
- 92.Tolcher TAY AW, Fearen I, Taylor A, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST). J Clin Oncol. 2009;27(suppl 15) (abstract 3503) [Google Scholar]
- 93.Kumar S, Mikhael JR, LaPlant BR, et al. Phase I trial of a novel combination of an HDAC inhibitor (LBH589) and an mTOR inhibitor (RAD001) in lymphoid and plasma cell malignancies. ASH Annual Meeting Abstracts. 2011:118. Abstract 2682. [Google Scholar]
- 94.Kumar S, Porrata LF, Ansell SM, et al. Phase I trial of sorafenib and everolimus in patients with lymphoma or multiple myeloma. ASH Annual Meeting Abstracts. 2010:116. Abstract 2802. [Google Scholar]
- 95.Younes A, Copeland A, Fanale MA, et al. Safety and efficacy of the novel combination of panobinostat (LBH589) and everolimus (RAD001) in relapsed/refractory Hodgkin and non-Hodgkin lymphoma. ASH Annual Meeting Abstracts. 2011:118. Abstract 3718. [Google Scholar]
- 96.Lemoine M, Derenzini E, Buglio D, et al. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012;119:4017–25. doi: 10.1182/blood-2011-01-331421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oki Y, Buglio D, Fanale M, et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013;19:6882–90. doi: 10.1158/1078-0432.CCR-13-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SJ, Kang HJ, Kim JS, et al. Phase I study of mTOR inhibitor everolimus plus CHOP in patients with advanced, aggressive T-cell lymphomas. ASH Annual Meeting Abstracts. 2011:118. Abstract 1642. [Google Scholar]
- 99.Guidetti A, Locatelli S, Viviani S, et al. Phosphorylation levels of extracellular-signal regulated kinase (ERK) and AKT in circulating lymphocytes predict response to targeted therapy with kinase inhibitors in refractory/relapsed Hodgkin lymphoma patients. ASH Annual Meeting Abstracts. 2011:118. Abstract 3705. [Google Scholar]
- 100.Walsh K, McKinney MS, Love C, et al. PAK1 mediates resistance to PI3K inhibition in lymphomas. Clin Cancer Res. 2013;19:1106–15. doi: 10.1158/1078-0432.CCR-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nanjundan M, Byers LA, Carey MS, et al. Proteomic profiling identifies pathways dysregulated in non—small-cell lung cancer and an inverse association of AMPK and adhesion pathways with recurrence. J Thorac Oncol. 2010;5:1894–904. doi: 10.1097/JTO.0b013e3181f2a266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindholm EM, Krohn M, Iadevaia S, et al. Proteomic characterization of breast cancer xenografts identifies early and late bevacizumab-induced responses and predicts effective drug combinations. Clin Cancer Res. 2014;20:404–12. doi: 10.1158/1078-0432.CCR-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng Z, Shi YX, Tsao T, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120:2679–89. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang H, Cheung LW, Li J, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012;22:2120–9. doi: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Craig DW, O'Shaughnessy JA, Kiefer JA, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013;12:104–16. doi: 10.1158/1535-7163.MCT-12-0781. [DOI] [PubMed] [Google Scholar]
- 106.Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111ra121. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Westin JR, Kurzrock R. It's about time: lessons for solid tumors from chronic myelogenous leukemia therapy. Mol Cancer Ther. 2012;11:2549–55. doi: 10.1158/1535-7163.MCT-12-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]