Abstract
The prevalence of diabetes is highest in older adults, a population that is increasing. Diabetes self-care is complex with important recommendations for nutrition, physical activity, checking glucose levels, and taking medication. Older adults with diabetes have unique issues which impact self-care. As people age, their health status, support systems, physical and mental abilities, and nutritional requirements change. Furthermore, comorbidities, complications, and polypharmacy complicate diabetes self-care. Depression is also more common among the elderly and may lead to deterioration in self-care behaviors. Because of concerns about cognitive deficits and multiple comorbidities, adults older than 65 years are often excluded from research trials. Thus, little clinical evidence is available and the most appropriate treatment approaches and how to best support older patients’ self-care efforts are unclear. This review summarizes the current literature, research findings, and expert and consensus recommendations with their rationales.
Keywords: diabetes, self-care, education, depression, older adults
Diabetes in the United States is most prevalent among the burgeoning population of obese and older Americans. More than one-in-four U.S. adults aged 65 years and older have diabetes with the vast majority (90%–95%) diagnosed with type 2 diabetes (Caspersen, Thomas, Boseman, Beckles, & Albright, 2012; Engelgau et al., 2004; CDC., 2011). Projections for 2050 show the largest percentage increase is for those aged 75 years and older and the second greatest increase is among those 65 to 75 years of age (460%, 241% increase respectively; Boyle, Thompson, Gregg, Barker, & Williamson, 2010; Caspersen et al., 2012; Cowie et al., 2009; Finucane et al., 2011; Wang, McPherson, Marsh, Gortmaker, & Brown, 2011). Further, although many associate the onset of Type 1 diabetes with adolescence, Type 1 diabetes onset peaks a second time between ages 50 and 70 years (Krolewski, Warram, Rand, & Kahn, 1987; Warram & Krolewski, 2005). Diabetes’s costs represent 23% of current health care expenditures, of which more than half are attributable to adults age 65 and older (American Diabetes Association [ADA], 2013a). Thus, prevention of diabetes is the ultimate goal; however, effective management for older adults already diagnosed with diabetes is critical to diminish risks of complications and reduce diabetes’ economic burden.
Older adults with diabetes represent the full spectrum of health status ranging from excellent or good health with few or no comorbidities and intact cognitive and functional status to very poor health such as end-stage chronic illness management, severe cognitive impairment or poor functional status that may necessitate care in a long term care setting. For this reason, in 2012 the ADA convened a Consensus Development Conference on Diabetes and Older Adults (≥65 years) to review existing evidence and treatment guidelines for older adults with diabetes and to identify when individualizing treatment goals should be considered (Kirkman et al., 2012). Importantly, these treatment guidelines impact medication prescriptions and diabetes self-care recommendations (See Table 1). As less than 1% of clinical trials in diabetes focused on older adults (Lakey et al., 2013), most recommendations from the consensus panel were based on expert opinion. Glycemic goals for healthy older adults should be consistent with that of younger adults with diabetes; however, goals must be individualized for older adults with high comorbidity status, cognitive dysfunction or end-stage disease. These hemoglobin A1c (A1C) targets, a measure of average glucose levels for the prior two to three months, could be as high as 8.5% to prevent hypoglycemia while also preventing patient exposure to risks of glycosuria, dehydration, hyperglycemic hyperosmolar syndrome, and poor wound healing associated with high glucose levels (Kirkman et al., 2012).
Table 1.
Hemoglobin A1C and Fasting Glucose level Targets for Older Adults with Diabetes (Kirkman et al., 2012)
| Patient characteristics/health status | Rationale | Reasonable A1C goal Lower goals may be set if achievable without recurrent or severe hypoglycemia or undue treatment burden | Fasting or preprandial glucose (mg/dL) | Bedtime glucose (mg/dL) |
|---|---|---|---|---|
| Healthy (Few coexisting chronic illnesses, intact cognitive and functional status | Longer remaining life expectancy | <7.5% | 90–130 | 90–150 |
| Complex/intermediate (Multiple coexisting chronic illnesses,* 2+ instrumental ADL impairments, or mild to moderate cognitive impairment) | Intermediate remaining life expectancy, high treatment burden, hypoglycemia vulnerability, fall risk | <8.0% | 90–150 | 100–180 |
| Very complex/poor health (Long-term care or end-stage chronic illnesses,** moderate to severe cognitive impairment, or 2+ ADL dependencies | Limited remaining life expectancy makes benefit uncertain | <8.5† | 100–180 | 110–200 |
This represents a consensus framework for considering treatment goals for glycemia in older adults with diabetes. The patient characteristic categories are general concepts. Not every patient will fall clearly into a particular category. Consideration of patient/caregiver preferences is an important aspect of treatment individualization. Additionally, a patient’s health status and preferences may change over time. ADL, activities of daily living.
Coexisting chronic illnesses (at least 3) are conditions serious enough to require medications or lifestyle management and may include arthritis, cancer, congestive heart failure, depression, emphysema, falls, hypertension, incontinence, stage III or worse kidney disease, MI, and stroke. By multiple we mean at least three, but many patients may have five or more.
The presence of a single end-stage chronic illness such as stage III–IV congestive heart failure or oxygen-dependent lung disease, chronic kidney disease requiring dialysis, or uncontrolled metastatic cancer may cause significant symptoms or impairment of functional status and significantly reduce life expectancy.
A1C of 8.5% equates to an estimated glucose level of ~200mg/dL. Looser targets than this may expose patients to acute risks from glycosuria, dehydration, hyperglycemic hyperosmolar syndrome, and poor wound healing.
Although diabetes is more prevalent in older age groups, most randomized controlled intervention trials of adults with diabetes exclude those who are above 60 or 65 years of age because of concerns about comorbidities, possible changes in mental status, and other problems that may be increased in older people (Kirkman et al., 2012). Although some intervention studies include older adults, often they do not analyze the data by age, thus the impact of interventions on older individuals’ self-care and glycemic control cannot be determined. As a result, many questions remain about effectiveness of diabetes self-care and educational/behavioral interventions for older diabetes patients, particularly regarding how normal cognitive decline and increased comorbidities associated with aging impacts self-care of diabetes in everyday life. Thus, clinicians have little guidance on how best to support these patients in their self-care efforts (Kirkman et al., 2012) and are unsure of which behavioral/educational programs would provide benefit for older adults. Furthermore, issues with physician-patient communication impact how people with diabetes understand and discuss their diabetes self-care recommendations and prescriptions (Beverly, Brooks, Ritholz, Abrahamson, & Weinger, 2012; Beverly, Ganda, et al., 2012; Beverly, Hultgren, et al., 2011; Beverly, Ritholz, et al., 2012). Studies that include older persons with diabetes need to incorporate age in the analytic approach or perform secondary analyses for different age groups to determine how therapeutic interventions impact the elderly.
In this review, we discuss the current literature on diabetes, self-care and older adults. First, we discuss diabetes self-care challenges in the older population, followed by comorbidity, cognitive and psychosocial challenges. Next, we review unique challenges faced by older adults residing in long-term care facilities. Finally, we discuss research and interventions needed to improve self-care practices, clinical outcomes, and quality of life in older adults with diabetes.
Diabetes Self-Care Challenges
Diabetes self-care is complex, requiring management of medication regimens (taking the appropriate dose of medication at the correct time), following a diabetes meal plan to account for glucose levels and choosing healthy foods, blood glucose monitoring, exercising regularly and attending regular medical/educational assessment visits (McNally, Rohan, Pendley, Delamater, & Drotar, 2010) to maintain optimal glycemic control. To integrate these behaviors and continue diabetes self-care during difficult life situations, those with diabetes need skills in problem solving, coping and risk prevention. Importantly, support from health care providers provides the foundation necessary to implement and maintain these behaviors over time. Healthcare providers need to be aware of the impact of aging on self-care abilities and help their patients adjust self-care to adapt to psychosocial and physiologic changes associated with aging. However, how specific self-care behaviors need to be adapted to the aging process and to the presence of complications and co-morbidities has not been well studied.
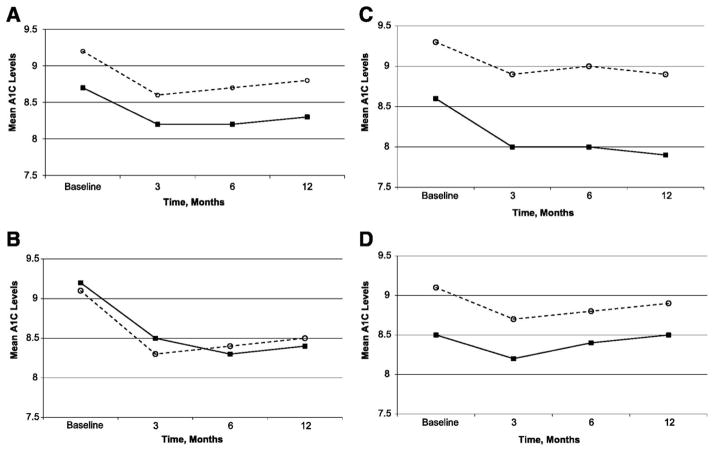
Many clinicians are reluctant to refer older adults to group education programs to gain the self-care support necessary to live with diabetes, feeling that older patients require individualized attention. A recent randomized controlled trial reported that a highly structured diabetes self-care education intervention with cognitive behavioral strategies embedded helped improve glycemia in poorly controlled diabetes patients more than standard group education or individual sessions with dietitians and/or nurse educators (Weinger et al., 2011). Importantly, a subgroup analysis of older adults in this study, although in a small sample (n=71) with age not exceeding 75 years, demonstrated that, on average, older adults benefited from group interventions more than standard one-to-one educational interventions and also more than middle-aged adults (Beverly et al., 2013; See Figure 1). How those over 75 years of age would respond to these interventions is not clear and needs further investigation. Thus, preliminary evidence suggests clinicians may safely recommend group interventions for older persons without dementia up to age 75 years (Beverly et al., 2013).
Figure 1.
Mean A1C levels over time for older versus younger adults for all intervention groups (n=222). A. All groups. B: Structured behavioral group intervention. C. Standard group education intervention (attention control). D. Individual education intervention (control). O= younger adults; ■ = older adults.
Nutrition
Nutrition is an integral component of diabetes self-care for all people with diabetes regardless of age. Following a healthy diet is a well-recognized factor that impacts one’s ability to achieve glycemic targets and prevent or delay the onset of severe diabetes complications; however, following a healthy diet is challenging for most people with diabetes (Delahanty & Halford, 1993; Nelson, Reiber, & Boyko, 2002; Neuhouser, Miller, Kristal, Barnett, & Cheskin, 2002; Oza-Frank, Cheng, Narayan, & Gregg, 2009; Pi-Sunyer et al., 1999; Vitolins et al., 2009). Although nutritional guidelines do not differ for younger versus older adults, older adults with diabetes may present with unique challenges that impact their ability to follow a healthy diet. For example, older adults may have limited finances or experience difficulties with food shopping and/or meal preparation. Furthermore, older adults are at greater risk for altered taste and smell perception, swallowing difficulties, dentition problems, altered gastrointestinal functioning, anorexia due to undernutrition and skipping meals due to cognitive dysfunction and/or depression (Kirkman et al., 2012). Weight loss should be approached with caution in the older adult as both intentional and unintentional weight loss may lead to severe nutritional deficiencies (Kirkman et al., 2012). Thus, obese older adults may require nutritional supplementation during an interval of intentional weight loss. Referral to a dietitian or Certified Diabetes Educator can be useful in assessing an older adult’s nutritional needs while developing strategies to address nutrition-specific self-care barriers.
As adults age they may require fewer calories due to decreased metabolic rates and reduced physical activity; however, older adults still require the same or higher levels of nutrients to promote optimal health outcomes (Lichtenstein, Rasmussen, Yu, Epstein, & Russell, 2008). Researchers at several U.S. universities have adapted the United States Department of Agriculture’s (USDA) MyPlate specifically for older adults (e.g., University of Florida’s MyPlate for older adults, Bobroff, 2011). MyPlate for older adults emphasizes the importance of choosing nutrient-dense food and maintaining fluid balance. It depicts foods in the following categories: 1) whole, enriched, and fortified grains and cereals, 2) bright-colored vegetables, 3) deep-colored fruit, 4) low- and non-fat dairy products, 5) dry beans, nuts, fish, poultry, lean meat and eggs, and liquid vegetable oils and soft spreads low in saturated and trans fat. MyPlate for older adults also provides guidance regarding fluid intake and physical activity. However, it is not tailored to the diet self-care needs of older adults with diabetes; nutrition guidelines and education tools specifically for this population are necessary for patients and for clinicians to provide excellent diabetes self-care support.
Few nutrition intervention studies have been designed specifically for older adults with diabetes; however, preliminary evidence suggests that medical nutrition therapy is beneficial for older adults (Kirkman et al., 2012). One study (Miller, Edwards, Kissling, & Sanville, 2002) evaluated a randomized nutrition intervention on blood glucose and lipoprotein levels in adults aged 65 years and older with type 2 diabetes. Study subjects participated in 10 weekly 2-hour group education sessions about interpreting carbohydrate, fat and cholesterol information on food labels. The nutrition education improved fasting plasma glucose (mean change= −18.8±6.2) and A1C (mean change= −0.5±0.1%) levels (Miller et al., 2002). Another study (Redmond et al., 2006) evaluated the impact of a nutrition intervention on older adults (mean age=73.6 years) with self-reported diabetes diagnosis from 10 senior centers in Georgia. Older adults participated in eight education sessions focusing on carbohydrate counting, portion control, and meal spacing. Following the intervention, the mean change in A1C levels for all participants decreased 0.24±1.35% (p=0.11). In sub-analyses, older adults with a pre-intervention A1C level >6.5% had a mean decrease in A1C levels of 0.66±1.58% (p=0.01) and adults with a pre-intervention A1C level >8.0%, on average, decreased in A1C levels 1.46±1.90% (p=0.01; Redmond et al., 2006). These studies show that nutrition-specific education can be beneficial among older adults with diabetes. More research is needed to confirm these findings and establish the necessary duration and quality of education to improve glycemic control in this population. Importantly, nutrition recommendations should take into account the older adults’ unique culture, values, preferences, and individual goals and abilities (Beverly, Wray, Chui, & LaCoe, 2014; Kirkman et al., 2012). For example, carbohydrate counting may not be needed by older adults who are on less intensive treatment regimens or have more relaxed glycemic goals.
Physical Activity
Daily physical activity is important to health for people of all ages. Diabetes does not pose a contraindication to the need for or benefit of physical activity for older adults. In fact, exercise provides distinct benefits to those with diabetes in terms of improving glycemic control, insulin sensitivity, promoting weight loss and reducing risk of cardiovascular disease. Guidelines for older adults with diabetes who are otherwise healthy (Kirkman et al., 2012) are consistent with recommendations of the national Centers for Disease Control and Prevention (CDC; Glasgow, Toobert, & Hampson, 1996) and the ADA (2013b): 30 minutes of moderate activity such as walking at a brisk pace 5 days a week and muscle strengthening activities 2 days each week. Despite the benefits of exercise, only 25% of older adults with diabetes who participated in a recent national survey met ADA guidelines for physical activity (Zhao, Ford, Li, & Balluz, 2011). Surprisingly, no differences were noted for those with and without disability. Factors associated with failure to meet guidelines were female gender, non-Hispanic black race, age greater than 75 years, obesity, and having a disability. Older adults may believe that exercise is not helpful unless it occurs for long periods of time and may feel incapable exercising for long durations. However, recent evidence from a sample of community-dwelling seniors with diabetes (DiPietro, Gribok, Stevens, Hamm, & Rumpler, 2013) suggests a greater improvement in blood glucose level on days when seniors walked for 15 minutes after breakfast, lunch and dinner (total 45 minutes) compared to days when they walked consistently for the same duration at only one time point during the day.
Aerobic exercise recommendations must be tailored for those with functional disability secondary to neuropathy, impaired vision or other cause to prevent injury. In one study (Pijpers et al., 2012), 30% of older adults with diabetes had sustained two or more falls over a 6-month period. The risk of recurrent falls was 1.7 (95% CI 1.11–2.51) times higher for those with diabetes compared to similar age adults without diabetes. Factors that explained 47% of the increased risk for recurrent falls were higher levels of pain, lower physical activity and grip strength, limitations in activities of daily living, and cognitive impairment. Although aerobic exercise may pose challenges for some older adults with diabetes, particularly those with neuromuscular and sensorimotor changes, all can participate and benefit from modest increases in regular exercise. Referral to supervised group exercise programs, such as those provided at senior centers or the Young Men’s Christian Association (YMCA), can improve activity level while promoting safety (Kirkman et al., 2012). In addition, all can participate in exercise programs that focus on muscle strengthening through resistance training. A recent meta-analysis (Hovanec, Sawant, Overend, Petrella, & Vandervoort, 2012) of three randomized trials examining the effect of resistance training in older adults with diabetes suggests that resistance training demonstrates moderate to large benefit in improving blood pressure, total and low-density lipoprotein (LDL) cholesterol, fasting insulin, and muscle strength.
Chair exercise is another alternative for older adults who are homebound, have limitations in mobility, or where safety of participation in a more traditional exercise program may be a concern, for example, those with peripheral neuropathy. In one trial (Niemela, Vaananen, Leinonen, & Laukkanen, 2011), older women assigned to a program of simple exercises performed while sitting in a rocking chair demonstrated improved grip strength, balance and walking at three months. Importantly, they were motivated to continue exercising following completion of their study participation.
Medication Prescriptions
A recent systematic review (Campbell et al., 2012) examined the impact of cognitive impairment on medication adherence in older adults. Cognitive dysfunction imposes many barriers to medication adherence. Patients may have difficulty understanding directions regarding how to take new medication and knowledge of why the medication was prescribed. Caregivers of older adults may rely on the patient to remember their medications or the caregiver may have cognitive difficulty themselves. Barriers to non-adherence associated with cognitive impairment in the elderly were being female, minority race or ethnicity, age ≥75 years, higher level of comorbidity and greater complexity of medication regimen. Clearly, periodic assessment of cognitive function as part of routine screening for older people with diabetes (Kirkman et al., 2012) is needed as deficits may not be readily apparent yet negatively affect diabetes self-care.
External medication barriers include insurance coverage and medication costs. Many older adults are prescribed multiple medications and may not be able to afford to pay for all of their medications all at once. For example, older adults with the Medicare prescription drug coverage (Part D) experience the coverage gap (i.e., “donut hole”) and subsequently struggle to pay for prescriptions. The passing of the Patient Protection and Affordable Care Act (PPACA) includes important improvements to Medicare prescription drug coverage and plans to eliminate the gap altogether by 2020. The aim of this new legislation is to assist older adults with diabetes and other health condition better afford prescription medications.
Hypoglycemia
Hypoglycemia is a common complication in older adults (Bertoni, Krop, Anderson, & Brancati, 2002), particularly for those in poor glycemic control and those who were recently hospitalized (Shorr, Ray, Daugherty, & Griffin, 1997). In one study (Munshi et al., 2011), subjects wore blinded continuous glucose monitoring (CGM) devices for three days. More than half experienced one or more hypoglycemia episodes detected by CGM lasting 46 minutes, on average with those occurring at night of longer duration. Most were undetected by either finger-stick glucose monitoring or presence of symptoms. Of note, hypoglycemia frequency and duration was similar for subjects with Type 2 and Type 1 diabetes.
Older age creates a greater vulnerability to hypoglycemia secondary to diminished counter-regulatory responses and changes in pharmacokinetics (reduced renal elimination) and pharmacodynamics (increased sensitivity to medications; Kirkman et al., 2012; Seaquist et al., 2013). Although the association between hypoglycemia and cognitive function is well known, the direction of the relationship has been unclear. Findings of a recent population-based prospective cohort study of 783 older adults with diabetes (Yaffe et al., 2013) suggest that the relationship is bidirectional. Over the 10-year period and controlling for comorbidities and change in cognitive function, subjects who had experienced a severe hypoglycemic event were 2 times more likely to develop dementia and subjects who had developed dementia during the follow up period were 3 times more likely to have a subsequent severe hypoglycemic event. Efforts to prevent hypoglycemia in older adults with diabetes are essential for health and well-being of this population.
Although few studies address hypoglycemia and diabetes self-care in older adults, the joint Workgroup from the ADA and the Endocrine Society recommends patient education to support patients and family members or other caregivers in the ability to prevent and treat hypoglycemia (Seaquist et al., 2013). Reinforcement of self-care behaviors, including early recognition and treatment of hypoglycemic symptoms, and reinforcement of their understanding of how medications work as well as the impact of food and exercise on glucose levels are important for hypoglycemia prevention (Seaquist et al., 2013).
Insulin Pump Therapy
Several studies have examined risk of hypoglycemia in subjects treated with intensive insulin regimens. Findings of these studies have been consistent with those wearing insulin pumps having significant hypoglycemia reduction (60–79%) compared to multiple daily injections (D. Cohen et al., 2003; Hoogma et al., 2006; Weintrob et al., 2003). A more recent technological innovation, the sensor-augmented insulin pumps programmed to interrupt insulin delivery at a preset glucose value, have been found to further improve safety (Bergenstal et al., 2013). Although older adults were not the population of interest in these clinical trials, study findings, as well as the ADA and American Geriatrics Society recommendation of A1C target ≤7% (Kirkman et al., 2012), support the consideration of insulin pumps in the diabetes management of healthy older adults.
Indications for insulin pump therapy for older adults are similar to established indications for younger adults with diabetes; however, age-related barriers such as impaired dexterity or visual impairment as well as patient therapy preferences must be considered as part of overall assessment (Stephens & Heffner, 2010) of health status prior to initiation of insulin pump therapy and on an ongoing basis as function may decline over time. Thus, frequent evaluation for older adults on insulin pump therapy is needed to account for any changes in cognitive decline or health status. Two trials (Herman et al., 2005; Raskin et al., 2003) have examined efficacy and safety of insulin pumps and multiple daily injections in older adults with Type 2 diabetes naïve to intensive insulin therapy. Findings were consistent in terms of A1C improvement, hypoglycemia, and treatment-specific technical or mechanical problems. A majority of subjects in both groups reported injection/infusion irritation or discomfort and technical problems, such as insulin pump/pen malfunction, particularly during the first 2 months of transition to the new therapy (Herman et al., 2005). This highlights the need for diabetes education centered on management of these technologies and incorporating practice sessions for troubleshooting potential issues. Despite the need to adapt to the use of a sophisticated technology, satisfaction, on average, was higher for those using insulin pumps experiencing less hassles, greater convenience and less life interference (Raskin et al., 2003).
As the primary insurer for the majority of Americans ≥65 years, Medicare Part B, provides coverage for insulin pumps under its durable medical equipment to those with Type 1 diabetes and some with Type 2 diabetes who meet one or more medical criteria: A1C >7%, frequent hypoglycemic episodes, and/or wide excursions in blood glucose levels. Inability to produce insulin must be documented by a C-peptide level. In addition, all individuals wishing to qualify for insulin pump coverage through Medicare must complete a diabetes self-care program, have been on an insulin regimen for the past 6 months, and monitored blood glucose levels a minimum of 4 times daily, and demonstrate basic proficiency in adjusting mealtime insulin doses according to blood glucose level. Consistent with the Medicare program, the individual is responsible for 20% of the Medicare approval amount for the pump and supplies after the yearly Part D deductible is met (Centers for Medicare & Medicaid Services, n.d.).
Comorbidity Challenges
Polypharmacy
Presence of one or more comorbid conditions is common for older adults with diabetes (Struijs, Baan, Schellevis, Westert, & van den Bos, 2006). The term polypharmacy is used when multiple medications are prescribed for multiple conditions (Nobili, Garattini, & Mannucci, 2011). Although there is no standard definition of the number of medications that defines polypharmacy, most define polypharmacy as taking five or six medications per day but do not distinguish between appropriate versus inappropriate use (Good, 2002). In one sample of older adults with diabetes, subjects, on average, reported taking 8.2±4.0 medications each day (Munshi et al., 2011). Self-reported medications may not include over the counter, herbal or medications such as Tylenol that may be taken on an “as needed” basis; therefore, self-report may underestimate the actual number of medications taken. Higher numbers of daily medications increase the complexity of medication self-care and the risk of medication error. Further complicating the issue is that medications are often prescribed by different practitioners and may be filled at different pharmacies making duplication and potential interactions difficult to easily identify.
Older individuals are at increased risk for adverse drug effects from most medications secondary to age-related changes in pharmacokinetics, notably, changes in renal function. Further, the risk for drug interactions is also higher. In a review of 17 studies of potentially harmful drug interactions in the elderly, Hines (Hines & Murphy, 2011) reported a variety of drug-drug interactions that often required hospitalization with the interaction between sulfonylureas and antimicrobial agents being among the more frequently occurring interactions. Review of all medications on a regular basis is needed with ongoing assessment of therapeutic response to medications currently prescribed. Drugs without clear indication should be discontinued. The Beers criteria provides guidance regarding specific drugs that should be avoided in the elderly (American Geriatrics Society Beers Criteria Update Expert Panel, 2012).
The Beers criteria were developed to improve care for older adults by reducing their exposure to potentially inappropriate medications and were recently updated by the American Geriatrics Society (American Geriatrics Society Beers Criteria Update Expert Panel, 2012). The criteria were developed through a systematic review of literature and grading of evidence to identify particular classes of medications to be avoided in the elderly. Diabetes medications that meet the Beer criteria as potentially inappropriate and should be avoided as first line treatment for vulnerable older adults are sliding scale insulin, and long-duration sulfonylureas (chlorpropamide and glyburide) due to their higher risk of hypoglycemia in the elderly.
Comorbidities and Complications
Diabetes self-care for older adults necessitates special attention to the clinical (e.g., comorbidity, complications) and functional (e.g., impairment, disability) heterogeneity of this population. The average person with diabetes has 3.5 other chronic conditions (See Table 2; Partnership for Solutions, 2004; Wolff, Starfield, & Anderson, 2002). The number of health conditions in addition to diabetes increases with age, such that 4 out of 5 adults aged 65–74 years and 5 out of 6 adults aged 75 and older have more than one other chronic condition (see Table 2; Partnership for Solutions, 2004). Older adults with diabetes also are at greater risk for geriatric syndromes, including cognitive impairment, depression, injurious falls, neuropathic pain, and urinary incontinence (Brown, Mangione, Saliba, & Sarkisian, 2003; Kirkman et al., 2012). Comorbidities and complications can have a negative impact on older adults’ diabetes self-care (Ciechanowski, Katon, & Russo, 2000; Kerr et al., 2007; Krein, Heisler, Piette, Makki, & Kerr, 2005; Schoenberg & Drungle, 2001), health status, and quality of life (Glasgow, Ruggiero, Eakin, Dryfoos, & Chobanian, 1997; Wray, Ofstedal, Langa, & Blaum, 2005). Furthermore, older adults’ comorbidities and complications may pose competing demands that require substantial time, effort, and money to manage effectively (Bayliss, Steiner, Fernald, Crane, & Main, 2003; Beverly, Wray, Chiu, & Weinger, 2011; Chernof et al., 1999; Jaen, Stange, & Nutting, 1994). Finally, high rates of comorbidity may require older adults with diabetes to take multiple medications, which can lead to drug side effects and drug-drug and drug-disease interactions (Brown et al., 2003).
Table 2.
Most Common Comorbidities in People with Diabetes (ADA, 2013b; Partnership for Solutions, 2004)
| Obesity |
| Hypertension |
| Dyslipidemia |
| Arthritis |
| Hearing impairment |
| Obstructive sleep apnea |
| Fatty liver disease |
| Low testosterone in men |
| Periodontal disease |
| Certain cancers |
| Liver cancer |
| Pancreatic cancer |
| Endometrial cancer |
| Colorectal cancer |
| Breast cancer |
| Bladder cancer |
| Cognitive impairment |
| Depression |
| Hip fracture |
Furthermore, physical changes associated with aging can negatively impact older adults’ diabetes self-care (Trief, 2007). Decreases in muscle mass, bone strength, joint flexibility, aerobic capacity, and visual and auditory acuity contribute to physical, functional, and cognitive decline (Trief, 2007); these declines can lead to disability (Egede, 2004), impairment of activities of daily living (Gregg et al., 2000; Volpato et al., 2002), poor perceived health (Black, 1999), and lower quality of life (Egede, Zheng, & Simpson, 2002). Thus, understanding the impact of comorbidity on diabetes self-care is critical for improving diabetes treatment in older adults.
Cognitive Challenges
Both older age and diabetes are risk factors for cognitive function impairment and the presence of both factors increases this risk (Hassing et al., 2004; Lu, Lin, & Kuo, 2009). Even in subjects with perceived normal cognition, diabetes may be associated with poorer executive function (Thabit, Kyaw Tun, McDermott, & Sreenan, 2012). Executive functions are higher level cognitive operations, such as problem solving, planning and organization that are key for diabetes self-care. Memory is an important component of executive function. Poor cognitive function impairs ability to perform many self-care tasks particularly those that rely on numeracy such as interpretation of blood glucose values, changing insulin doses, and drawing up insulin or dialing a dose for insulin administration via syringe or pen (Kirkman et al., 2012).
Although the exact pathophysiology affecting executive function and cognition for those with diabetes is not fully understood, hyperglycemia and hypoglycemia, vascular disease, and insulin resistance are thought to have important roles (Kodl & Seaquist, 2008). In one large sample of community dwelling older women (N=2,347), women with diabetes demonstrated poorer performance on a battery of tests of cognitive function compared to those without diabetes (Grodstein, Chen, Wilson, & Manson, 2001). In another sample (N=60) recruited from a geriatric diabetes clinic, approximately one third screened positive for cognitive dysfunction. Notably, these adults demonstrated other functional disabilities such as hearing or visual impairment, recent falls and difficulty performing activities of daily living (Munshi et al., 2006). Cognitive impairment, even if subtle, has particular relevance because nearly one third of non-institutionalized elderly Americans, particularly women, live alone (Greenberg, 2011), and may lack financial and social resources to support their successful diabetes self-care.
Psychosocial Challenges
Depression, Depressive Symptoms and Distress
Psychosocial changes, such as loss of family members and friends, fears about mortality, loneliness, isolation, and retirement, may also negatively impact diabetes self-care. For example, the loss of a loved one may lead to depression or depressive symptoms, which may feed into a negative cycle of poor self-care and worsening glycemic control (Rubin & Peyrot, 1994). Depression, elevated depressive symptoms and distress are associated with decrease in the frequency of diabetes self-care behaviors and with poor glycemic control (Fisher et al., 2007; Leyva, Zagarins, Allen, & Welch, 2011; Lustman et al., 2000). Importantly, an estimated 14% to 28% of older adults with diabetes have depression (Bell et al., 2005; Bruce et al., 2003; Chou & Chi, 2005; Kessler et al., 2003). Older adults with diabetes experience disproportionately high rates of depression, which are two to four times greater than that of the general population aged 65 and older (CDC, 2010).
Depression and depressive symptoms can decrease adherence to dietary, exercise and medication regimens and, in turn, contribute to worsening glycemic control (Ciechanowski et al., 2000; Lin et al., 2004, Lustman et al., 2000). Depression and depressive symptoms also are associated with the presence of serious complications (e.g., retinopathy, neuropathy, nephropathy, cardiovascular disease, hypertension, sexual dysfunction (H. W. Cohen, Gibson, & Alderman, 2000; de Groot, Anderson, Freedland, Clouse, & Lustman, 2001; Jacobson, 1996; Kovacs, Mukerji, Drash, & Iyengar, 1995; Lustman, Griffith, Gavard, & Clouse, 1992), poor physical functioning (Bell et al., 2005), increased hospitalization and mortality (Rosenthal, Fajardo, Gilmore, Morley, & Naliboff, 1998). Importantly, among 315 middle-aged and older type 2 diabetes patients, elevated depressive symptoms were associated with reluctance to discuss self-care with health care providers while poor glycemic control was not (Beverly, Ganda, et al., 2012). Identification of depression and depressive symptoms in older adults with diabetes is critical because this population has the highest suicide rate of any age group (National Institute of Mental Health, 2010; CDC, 2007). Thus, timely diagnosis and effective treatment of depression and depressive symptoms in older adults is necessary to mitigate risk of suicide and improve self-care and clinical outcomes.
Furthermore, many older adults may experience diabetes distress, which is distinct from depression as distress develops from living with diabetes. Diabetes distress may include frustration with self-care, fears about the future, concerns about diabetes complications, and difficulties with family member and/or friends (Fisher, Glasgow, & Strycker, 2010; Nicolucci et al., 2013). Older adults may feel overwhelmed with the never-ending demands of self-care or confusion regarding conflicting treatment recommendations for multiple health conditions. Importantly, interventions designed to address diabetes-specific barriers and correspondent problem-solving strategies can lower diabetes distress in older adults (Beverly et al., 2013; Fisher et al., 2013; Munshi et al., 2013). Lowering diabetes distress in older adults may be all the more important given recent research demonstrating an association between diabetes distress and A1C levels (Fisher, Glasgow, et al., 2010; Fisher, Mullan, et al., 2010).
Long-Term Care Facilities
Nearly 33% of all long-term care residents have diabetes (Dybicz, Thompson, Molotsky, & Stuart, 2011; Zhang et al., 2010). Older adults residing in long-term care facilities are more likely to have certain comorbid conditions (e.g., cardiovascular disease, renal failure, renal insufficiency, dyslipidemia), take medications for comorbidities, visit the emergency department, be hospitalized with longer lengths of stays and suffer fractures from falls (Dybicz et al., 2011; Maurer, Burcham, & Cheng, 2005; Zhang et al., 2010). Further, long-term care residents with diabetes require more staff time and attention to manage the complexities of diabetes self-care (Travis, Buchanan, Wang, & Kim, 2004). Self-care challenges in long-term care include nutrition therapy, physical activity, blood glucose monitoring and use of oral antihyperglycemic agents and/or insulin administration. Medical nutrition therapy for weight loss is not recommended for older adults in nursing homes because of the increased risk for malnutrition, dehydration and loss of lean body mass (Kyrou & Tsigos, 2009). For this reason a healthy, regular diet is recommended for nursing home residents with diabetes (Schafer et al., 2004). In fact, most older residents tend to be underweight rather than overweight (Schafer et al., 2004). Interestingly, tube feeding is common in nursing home residents, and approximately half of the residents receiving enteral feeding have diabetes (Arinzon, Shabat, Shuval, Peisakh, & Berner, 2008). Research is needed to determine the best diabetes-specific formulas to improve glycemic control in residents receiving enteral nutrition.
Nursing home residents with good functional status should be encouraged to participate in light to moderate physical activity (e.g., water aerobics, walking, resistance training; Migdal, Yarandi, Smiley, & Umpierrez, 2011). The presence of comorbid conditions and complications (e.g., retinopathy, neuropathy, foot wound, hearing impairment) should be noted prior to engaging in a physical activity program (Migdal et al., 2011). A1C monitoring in long-term care facilities is inconsistent, with 16% to 26% of residents not having A1C levels measured in the past 12 months (Garcia & Brown, 2011; Meyers, Broton, Woo-Rippe, Lindquist, & Cen, 2007; Pham, Pinganaud, Richard-Harston, Decamps, & Bourdel-Marchasson, 2003). Further, a recent survey study found that only 7% of long-term care facilities had policies for A1C testing despite the American Diabetes Association (ADA), American Geriatrics Society (AGS) and American Medical Directors Association (AMDA) guideline recommendations for A1C monitoring frequency (Feldman, Rosen, & DeStasio, 2009). In addition, only 31% of facilities had policies for blood glucose monitoring. Both the ADA and AMDA recommend blood glucose monitoring be individualized based on the healthcare needs (e.g., multiple daily insulin injections, insulin pump therapy) and goals for each long-term care resident (ADA, 2013b; AMDA, 2008). Blood glucose monitoring is critical in long-term care facilities to prevent serious hypoglycemia or hyperglycemia and their sequelae.
The population of older people with diabetes is growing and represents a heterogeneous group with varying health status and needs. Few studies are available to guide clinicians regarding the most effective approaches to supporting diabetes self-care or on individualizing diabetes care. For example, whether those older than 75 years benefit from group diabetes education and self-care support is not clear. Improved assessment techniques to screen diabetes patients for self-care problems are needed particularly for those in primary care.
Many specific personal, external and environmental factors influence one’s ability to perform diabetes self-care and these factors are not typically stable for the older adult. Assessment of barriers allows health providers to individualize clinical approaches geared toward improving diabetes self-care and glycemic and other outcomes. For example, the person’s support systems change with marriages, births of grandchildren, and deaths among families and friends. As family become less available, resources such as alternative caregivers, need to be considered. In addition, comorbidities and complications in particular can be significant barriers. Thus, clinicians need to incorporate assessment of several of these factors into routine visits with elderly patients.
Although cognitive issues such as subtle changes in executive functions and memory are often anticipated and recognized, how these impact self-care behaviors is not well known. Today’s healthcare environment requires effective, efficient, and grounded in evidence; thus, research in all aspects of self-care to create a strong evidence base for this growing population of older adults with diabetes. In addition, healthcare providers need training in how to support older people with diabetes and psychosocial problems to help them live well with diabetes.
Finally, contemporary issues, such as clinical (e.g., geriatric syndromes, comorbidity, diabetes complications), functional (e.g., cognitive impairment, disability) and situational (e.g., long-term care, homebound) heterogeneity, challenge clinicians and researchers to provide comprehensive care to older adults with diabetes. Psychosocial and physiologic changes associated with aging can impact older adults’ ability to carry out self-care behaviors and follow through with treatment recommendations. Thus, nurse clinicians and researchers need to be aware of older patients’ individual circumstances and the potential impact these circumstances can have on diabetes self-care. The elderly with diabetes represent a group with a wide range of physical, cognitive, and socioeconomic resources. More research is needed to develop the evidence base for best self-care practices to improve clinical outcomes and quality of life in older adults with diabetes.
Acknowledgments
Funding
The author (s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health Grants R01 DK060115 and P30 DK036836.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Katie Weinger, Email: katie.weinger@joslin.harvard.edu, Joslin Diabetes Center - Behavioral Research, Harvard Medical School - Psychiatry, One Joslin Place, Boston, Massachusetts 02215, United States.
Elizabeth A. Beverly, Ohio University Heritage College of Osteopathic Medicine, Athens, Ohio, United States
Arlene Smaldone, Columbia University School of Nursing, New York, New York, United States.
References
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013a;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013b;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Medical Directors Association. Diabetes management in the long-term care setting: Clinical practice guideline. Columbia, MD: Author; 2008. [Google Scholar]
- Arinzon Z, Shabat S, Shuval I, Peisakh A, Berner Y. Prevalence of diabetes mellitus in elderly patients received enteral nutrition long-term care service. Archives of Gerontology and Geriatrics. 2008;47(3):383–393. doi: 10.1016/j.archger.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Annals of Family Medicine. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Smith SL, Arcury TA, Snively BM, Stafford JM, Quandt SA. Prevalence and correlates of depressive symptoms among rural older African Americans, Native Americans, and Whites with diabetes. Diabetes Care. 2005;28(4):823–829. doi: 10.2337/diacare.28.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Kaufman FR. Threshold-based insulin-pump interruption for reduction of hypoglycemia. New England Journal of Medicine. 2013;369:224–232. doi: 10.1056/NEJMoa1303576. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25(3):471–475. doi: 10.2337/diacare.25.3.471. [DOI] [PubMed] [Google Scholar]
- Beverly EA, Brooks KM, Ritholz MD, Abrahamson MJ, Weinger K. Assessment of emotional struggles in type 2 diabetes: Patient perspectives. Diabetes Care. 2012;35(8):e62. doi: 10.2337/dc12-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Fitzgerald S, Sitnikov L, Ganda OP, Caballero AE, Weinger K. Do older adults aged 60–75 years benefit from diabetes behavioral interventions? Diabetes Care. 2013;36(6):1501–1506. doi: 10.2337/dc12-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Ganda OP, Ritholz MD, Lee Y, Brooks KM, Lewis-Schroeder NF, Weinger K. Look who’s (not) talking: Diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care. 2012;35(7):1466–1472. doi: 10.2337/dc11-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Hultgren BA, Brooks KM, Ritholz MD, Abrahamson MJ, Weinger K. Understanding physicians’ challenges when treating type 2 diabetic patients’ social and emotional difficulties: A qualitative study. Diabetes Care. 2011;34(5):1086–1088. doi: 10.2337/dc10-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Ritholz MD, Brooks KM, Hultgren BA, Lee Y, Abrahamson MJ, Weinger K. A qualitative study of perceived responsibility and self-blame in type 2 diabetes: reflections of physicians and patients. Journal of General Internal Medicine. 2012;27(9):1180–1187. doi: 10.1007/s11606-012-2070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Wray LA, Chui CJ, LaCoe CL. Listening to older adults’ values and preferences for type 2 diabetes care: A qualitative study. Diabetes Spectrum. 2014;27:1, 44–49, 1944–7353. doi: 10.2337/diaspect.27.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Wray LA, Chiu CJ, Weinger K. Perceived challenges and priorities in co-morbidity management of older patients with Type 2 diabetes. Diabetic Medicine. 2011;28(7):781–784. doi: 10.1111/j.1464-5491.2011.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SA. Increased health burden associated with comorbid depression in older diabetic Mexican Americans. Results from the Hispanic Established Population for the Epidemiologic Study of the Elderly survey. Diabetes Care. 1999;22(1):56–64. doi: 10.2337/diacare.22.1.56. [DOI] [PubMed] [Google Scholar]
- Bobroff LB. MyPlate for older adults. Gainsville, FL: University of Florida; 2011. [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. Journal of the American Geriatrics Society. 2003;51(5 Suppl Guidelines):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- Bruce DG, Casey GP, Grange V, Clarnette RC, Almeida OP, Foster JK, Davis TM. Cognitive impairment, physical disability and depressive symptoms in older diabetic patients: The Fremantle Cognition in Diabetes Study. Diabetes Research and Clinical Practice. 2003;61(1):59–67. doi: 10.1016/s0168-8227(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication adherence in older adults with cognitive impairment: A systematic evidence-based review. American Journal of Geriatric Pharmacotherapy. 2012;10(3):165–177. doi: 10.1016/j.amjopharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. American Journal of Public Health. 2012;102(8):1482–1497. doi: 10.2105/AJPH.2011.300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Web-based injury statistics query and reporting system (WISQARS) 2007 Available from http://www.cdc.gov/injury/wisqars/index.html.
- Centers for Disease Control and Prevention. Current depression among adults - United States, 2006–2008. Morbidity and Mortality Weekly Reports. 2010 Oct 1; Retrieved from http://www.cdc.gov/mmwr/pdf/wk/mm5938.pdf. [PubMed]
- Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. 2011 Retrieved from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- Centers for Medicare & Medicaid Services. Your Medicare coverage. n.d Retrieved from http://www.medicare.gov/coverage/durable-medical-equipment-coverage.html.
- Chernof BA, Sherman SE, Lanto AB, Lee ML, Yano EM, Rubenstein LV. Health habit counseling amidst competing demands: Effects of patient health habits and visit characteristics. Medical Care. 1999;37(8):738–747. doi: 10.1097/00005650-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Chou KL, Chi I. Prevalence of depression among elderly Chinese with diabetes. International Journal of Geriatric Psychiatry. 2005;20(6):570–575. doi: 10.1002/gps.1328. [DOI] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Archives of Internal Medicine. 2000;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- Cohen D, Weintrob N, Benzaquen H, Galatzer A, Fayman G, Phillip M. Continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with type I diabetes mellitus: A randomized open crossover trial. Journal of Pediatric Endocrinology and Metabolism. 2003;16(7):1047–1050. doi: 10.1515/jpem.2003.16.7.1047. [DOI] [PubMed] [Google Scholar]
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: Association with use of tricyclic agents. American Journal of Medicine. 2000;108(1):2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Delahanty LM, Halford BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care. 1993;16(11):1453–1458. doi: 10.2337/diacare.16.11.1453. [DOI] [PubMed] [Google Scholar]
- DiPietro L, Gribok A, Stevens MS, Hamm LF, Rumpler W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care. 2013;36(10):3262–3268. doi: 10.2337/dc13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybicz SB, Thompson S, Molotsky S, Stuart B. Prevalence of diabetes and the burden of comorbid conditions among elderly nursing home residents. American Journal of Geriatric Pharmacotherapy. 2011;9(4):212–223. doi: 10.1016/j.amjopharm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Egede LE. Diabetes, major depression, and functional disability among U.S. adults. Diabetes Care. 2004;27(2):421–428. doi: 10.2337/diacare.27.2.421. [DOI] [PubMed] [Google Scholar]
- Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25(3):464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Narayan KM. The evolving diabetes burden in the United States. Annals of Internal Medicine. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- Feldman SM, Rosen R, DeStasio J. Status of diabetes management in the nursing home setting in 2008: A retrospective chart review and epidemiology study of diabetic nursing home residents and nursing home initiatives in diabetes management. Journal of the American Medical Directors Association. 2009;10(5):354–360. doi: 10.1016/j.jamda.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, Laurencin G. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–1036. doi: 10.2337/dc09-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Hessler D, Glasgow RE, Arean PA, Masharani U, Naranjo D, Strycker LA. REDEEM: A pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36(9):2551–2558. doi: 10.2337/dc12-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia TJ, Brown SA. Diabetes management in the nursing home: A systematic review of the literature. Diabetes Educator. 2011;37(2):167–187. doi: 10.1177/0145721710395330. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20(4):562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care. 1996;19(8):835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- Good CB. Polypharmacy in elderly patients with diabetes. Diabetes Spectrum. 2002;15(4):240–248. [Google Scholar]
- Greenberg S. A profile of older Americans: 2011. Washington, DC: Administration on Aging, U.S. Department of Health and Human Services; 2011. [Google Scholar]
- Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, Narayan KM. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23(9):1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Chen J, Wilson RS, Manson JE. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24(6):1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Grant MD, Hofer SM, Pedersen NL, Nilsson SE, Berg S, Johansson B. Type 2 diabetes mellitus contributes to cognitive decline in old age: A longitudinal population-based study. Journal of the International Neuropsychological Society. 2004;10(4):599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, Raskin P. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568–1573. doi: 10.2337/diacare.28.7.1568. [DOI] [PubMed] [Google Scholar]
- Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: A review. American Journal of Geriatric Pharmacotherapy. 2011;9(6):364–377. doi: 10.1016/j.amjopharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, Bolli GB. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: Results of the 5-nations trial. Diabetic Medicine. 2006;23(2):141–147. doi: 10.1111/j.1464-5491.2005.01738.x. [DOI] [PubMed] [Google Scholar]
- Hovanec N, Sawant A, Overend TJ, Petrella RJ, Vandervoort AA. Resistance training and older adults with type 2 diabetes mellitus: Strength of the evidence. Journal of Aging Research. 2012;2012:284635. doi: 10.1155/2012/284635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AM. The psychological care of patients with insulin-dependent diabetes mellitus. New England Journal of Medicine. 1996;334(19):1249–1253. doi: 10.1056/NEJM199605093341907. [DOI] [PubMed] [Google Scholar]
- Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: A model for the delivery of clinical preventive services. Journal of Family Practice. 1994;38(2):166–171. [PubMed] [Google Scholar]
- Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, Piette JD. Beyond comorbidity counts: How do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? Journal of General Internal Medicine. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Swift CS. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocrine Reviews. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Mukerji P, Drash A, Iyengar S. Biomedical and psychiatric risk factors for retinopathy among children with IDDM. Diabetes Care. 1995;18(12):1592–1599. doi: 10.2337/diacare.18.12.1592. [DOI] [PubMed] [Google Scholar]
- Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28(1):65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- Krolewski AS, Warram JH, Rand LI, Kahn CR. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. New England Journal of Medicine. 1987;317(22):1390–1398. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Obesity in the elderly diabetic patient: Is weight loss beneficial? No. Diabetes Care. 2009;32(Suppl 2):S403–S409. doi: 10.2337/dc09-S348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey WC, Barnard K, Batch BC, Chiswell K, Tasneem A, Green JB. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56(6):1226–1235. doi: 10.1007/s00125-013-2890-4. [DOI] [PubMed] [Google Scholar]
- Leyva B, Zagarins SE, Allen NA, Welch G. The relative impact of diabetes distress vs depression on glycemic control in hispanic patients following a diabetes self-management education intervention. Ethnicity & Disease. 2011;21(3):322–327. [PubMed] [Google Scholar]
- Lichtenstein AH, Rasmussen H, Yu WW, Epstein SR, Russell RM. Modified MyPyramid for Older Adults. Journal of Nutrition. 2008;138(1):5–11. doi: 10.1093/jn/138.1.5. [DOI] [PubMed] [Google Scholar]
- Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS One. 2009;4(1):e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Gavard JA, Clouse RE. Depression in adults with diabetes. Diabetes Care. 1992;15(11):1631–1639. doi: 10.2337/diacare.15.11.1631. [DOI] [PubMed] [Google Scholar]
- Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(9):1157–1162. doi: 10.1093/gerona/60.9.1157. [DOI] [PubMed] [Google Scholar]
- McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33(6):1159–1162. doi: 10.2337/dc09-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RM, Broton JC, Woo-Rippe KW, Lindquist SA, Cen YY. Variability in glycosylated hemoglobin values in diabetic patients living in long-term care facilities. Journal of the American Medical Directors Association. 2007;8(8):511–514. doi: 10.1016/j.jamda.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Migdal A, Yarandi SS, Smiley D, Umpierrez GE. Update on diabetes in the elderly and in nursing home residents. Journal of the American Medical Directors Association. 2011;12(9):627–632. e622. doi: 10.1016/j.jamda.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Miller CK, Edwards L, Kissling G, Sanville L. Nutrition education improves metabolic outcomes among older adults with diabetes mellitus: results from a randomized controlled trial. Preventive Medicine. 2002;34(2):252–259. doi: 10.1006/pmed.2001.0985. [DOI] [PubMed] [Google Scholar]
- Munshi MN, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, Weinger K. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29(8):1794–1799. doi: 10.2337/dc06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi MN, Segal AR, Suhl E, Staum E, Desrochers L, Sternthal A, Weinger K. Frequent hypoglycemia among elderly patients with poor glycemic control. Archives of Internal Medicine. 2011;171(4):362–364. doi: 10.1001/archinternmed.2010.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi MN, Segal AR, Suhl E, Ryan C, Sternthal A, Giusti J, Weinger K. Assessment of barriers to improve diabetes management in older adults: A randomized controlled study. Diabetes Care. 2013;36(3):543–549. doi: 10.2337/dc12-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. Suicide in the US: Statistics and prevention. Rockville, MD: Author; 2010. NIH Publication No. 06-4594. [Google Scholar]
- Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: Findings from the third national health and nutrition examination survey (NHANES III) Diabetes Care. 2002;25(10):1722–1728. doi: 10.2337/diacare.25.10.1722. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Miller DL, Kristal AR, Barnett MJ, Cheskin LJ. Diet and exercise habits of patients with diabetes, dyslipidemia, cardiovascular disease or hypertension. Journal of the American College of Nutrition. 2002;21(5):394–401. doi: 10.1080/07315724.2002.10719241. [DOI] [PubMed] [Google Scholar]
- Nicolucci A, Kovacs Burns K, Holt RI, Comaschi M, Hermanns N, Ishii H, Group DS. Diabetes attitudes, wishes and needs second study (DAWN2): Cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Medicine. 2013;30(7):767–777. doi: 10.1111/dme.12245. [DOI] [PubMed] [Google Scholar]
- Niemela K, Vaananen I, Leinonen R, Laukkanen P. Benefits of home-based rocking-chair exercise for physical performance in community-dwelling elderly women: A randomized controlled trial. Aging Clinical and Experimental Research. 2011;23(4):279–287. doi: 10.3275/7230. [DOI] [PubMed] [Google Scholar]
- Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: Challenges for the internist of the third millenium. Journal of Comorbidity. 2011;1:28–44. doi: 10.15256/joc.2011.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza-Frank R, Cheng YJ, Narayan KM, Gregg EW. Trends in nutrient intake among adults with diabetes in the United States: 1988–2004. Journal of the American Dietetic Association. 2009;109(7):1173–1178. doi: 10.1016/j.jada.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Partnership for Solutions. Diabetes: The impact of multiple chronic conditions. Baltimore, MD: Johns Hopkins University; 2004. [Google Scholar]
- Pham M, Pinganaud G, Richard-Harston S, Decamps A, Bourdel-Marchasson I. Prospective audit of diabetes care and outcomes in a group of geriatric French care homes. Diabetes & Metabolism. 2003;29(3):251–258. doi: 10.1016/s1262-3636(07)70034-4. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Maggio CA, McCarron DA, Reusser ME, Stern JS, Haynes RB, McMahon M. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care. 1999;22(2):191–197. doi: 10.2337/diacare.22.2.191. [DOI] [PubMed] [Google Scholar]
- Pijpers E, Ferreira I, de Jongh RT, Deeg DJ, Lips P, Stehouwer CD, Nieuwenhuijzen Kruseman AC. Older individuals with diabetes have an increased risk of recurrent falls: Analysis of potential mediating factors: the Longitudinal Ageing Study Amsterdam. Age and Ageing. 2012;41(3):358–365. doi: 10.1093/ageing/afr145. [DOI] [PubMed] [Google Scholar]
- Raskin P, Bode BW, Marks JB, Hirsch IB, Weinstein RL, McGill JB, Reinhardt RR. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: A randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598–2603. doi: 10.2337/diacare.26.9.2598. [DOI] [PubMed] [Google Scholar]
- Redmond EH, Burnett SM, Johnson MA, Park S, Fischer JG, Johnson T. Improvement in A1C levels and diabetes self-management activities following a nutrition and diabetes education program in older adults. Journal of Nutrition for the Elderly. 2006;26(1–2):83–102. doi: 10.1300/J052v26n01_05. [DOI] [PubMed] [Google Scholar]
- Rosenthal MJ, Fajardo M, Gilmore S, Morley JE, Naliboff BD. Hospitalization and mortality of diabetes in older adults. A 3-year prospective study. Diabetes Care. 1998;21(2):231–235. doi: 10.2337/diacare.21.2.231. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Peyrot M. Psychosocial problems in diabetes management: Impediments to intensive self-care. Practical Diabetology. 1994;13:8–14. [Google Scholar]
- Schafer RG, Bohannon B, Franz MJ, Freeman J, Holmes A, McLaughlin S, American Diabetes A. Diabetes nutrition recommendations for health care institutions. Diabetes Care. 2004;27(Suppl 1):S55–S57. doi: 10.2337/diacare.27.2007.s55. [DOI] [PubMed] [Google Scholar]
- Schoenberg NE, Drungle SC. Barriers to non-insulin dependent diabetes mellitus (NIDDM) self-care practices among older women. Journal of Aging and Health. 2001;13(4):443–466. doi: 10.1177/089826430101300401. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Vigersky R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Archives of Internal Medicine. 1997;157(15):1681–1686. [PubMed] [Google Scholar]
- Stephens EA, Heffner J. Evaluating older patients with diabetes for insulin pump therapy. Diabetes Technology & Therapeutics. 2010;12(Suppl 1):S91–S97. doi: 10.1089/dia.2010.0024. [DOI] [PubMed] [Google Scholar]
- Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GA. Comorbidity in patients with diabetes mellitus: Impact on medical health care utilization. BMC Health Services Research. 2006;6:84. doi: 10.1186/1472-6963-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabit H, Kyaw Tun T, McDermott J, Sreenan S. Executive function and diabetes mellitus--a stone left unturned? Current Diabetes Reviews. 2012;8(2):109–115. doi: 10.2174/157339912799424555. [DOI] [PubMed] [Google Scholar]
- Travis SS, Buchanan RJ, Wang S, Kim M. Analyses of nursing home residents with diabetes at admission. Journal of the American Medical Directors Association. 2004;5(5):320–327. [PubMed] [Google Scholar]
- Trief PM. Depression in elderly diabetes patients. Diabetes Spectrum. 2007;20(2):71–75. [Google Scholar]
- Vitolins MZ, Anderson AM, Delahanty L, Raynor H, Miller GD, Mobley C, Mayer-Davis E. Action for Health in Diabetes (Look AHEAD) trial: Baseline evaluation of selected nutrients and food group intake. Journal of the American Dietetic Association. 2009;109(8):1367–1375. doi: 10.1016/j.jada.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25(4):678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Warram JH, Krolewski AS. Epidemiology of Diabetes Mellitus. In: Kahn CR, King GL, Moses AC, Weir GC, Jacobson AM, Smith RJ, editors. Joslin’s diabetes mellitus. 14. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. pp. 341–354. [Google Scholar]
- Weinger K, Beverly EA, Lee Y, Sitnokov L, Ganda OP, Caballero AE. The effect of a structured behavioral intervention on poorly controlled diabetes: A randomized controlled trial. Archives of Internal Medicine. 2011;171(22):1990–1999. doi: 10.1001/archinternmed.2011.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, Phillip M. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: A randomized open crossover trial. Pediatrics. 2003;112(3 Pt 1):559–564. doi: 10.1542/peds.112.3.559. [DOI] [PubMed] [Google Scholar]
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- Wray LA, Ofstedal MB, Langa KM, Blaum CS. The effect of diabetes on disability in middle-aged and older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(9):1206–1211. doi: 10.1093/gerona/60.9.1206. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, Strotmeyer ES Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Internal Medicine. 2013;173(14):1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Decker FH, Luo H, Geiss LS, Pearson WS, Saaddine JB, Albright A. Trends in the prevalence and comorbidities of diabetes mellitus in nursing home residents in the United States: 1995–2004. Journal of the American Geriatrics Society. 2010;58(4):724–730. doi: 10.1111/j.1532-5415.2010.02786.x. [DOI] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: Prevalence and correlates of meeting physical activity recommendations. Journal of the American Geriatrics Society. 2011;59(1):132–137. doi: 10.1111/j.1532-5415.2010.03236.x. [DOI] [PubMed] [Google Scholar]