Abstract
Background context
Traumatic injuries occurring at the conus medullaris of the spinal cord cause both permanent damage to the central nervous system, and to the cauda equina nerve roots.
Purpose
This proof of concept study determined whether implanting the nerve roots into a biodegradable scaffold would improve regeneration after injury.
Study design/setting
All experimental work involving rats was performed according to approved guidelines by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC). Surgical procedures were performed on 32 Sprague Dawley rats. Four ventral cauda equina nerve roots were re-implanted either directly into the ventral cord stump or through a poly(lactic-co-glycolic acid) (PLGA) scaffold. These experimental groups were compared to a control group in which the nerves were inserted into a muscle fascia barrier that was placed between the spinal cord and nerve roots. Animals were sacrificed at four weeks.
Methods
This work was funded by the authors' institution; Morton Cure Paralysis Fund; The Craig H. Neilsen Foundation; and NIBIB grant R01 EB 02390. There was no conflict of interest between the study funding and the conclusions drawn.
Results
There was no difference in motor neuron counts in the spinal cord rostral to the injury in all treatment groups, implying equal potential for regeneration into implanted nerve roots. One-way ANOVA testing, with Tukey's post-test, showed a statistically significant improvement in axon regeneration through the injury in the PLGA scaffold treatment group compared to the control (p<0.05, scaffold n=11, control n=11).
Conclusion
This pilot study demonstrated that a PLGA scaffold improved regeneration of axons into peripheral nerve roots. However, the number of regenerating axons observed was limited and did not lead to functional recovery. Future experiments will employ a different scaffold material and possible growth factors or enzymes to increase axon populations.
Introduction
Spinal cord injury is a life altering injury that can have a devastating impact on the quality of life and medical costs of patients and their families [1]. Injuries that occur at the conus medullaris level can result in paralysis of the lower extremities and loss of autonomic function [2]. In conus medullaris injuries, spinal roots from the cauda equina are avulsed from the spinal cord; as well as direct trauma occurring to the cord at the first and second lumbar vertebra. It has been previously shown that directly repairing avulsed spinal nerves in other areas of the spinal cord can lead to functional recovery [3]. Neurophysiologic data has shown that if the alpha motor neuron is intact, it will produce new axons capable of entering into the implanted roots, forming neuromuscular synapses, and conducting impulses causing muscle contractions [4]. Contusion trauma related to the conus medullaris region is dissimilar to a spinal nerve avulsion but direct implantation of the cauda equina nerve roots could be a viable option to restore function if there was appropriate guidance for regenerating axons.
Extensive research has been completed in the area of peripheral nerve repair using autologous nerve grafts into the injured area [5]. These grafts provide a supportive environment for axon growth, but have adverse neurologic effects in the area that the donor nerve was harvested. The negative result of autologous nerve graft harvesting has led to the development of a variety of conduit materials to bridge the injury and guide axon growth [6, 7]. Our lab has reported that the biomaterial poly(lactic-co-glycollic acid) (PLGA) can be used in a peripheral nerve transection model of axon regeneration to bridge the gap created between both ends of the cut nerve [8].
A vast array of biomaterials has been investigated for their utility in bridging injured areas within the spinal cord [9-11]. We previously reported that biodegradable scaffolds fabricated from PLGA promote axonal regeneration in the central nervous system [12, 13]. In the present study we pilot a combinatory approach using techniques from both peripheral nerve repair and spinal cord repair. The principles of both fields were used through the implantation of cauda equina peripheral nerve roots into a PLGA scaffold that was attached to the injured conus medullaris region of the spinal cord. The scaffold contains four channels with the goal of placing an avulsed motor nerve root into each channel. It is proposed that the channels will guide the regenerating motor axons from the rostral, healthy spinal cord through the injured region into the implanted roots.
This study examined two experimental groups; 1) a biodegradable PLGA scaffold guiding nerve growth through the injury and 2) a direct implantation of the cut nerve roots onto the ventral spinal cord at the site of injury. These two groups were compared to a control group containing a fixed barrier of muscle fascia between the nerve root and spinal cord. The findings from this pilot study will have an impact on future conus medullaris injury treatment models.
Materials and Methods
Injury model
All experimental work was performed according to approved guidelines from the Mayo Clinic Institutional Animal Care and Use Committee (IACUC). Sprague Dawley rats weighing approximately 300 grams at the time of surgery were anesthetized with an intraperitoneal injection of ketamine and xylazine and underwent a laminectomy at the thoracic-lumbar junction. The distal 3-4 mm of the conus medullaris region was transected and removed after identifying 2 ventral lumbar motor roots on each ventral lateral side. These transected roots were cut and immediately implanted back onto the cut spinal cord using fibrin glue and one of three surgical root implantation modalities described in detail below.
PLGA scaffold preparation
Prior to surgical implantation of the transected roots onto the conus medullaris, the PLGA scaffolds with four parallel channels were fabricated by injection molding and solvent evaporation as previously described [14, 15]. Briefly, cylindrical, Teflon molds with a diameter of 3.0 mm were fitted with Delrin spacers containing an array of four, uniformly spaced stainless-steel wires. The wires were coated with Ease Release 200 (Mann Formulated Products, Easton, PA) to facilitate removal of the wires after forming the scaffold mold. A solution of poly (lactic-co-glycolic acid) (PLGA, Alkermes, Cambridge, MA), with a copolymer ration of 85:15 lactide:glycolide was made by adding 1.0 g PLGA to 2.0 mL dichloromethane in a glass vial followed by shaking vigorously for three hours. The PLGA solution was then injected via a syringe and 16-gauge needle into each mold until solution was observed flowing out of the opposite end of the scaffold mold, ensuring the removal of all air pockets. Polymer filled molds were then vacuum dried for 24 hours followed by a 30 minute washing in ethanol to sterilize for in vivo implantation. This was followed by another 24-hour vacuum drying period. Finished scaffolds were stored in desiccated, sterilized glass vials at four degrees Celsius.
Nerve root implantation techniques
Three different surgical techniques were used to implant the amputated nerve roots; direct repair, scaffold repair, and control muscle fascia barrier repair (figure 1). In the direct repair group, four previously transected ventral nerve roots were implanted directly onto the ventral horns in the conus medullaris region using fibrin glue. In the scaffold repair group, a biodegradable polymer scaffold containing four channels was placed at the site of injury with one end contacting the remaining conus medullaris. At the other end of the scaffold, one previously transected ventral nerve root from the cauda equina was implanted into of the four channels and held in place by fibrin glue. Fibrin glue was chosen to secure the nerve roots in place instead of using a suture technique due the extremely small size of the rodent ventral motor nerve roots (approximately 0.1 mm in diameter). Additionally, these ventral motor nerve roots lack a substantial connective tissue sheath that could allow the use of microsurgical suture technique. In the control group, muscle fascia was placed between the spinal cord and the four implanted cauda equina nerve roots as a barrier to regeneration.
Figure 1.
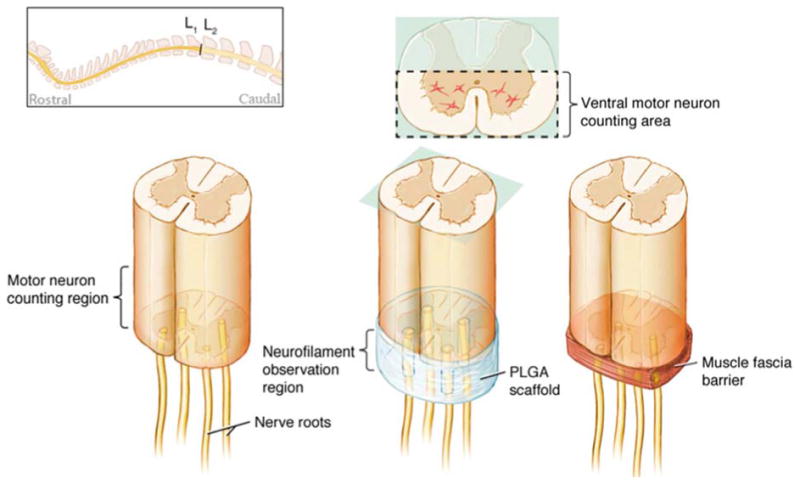
Top left schematic shows the lumbar region (L1-L2) where the injury site was located. Left diagram depicts the experimental group consisting of nerve roots directly implanted onto cut spinal cord. Central diagram shows the PLGA scaffold experimental group with a single nerve root inserted into each of the four channels. The right diagram shows the control group with a muscle fascia inserted between the cord and the four implanted nerve roots. The motor neuron counting region for each group was immediately rostral to the injury as seen in left diagram. Axon regeneraton was observed in the region labeled “neurofilament observation region” for all 3 groups.
Post-surgical care
Immediately following surgery, animals were placed on a heating pad to maintain body temperature until fully recovered from anesthesia. For seven days following surgery, the antibiotic Baytril (0.05mg/kg) was administered subcutaneously to prevent surgical site infection. The rats' bladders were manually expressed twice daily. If the animals appeared dehydrated, lactated ringer solution was administered subcutaneously. 65 mgs/kg Tylenol was administered orally to minimize autotomy in rats after surgery. All animals were euthanized at the end of four weeks and spinal columns containing the cord, scaffold and roots were removed en bloc.
Tissue embedding and neurofilament antibody labeling
Tissue was fixed in 4% paraformaldehyde. The spinal columns were processed and embedded en bloc in paraffin by NeuroScience Associates (Knoxville, TN). Eight micrometer serial section slides were prepared in axial orientation. Tissue sections within the injury site were identified under light microscopy and were deparaffinized for neurofilament antibody application using two washes of xylene and rehydrated in decreasing stepwise concentrations of ethyl alcohol (100% ethyl alcohol, 95%, 80%). After rehydration, proteinase K was applied for 20 minutes to retrieve target antigen sites. Three cycles of potassium buffered sulfate were then applied to remove proteinase K followed by serum blockage of non-specific binding sites. Primary mouse anti-human neurofilament antibodies (clone 2F11, DAKO) were incubated overnight on tissue. Excess primary antibody was removed with Tween 20 and donkey anti-mouse horseradish peroxidase secondary antibody was incubated on the tissue for 1 hour. Excess secondary was washed away with Tween 20 and diaminobenzidine was applied. Finally, tissue was dehydrated by stepwise increasing ethyl alcohol concentrations.
Motor neuron counting rostral to injury site
In tissue sections rostral to the injury site, motor neuron populations were identified using hematoxylin and eosin stain. For each animal, two serial axial sections of intact spinal cord tissue were selected at approximately 160 micrometers rostral to the beginning of the astrocytic scar. A blinded observer then counted all motor neurons in each section, recording the number of motor neurons present in each section independently. The average of the two sample sections was calculated to produce a single number representing motor neurons rostral to the injury in the respective animal. Motor neurons were identified by their characteristic angular morphology, central nuclei, and Nissl staining pattern. Neurolucida (MBF Bioscience) software was used to record and tabulate observed motor neurons. Micrographs were captured of each serial slide beginning immediately rostral to the injury site and continuing through to intact caudal nerves (Axiovision software, Zeiss Axiocam MRc digital camera, and Zeiss Imager Z1 light microscope). A blinded observer viewed the pictures and recorded which animals had axons regenerating through the injury site. GraphPad Prism was used for one-way ANOVA and Tukey's multiple comparisons testing for all data sets.
Results
Figure 1 depicts the three different surgical groups. A total of 10 animals that underwent the direct nerve root repair surgery survived through the four-week recovery period. Eleven animals survived the PLGA scaffold implant surgery and four-week recovery, and eleven animals survived the control muscle fascia barrier implant surgery and four-week recovery. The number of motor neurons in the ventral half of spinal cord tissue 160 µm rostral to the lesion was not different in the three groups (figure 2). There was a mean range of 44 – 54 neurons per cross section counted in the experimental groups.
Figure 2.
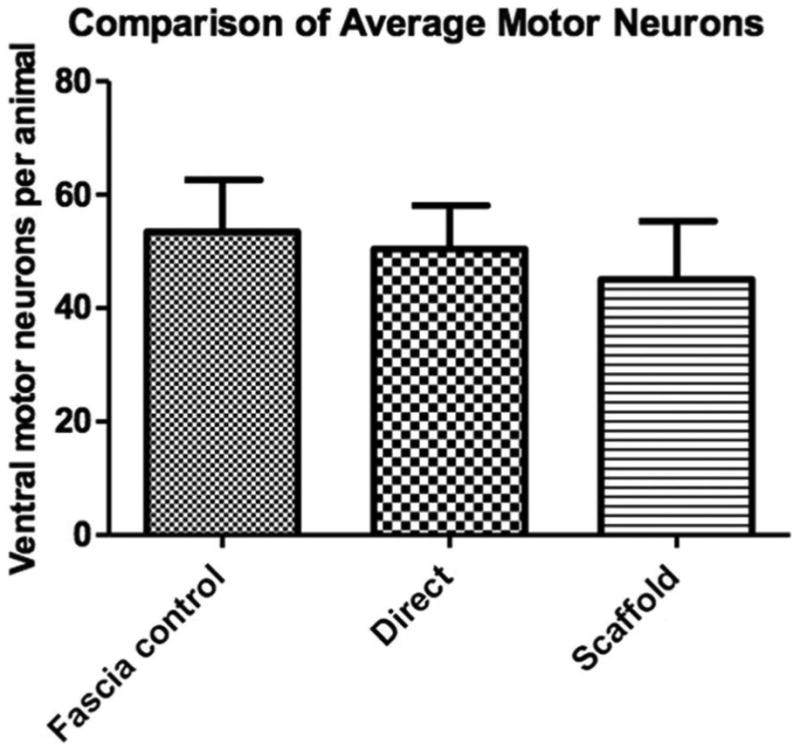
No significant difference in motor neuron numbers 160 µm rostral to the injured area. Motor neuron cell bodies were identified in both ventral gray matter horns of the intact spinal cord. Error bars represent standard error of the mean.
Post-mortem histology showed that the fibrin glue was successful in securing the nerve roots onto the spinal cord for the entire duration of the experiment when using the scaffold implantation technique, as well as when directly implanting the nerve roots onto the spinal cord and when implanting onto the muscle fascia control. Positive and specific staining of neurofilaments was confirmed in both normal ventral nerve roots and scaffold implanted nerve roots that were in the same tissue section (figure 3). The intact roots were passing from intact spinal cord tissue above the lesion and exiting through intervertebral foramina caudal to the injury region. These showed a regular distribution of axons that was readily distinguishable from the regenerating axons in the channels of the scaffold that had a more irregular growth pattern (figure 3).
Figure 3.
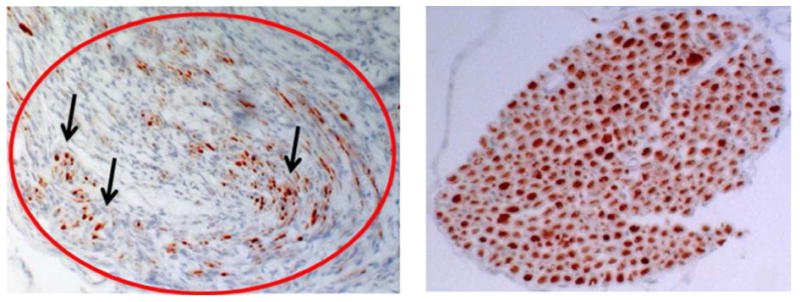
(Left) neurofilament antibody marked comparison of regeneration occurring through the implanted nerve root outlined in red. Black arrows indicate axons labeled by neurofilament antibody conjugated to diaminobenzidine. (Right) positive control nerve root showing intense labeling of axons. Both images were captured at 200x magnification light microscopy.
For the scaffold sections, the profiles of four channels were identified in each section (as in figure 3) and scored as positive if they contained at least five regenerating neurofilament profiles at approximately mid-scaffold level. Channels with four or less profiles were scored as negative for regeneration. These results are shown in figure 4. In the direct implantation and muscle fascia groups, the implanted roots were identified distal to their tissue insertion and then reviewed and scored using the same serial section observations for neurofilament labeling.
Figure 4.
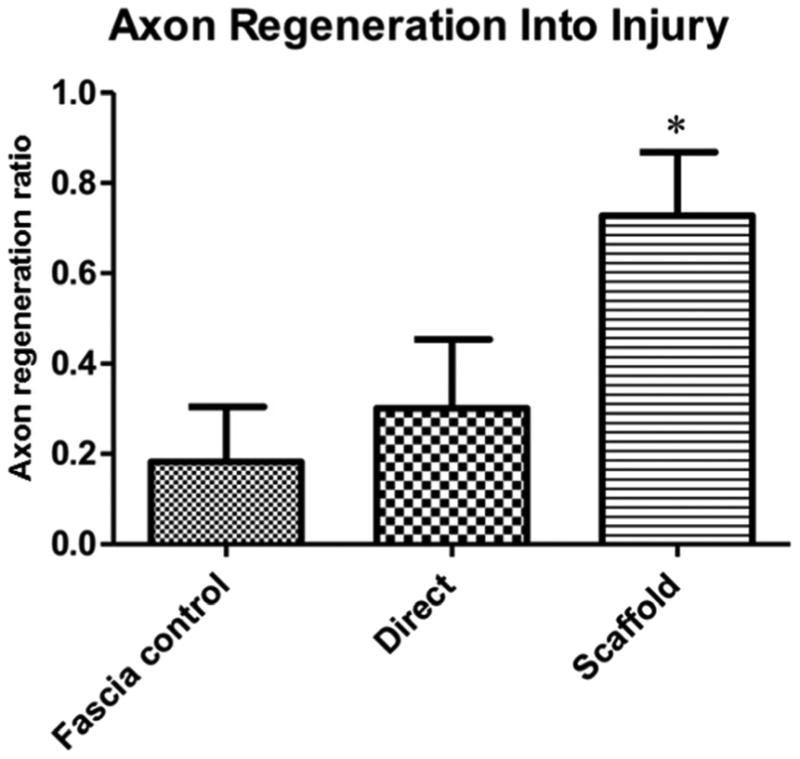
Comparison of the ratio of animals within each surgical group presenting >/=5 regenerating axons within the scaffold. There was no difference between those with direct implantation into the cord compared with the negative control where the root was inserted into a muscle fascia barrier. Asterisk indicates a significant difference between the scaffold and fascia control group (*p value < 0.05; One Way ANOVA and Tukey's post-test of significance).
In addition, trichrome staining of select tissue specimens showed a presence of PLGA material at the four-week endpoint of the experiment. In agreement with our previously published studies, there appeared to be a fibrous rim of connective tissue around a centralized core that contained the regenerating axons within the PLGA scaffolds (data not shown) [16]. There was little inflammation in response to the implanted scaffold material as judged in trichrome stained sections.
Discussion
This pilot study demonstrates that implantation of ventral motor nerve roots into a biodegradable scaffold supported axonal regeneration through the scaffold. The channels in the scaffold served as a mechanical bridge for axons to regenerate between the cord and the nerve root in 73% of animals which was significantly more than when the roots were inserted directly into the ventral cord (30%) or when the roots were inserted into a fascia barrier (18%). Although these differences are large, they should be interpreted cautiously. The number of axons regenerating through any individual channel was small and would not be expected to lead to functional recovery. Function was not assessed in this short-term study. In addition, the source of the axons regenerating through the scaffold was not identified. In studies of biodegradable scaffolds in spinal cord injury, axons regenerate in both rostral to caudal and caudal to rostral directions [13]. In the present study, since ventral roots were inserted into the scaffold channels, it is most likely that the regenerating axons originated from within the spinal cord. In future studies, it would be necessary to use retrograde axonal tracing to determine whether axons were originating from specific populations of neurons in the cord as in previous studies [17-19].
A four-channel design was utilized for this study as a simplified design of our previously published scaffold designs [14]. We chose the simplified design to accommodate the implantation of four ventral motor nerve roots to study regeneration through the scaffold as a pilot study. We used a scaffold fabricated from PLGA because it is approved by the U.S. Food and Drug Administration, is easily molded into a four-channel scaffold, and we have previously shown it is capable of supporting regeneration when implanted into the nervous system [18, 20]. Specifically, this material has been used successfully in both peripheral nerve [17, 18] and spinal cord injury repair models [12, 13, 21]. Other material may have better mechanical tissue compatibility for interfacing between the spinal cord and implanted nerve roots. PLGA is a stiff material. Hydrogels such as oligo-polyethylene derivatives may more closely mimic the mechanical environment of the spinal cord and potentially provide better substrates for regeneration. We previously compared multiple scaffold biomaterials implanted into a transected region of rodent spinal cord and examined the mechanical properties (3-point bending and compression modulus) as well as their interactions with spinal cord tissue, ability to support axon regeneration, and glial scar/cyst cavity formation. While PLGA scaffolds created a significantly smaller cyst volume with the highest 3-point bending properties, they did not yield a centralized pattern of axonal regeneration through the channels, nor did the PLGA scaffold support maximal regeneration compared to the other biomaterials tested [16]. Due to these findings, as well as the successful use of autologous nerve grafts, we chose the biomaterial which formed the smallest cyst combined with the implanted nerve root to support maximal nerve regeneration.
Regeneration may also be improved by providing a cellular matrix within the scaffold channels to improve regeneration. Both neural progenitor cells and Schwann cells have been demonstrated to improve regeneration within scaffolds in the spinal cord [12, 22]. In addition small molecules released from microspheres within scaffolds also enhance regeneration after spinal cord injury [22]. There are therefore a number of strategies that could be utilized with this repair model that may significantly enhance regeneration and functional recovery. Future directions will examine the interactions between the PLGA implant, the nerve root, and the host tissue environment over multiple time points. It is also possible that a four-week time course for recovery is too short to accurately evaluate functional recovery due to the necessary time needed for nerve regrowth and proper re-innervation of target muscles.
While this proof-of-principle study shows successful regeneration of motor neuron axons into an implanted biomaterial scaffold, the sharp transection injury model utilized is not an ideal representation of nerve root avulsions or stretch injuries, as seen in the clinical setting. Acute nerve transection followed by implantation was chosen for this study for multiple reasons. First, the animals would only have to undergo a single major survival surgery as an alternative to cutting the nerves and waiting a period of days, then re-exposing the surgery site for a second major survival surgery to implant the nerves. Second, the acute implantation provides the highest possibility of regeneration. As a pilot study, this simplified surgical protocol to observe regeneration establishes a starting point for the development of more complex, clinically relevant nerve root avulsion models with an extended period of time between avulsion and implantation into a scaffold.
The injury model utilized here represents the first use of a cauda equina ventral motor nerve root injury model for tissue engineering and nerve regeneration purposes. From the clinical perspective, further work with this model may lead to repair strategies after conus medullaris injury. In addition, this type of repair may be relevant as a potential repair strategy for nerve resections that occur during removal of sacral and pelvic neoplasms. In these cases, sacral nerves subserving bowel, bladder and sexual function may be lost. The ability to repair these relatively short nerves and restore function would lead to very significant improvements in quality of life for these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birmingham TUoAa. National Spinal Cord Injury Statistical Center. The University of Alabama at Birmingham; 2012. http://www.nscisc.uab.edu. [Google Scholar]
- 2.Kingwell SP, Curt A, Dvorak MF. Factors affecting neurological outcome in traumatic conus medullaris and cauda equina injuries. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC.2008.25.11.E7. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim AG, Kirkwood PA, Raisman G, Li Y. Restoration of hand function in a rat model of repair of brachial plexus injury. Brain. 2009;132:1268–76. doi: 10.1093/brain/awp030. [DOI] [PubMed] [Google Scholar]
- 4.Carlstedt T. Functional recovery after ventral root avulsion and implantation in the spinal cord. Clin Neurol Neurosurg. 1993;95(Suppl):S109–11. doi: 10.1016/0303-8467(93)90046-j. [DOI] [PubMed] [Google Scholar]
- 5.Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92:245–76. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63–8. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- 7.de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26:E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer R, Knight AM, Borntraeger A, et al. Rat sciatic nerve repair with a poly-lactic-co-glycolic acid scaffold and nerve growth factor releasing microspheres. Microsurgery. 2011;31:293–302. doi: 10.1002/micr.20869. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JA, Windebank AJ, Moore MJ, Spinner RJ, Currier BL, Yaszemski MJ. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery. 2002;51:742–52. [PubMed] [Google Scholar]
- 10.Angius D, Wang H, Spinner RJ, Gutierrez-Cotto Y, Yaszemski MJ, Windebank AJ. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials. 2012;33:8034–9. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27:1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson HE, Rooney GE, Gross L, et al. Neural stem cell- and schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15:1797–805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen BK, Knight AM, de Ruiter GC, et al. Axon regeneration through scaffold into distal spinal cord after transection. J Neurotrauma. 2009;26:1759–71. doi: 10.1089/neu.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore MJ, Friedman JA, Lewellyn EB, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–29. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Mikos AG, Lyman MD, Freed LE, Langer R. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15:55–8. doi: 10.1016/0142-9612(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 16.Chen BK, Knight AM, Madigan NN, et al. Comparison of polymer scaffolds in rat spinal cord: a step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials. 2011;32:8077–86. doi: 10.1016/j.biomaterials.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ruiter GC, Malessy MJ, Alaid AO, et al. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–50. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Ruiter GC, Spinner RJ, Malessy MJ, et al. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63:144–53. doi: 10.1227/01.NEU.0000335081.47352.78. discussion 53-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao L, de Ruiter GC, Wang H, et al. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31:5789–97. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 20.de Ruiter GC, Onyeneho IA, Liang ET, et al. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res A. 2008;84:643–51. doi: 10.1002/jbm.a.31298. [DOI] [PubMed] [Google Scholar]
- 21.Krych AJ, Rooney GE, Chen B, et al. Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord. Acta Biomater. 2009;5:2551–9. doi: 10.1016/j.actbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rooney GE, Knight AM, Madigan NN, et al. Sustained delivery of dibutyryl cyclic adenosine monophosphate to the transected spinal cord via oligo [(polyethylene glycol) fumarate] hydrogels. Tissue Eng Part A. 2011;17:1287–302. doi: 10.1089/ten.tea.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]


