Abstract
Obesity is a widespread health concern that is associated with an increased prevalence of hypertension and cardiovascular disease. Both obesity and hypertension have independently been associated with increased levels of inflammatory cytokines and immune cells within specific brain regions, as well as increased activity of the renin-angiotensin system (RAS). To test the hypothesis that high-fat diet (HFD) induced obesity leads to an angiotensin-II (Ang-II)-dependent increase in inflammatory cells within specific forebrain regions that are important for cardiovascular regulation, we first assessed microglial activation, astrocyte activation, inflammation and RAS component gene expression within selected metabolic and cardiovascular control centers of the forebrain in adult male C57BL/6 mice given either a HFD or a low-fat diet (LFD) for 8 weeks. Subsequently, we assessed the necessity of the paraventricular nucleus of the hypothalamus (PVN) angiotensin type-1a (AT1a) receptor for these responses by using the Cre/lox system in mice to selectively delete the AT1a receptor from the PVN. These studies reveal that in addition to the arcuate nucleus of the hypothalamus (ARC), the PVN and the subfornical organ (SFO), two brain regions that are known to regulate blood pressure and energy balance, also initiate proinflammatory responses after the consumption of a diet high in fat. They further indicate that some, but not all, of these responses are reversed upon deletion of AT1a specifically within the PVN.
Keywords: microglia, astrocyte, high-fat diet, angiotensin type-1 receptor, paraventricular nucleus
Introduction
Obesity is an epidemic health concern that is associated with an increased prevalence of hypertension, as well as an enhanced risk for cardiovascular morbidity and mortality [1–6]. Due to their high incidence of co-morbidity, determining effective strategies that combat both obesity and hypertension are in high demand and both the renin-angiotensin system (RAS) and inflammatory cells within the brain have been implicated as promising targets in this regard. Obesity and hypertension are independently acknowledged as mild inflammatory conditions that are often accompanied by elevated RAS activity [7–13]. In rodents, high-fat diet (HFD) induced obesity leads to increased inflammatory factors and immune cells in peripheral tissues and in brain regions that are essential for maintaining of energy balance [13–16] and it is possible that this accumulation of astrocytes and microglia (the resident immune cells of the brain) in these specific brain regions then plays an integral role in the dysregulation of energy balance [17]. During hypertension, a similar scenario occurs; however, in these instances, the elevated levels of inflammatory cells and factors have primarily been localized to brain regions and peripheral tissues that are important for the regulation of cardiovascular function [10, 18–22].
Despite the similarities between obesity and hypertension-related CNS immune cell activation, in many instances, the proposed mechanisms contributing to the shift toward a proinflammatory state within the brain during these conditions differ. In the case of obesity, several studies have implicated the proinflammatory effects of free fatty acids [23], leptin [24], and gut microbiota [25] as contributing mechanisms to the activation of CNS immune cells, while during hypertension, the proinflammatory actions of angiotensin-II (Ang-II) have received much attention [10, 18, 19, 26]. Of relevance, obesity is also associated with increased RAS activity [8] and although the proinflammatory effects of Ang-II have been characterized [10, 18, 19], whether obesity-related inflammation within specific brain regions depends on angiotensin signaling is not clear. Furthermore, the effect of HFD feeding and other obesity-related factors on microglial activation, inflammation and RAS activity in cardiovascular and other metabolic control centers in the mouse forebrain is largely unknown.
Here we hypothesized that an important link between HFD-induced metabolic and cardiovascular dysregulation is a RAS-dependent increase in inflammation within specific forebrain regions that regulate energy balance and blood pressure. It is recognized that obesity leads to an accumulation of immune cells within ARC [13, 17, 27], a brain region that is critical for the regulation of energy balance [28]. The present studies sought to determine whether HFD consumption also leads to proinflammatory responses within the paraventricular nucleus of the hypothalamus (PVN) and the subfornical organ (SFO), two brain regions that regulate blood pressure and energy balance. We further evaluated whether deletion of angiotensin type-1a (AT1a) receptors within the PVN impacts microglial and astrocytic activation during HFD-feeding.
Materials and Methods
Animals
For the experiments conducted in adult male C57BL/6 mice, animals were obtained from Harlan laboratories (Tampa, FL). The Cre/lox system was used to generate mice with specific deletion of AT1a within the PVN (PVN AT1a KO) on a C57BL/6 mixed background as previously-described [29]. In brief, PVN AT1a KO (homozygous for AT1a flox and expressing Sim1Cre) and littermate control mice (homozygous for AT1aflox/flox) were generated by crossing AT1a flox mice (obtained from Dr. Alan Daugherty, University of Kentucky [30]) with Sim1Cre mice (generated by Dr. B. Lowell, Beth Israel Deaconess Medical Center and Harvard Medical School; [31]). It is important to consider that PVN AT1a KO mice and littermate control mice were born at the University of Florida animal facilities, while the cohorts of C57BL/6 wild-type mice were obtained from Harlan laboratories. As a consequence, these separate cohorts of mice were exposed to different prenatal and birthing conditions, which are known to impact physiology even during adulthood. In the present manuscript, direct comparisons are therefore only made between cohorts of mice subjected to the same breeding conditions. Unless otherwise noted, mice were 8–10 wks of age at the initiation of all studies, were individually-housed, maintained on a 12h light/dark cycle and were given ad libitum access to water and to a HFD (60% kcal fat; Research Diets, New Brunswick, NJ [D12492]) or a low-fat diet (LFD; 10% kcal fat; Research Diets, New Brunswick, NJ [D12450B]). All studies were approved by University of Florida Institutional Animal Care and Use Committee and were in compliance with the animal welfare guidelines of the United States National Institute of Health.
Experimental design
Experiments conducted in wild-type mice were initiated one week after their arrival to the University of Florida animal facilities. At this time, mice were divided into two groups matched for body weight: those given HFD and those given LFD. Experiments conducted in PVN AT1a KO mice and controls were initiated when the mice were 8–9 weeks of age, at which time all PVN AT1a KO mice and controls were given ad libitum access HFD. In all cases, mice remained on HFD or LFD for 8 weeks, after which mice were fasted for 2 h and anesthetized using sodium pentobarbital. Mice were then either euthanized via de-capitation to collect brains for gene expression analysis or were trans-cardially perfused with 0.15 M NaCl followed by 4% paraformaldehyde. Brains were collected from perfused mice and post-fixed in 4 % paraformaldehyde for 4 h. Afterwards, brains were stored in 30% sucrose at 4 °C until processing for immunohistochemistry (IHC). Brains collected for gene expression analysis were flash-frozen in dry ice-cooled isopentane and stored at −80 C until processing.
Body and adipose mass
Body mass was assessed at the initiation of the study and weekly, thereafter. At the termination of the experiments, mice were euthanized and adiposity was evaluated by manually removing the epididydmal, inguinal, mesenteric and retroperitoneal white adipose tissue pads and then weighing them on a calibrated scale.
Immunohistochemistry
Four series of 25 μm coronal brain sections were taken on a Leica CM3050 S cryostat and placed in cryoprotective solution for storage at −20°C. For assessment of Iba-1 or GFAP immunoreactivity, sections were washed in 50 mM KPBS and then placed in a blocking solution (50 mM KPBS with 2% bovine serum albumin and 0.1% Triton-X) at room temperature for 2 h. Subsequently, sections were incubated in rabbit anti Iba-1 (1:3000; Wako Chemicals, Richmond, VA) or mouse anti GFAP (1:1500; EnCor Biotechnology, Gainesville, FL) at 4°C in the blocking solution overnight. The following day, sections were brought to room temperature, rinsed, and incubated for 2 h in the secondary Ab (Cy3 anti-rabbit for Iba-1 and Alexa 488 anti-mouse for GFAP; Jackson ImmunoResearch; 1:500) made in blocking solution. Sections were then rinsed, sequentially mounted onto microscope slides and cover-slipped with polyvinyl alcohol mounting medium with DABCO.
Imaging and Analysis
Brain regions of interest (ROI) were identified using anatomical landmarks and coordinates described by Franklin and Paxinos [32]. Fluorescence images were captured using a Zeiss AxioImager M2 microscope (Carl Zeiss, Thornwood, New York). Image capture and analysis was performed at 20× for Iba-1 staining of microglia and 10× for GFAP staining of astrocytes. For the assessment of microglial size and number, 12 μm z-stacks (containing 20 images) were taken through Iba-1 stained sections at 20× magnification. For assessment of GFAP immunoreactivity, 10× images were captured for all brain regions assessed.
Images of brain sections were analyzed for number of and size of Iba-1 positive cell-bodies or for the area fraction of GFAP staining within the brain regions of interest using Image J (NIH). In a subset of samples, the size of the microglia was manually verified using Axiovision. Captured images were converted into greyscale and binary formats and thresholds for black and white balance were adjusted to the same level for each ROI. Counts were taken from matched sections representing the ROI with reference to Franklin and Paxinos [32]. In the case of bilateral nuclei, counts were taken from both sides and averaged.
RNA extraction and cDNA synthesis
For gene expression analysis, the PVN, SFO, and mediobasal hypothalamus (MBH; containing the ARC, VMH and ME) were isolated using a cryostat and a micro-punch kit. Brains were sectioned at 100 μm and punches were taken from brain slices through the entirety of the regions of interest and submerged in RLT buffer containing β-mercaptoethanol. RNeasy columns (Qiagen, Valencia, CA) were used to isolate RNA from the regions of interest. DNase treatment (Qiagen, Valencia, CA) was performed to minimize genomic DNA contamination of RNA extracts. Subsequently, iScript (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 200 ng total RNA.
Semi-quantitative real-time PCR
Gene expression was assessed using semi-quantitative real-time PCR. For semi-quantitative real-time PCR analysis of angiotensin-converting enzyme (ACE; Mm00802048), angiotensin type-1a receptor (AT1a; Mm01166161), monocyte chemoattractant protein (MCP-1; Mm00441242), tumor necrosis factor α (TNF-α; Mm00443260), interleukin-6 (IL-6; Mm00446190), interleukin-1β (IL-1β; Mm00434228), cluster of differentiation molecule 11b (CD11b; Mm00434455) and glial fibrillary acidic protein (GFAP; Mm01253033) diluted (1:5), cDNA samples were run in duplicate using a StepOne Real-time PCR system, Taqman Universal Gene Expression Master Mix and validated Taqman probes (Applied Biosystems, Foster City, CA). Expression patterns of genes of interest were normalized to constitutively-expressed GAPDH (Mm99999915) and relative expression was quantified using the 2ΔΔCt method [29].
Statistics
In all of these experiments, comparisons were made between two groups and an unpaired Student’s t-test was used to determine statistical significance. Statistical significance was set at p < 0.05.
Results
Short and long-term HFD consumption increases body mass and adiposity
As depicted in Figure 1, mice given HFD for 8 wks had significantly increased body mass relative to LFD-fed controls (p < 0.0001). Similarly, the masses of the white adipose tissue depots (iWAT, mWAT, rpWAT and eWAT; p < 0.0001) were also significantly increased subsequent HFD-feeding.
Figure 1.
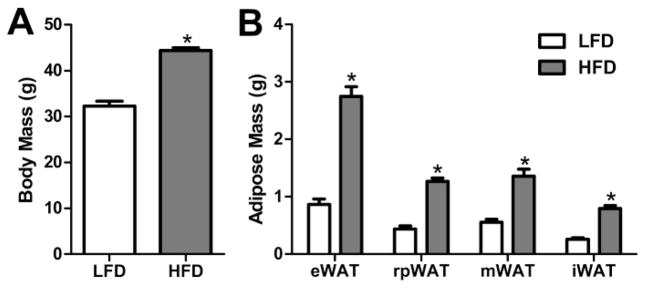
Body mass and Adipose Mass of mice given HFD or LFD for 8 wks. (A) Body Mass and (B) epidydmal (eWAT), retroperitoneal (rpWAT), mesenteric (mWAT) or inguinal (iWAT) white adipose tissue weights after 8 wks of HFD (n = 15) or LFD (n = 18) consumption. Bars = SEM. * = (p < 0.05).
The effect of HFD consumption on the size and number of Iba-1 positive cells in specific forebrain nuclei
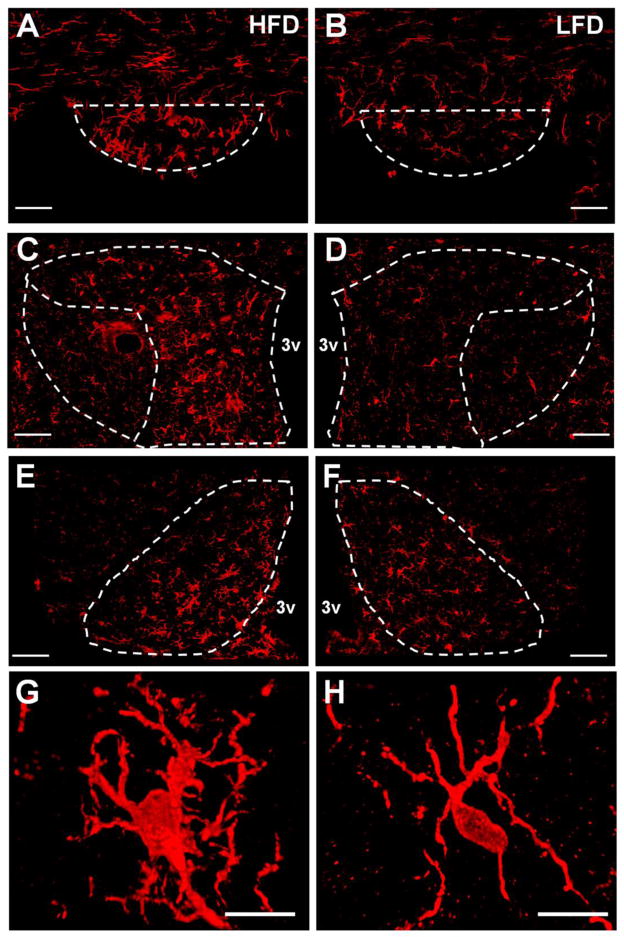
When microglia sense threats to the brain they respond by migrating to the damaged tissue [10, 13, 33] and undergo a phenotypic transformation from their ‘resting’ state, in which they have small soma and long fine processes to an activated state, characterized by enlarged cell bodies and ramified processes [10, 34, 35, and also Figure 2]. It has been demonstrated that obesity leads to microglial activation within the arcuate nucleus of the hypothalamus (ARC) [36]. Consistent with these previous results, we found that after 8 wks, consumption of HFD led to increases in the number of Iba-1 positive microglia (p = 0.02) within the ARC as well as the size of their cell bodies (p = 0.005) ; Figures 2 and 3). Our data also demonstrate for the first time that, relative to LFD-fed controls, mice fed HFD for 8 wks had a larger number of microglia within the SFO (p = 0.03) and the parvocellular portion of the PVN (PaPVN; p = 0.025), two brain regions that are important for the regulation of blood pressure and contain high densities of the AT1a receptor. The size of the Iba-1-positive cell-bodies was also larger within the PaPVN (p = 0.004) and SFO (p = 0.005) of mice given HFD, suggestive of an increase in microglial activation. In contrast, the size (p = 0.35) and number of microglia (p = 0.91) within the magnocellular region of the PVN were not different between the groups (Figure 3).
Figure 2.
Iba-1 immunoreactivity in the SFO, PVN and ARC of mice after 8 wks on HFD or LFD. Projection images generated from 20× immunofluorescence z-stacks through the SFO [(A) HFD and (B) LFD; scale bars = 50 μm, paPVN and maPVN [(C) HFD or (D) LFD; scale bars = 50 μm], and ARC [(E) HFD and (F) LFD; scale bars = 50 μm] of mice maintained on HFD or LFD for 8 wks. Projections images generated from 63x z-stacks (scale bar = 10 μm) of active (G) and resting (H) microglia in the paPVN of mice maintained on HFD and LFD, respectively. 3v = third cerebral ventricle.
Figure 3.
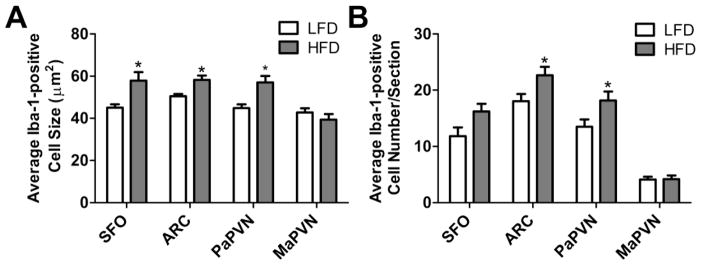
Quantification of the (A) size and (B) number of Iba-1 positive cells in the SFO, ARC. PaPVN and MaPVN in mice given HFD (n = 10) or LFD (n = 8) for 8 wks. Bars = SEM. * = (p < 0.05).
The effect of HFD consumption on the area occupied by GFAP positive cells in specific forebrain nuclei
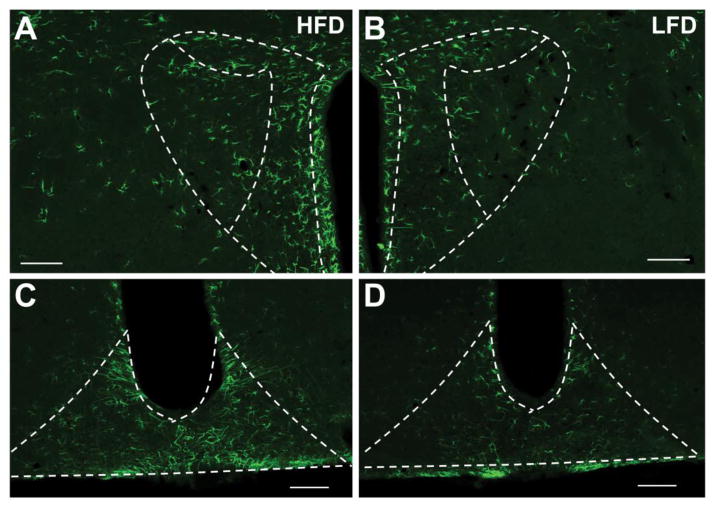
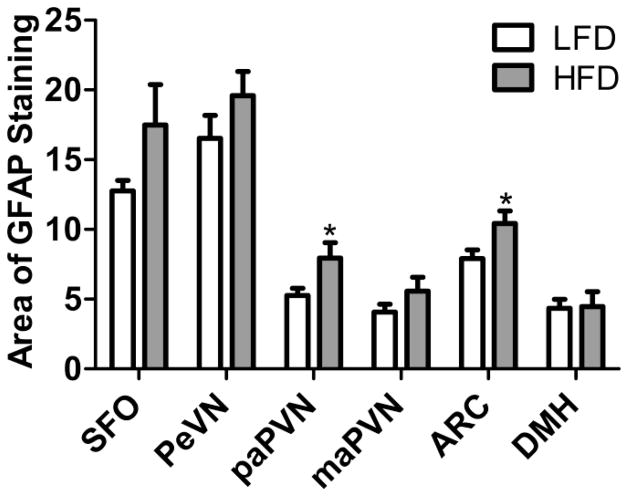
Previous studies have ascertained that the level of GFAP, which is largely expressed in astrocytes, is increased in specific brain regions in response to various threats to the internal milieu of the brain [13, 37, 38]. Figures 4 and 5 depict the extent to which maintenance on a HFD for 8 wks impacts immunostaining for this astrocytic marker within the SFO, ARC, PaPVN, PeVN, PVN, and DMH. After 8 wks of HFD consumption, GFAP-positive cells occupy a greater area of the PaPVN (p = 0.021) and ARC (p = 0.015). Conversely, the levels of GFAP staining within the SFO (p = 0.075), the magnocellular portion of the PVN (p = 0.23), the PeVN (p = 0.22) and the DMH (p = 0.91) were not significantly different between the mice fed HFD or LFD (Figure 5).
Figure 4.
GFAP immunoreactivity in the PVN and ARC after 8 wks on HFD or LFD. 10 × immunofluorescence images of the PVN and ARC of mice given HFD (A and C) or LFD (B and D) for 8 wks. Scale bars = 100 μm.
Figure 5.
Quantification of the % Area of GFAP positive staining in the SFO, PeVN, PaPVN, MaPVN, ARC and DMH of mice given HFD (n = 10) or LFD (n = 8) for 8 wks. Bars = SEM. * = (p < 0.05).
The effect of HFD consumption on inflammatory and RAS gene expression in specific brain nuclei
We next determined whether changes in Iba-1 and GFAP staining were associated with altered gene expression for microglial and astrocytic markers, as well as proinflammatory factors and components of the RAS (Figure 6). Within the SFO, HFD-fed mice had elevated levels of mRNAs for AT1a (p = 0.031), CD11b (p = 0.034), while the levels of GFAP (p = 0.08), ACE (p = 0.45), MCP-1 (p = 0.20), IL-6 (p = 0.32) and TNFα (p = 0.22) were similar between the groups (Figure 6A). Mice given HFD also had elevated levels of GFAP (p = 0.016) and IL-6 mRNAs (p = 0.0066) within the PVN, while the mRNA levels of the other genes assessed were unaltered (Figure 6B). Lastly, within the MBH (including the ARC, VMH and ME), the levels of GFAP mRNA were significantly increased in the mice fed HFD relative to LFD-fed controls (p = 0.017), while, the levels of mRNAs for ACE (p = 0.48), AT1a (p = 0.15), MCP-1 (p = 0.24), IL-6 (p = 0.37) and CD11b (p = 0.18) were unaltered (Figure 6C).
Figure 6.
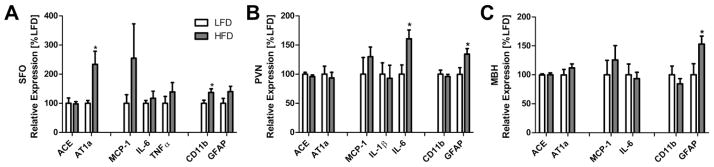
Gene expression in the SFO (left), PVN (center) and mediobasal hypothalamus (MBH; right) of mice given HFD (n = 9) or LFD (n = 7) for 8 wks. AT1a = angiotensin type-1a receptor; ACE = angiotensin-converting enzyme; MCP-1 = monocyte chemotactic protein-1; TNFα = tumor necrosis factor α; IL-6 = interleukin 6; IL1β = interleukin 1β; CD11b = cluster of differentiation molecule 11B; GFAP = glial fibrillary acidic protein. Bars = SEM.* = (p < 0.05).
Obesity-related microglial activation, astrogliosis and inflammation in PVN AT1a KO mice
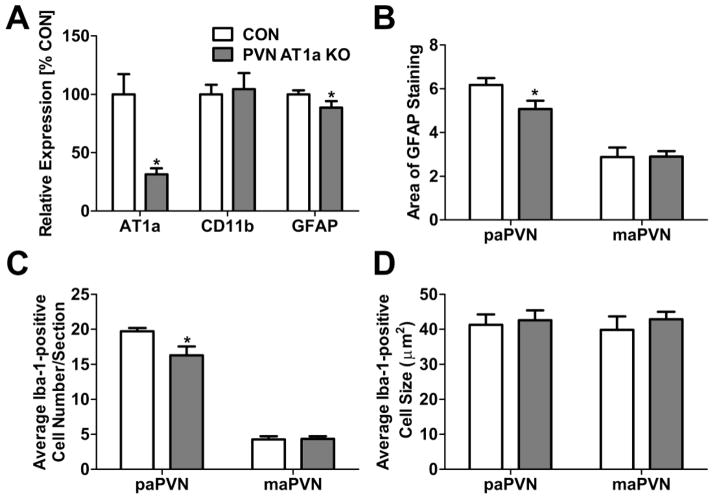
In a final set of experiments, we sought to investigate the role of PVN AT1a in the HFD-induced increase in microglial and astrocytic staining within the parvocellular PVN. Importantly, co-expression of the Sim1-Cre-recombinase transgene and the AT1a receptor flox gene elicited a significant decrease in AT1a mRNA in isolated PVNs (p = 0.001; Figure 7A). This is consistent with our previously-published receptor autoradiography results, indicating that AT1a receptor deletion is localized specifically to this forebrain region [29]. After 8 weeks of HFD consumption, mice lacking the PVN AT1a receptor weighed more than controls (34.5 ± 0.87 g [CON] vs. 38.0 ± 1.04 [KO] ; p = 0.015) and exhibited decreased GFAP mRNA (p = 0.046; Figure 7A) and immunoreactivity (p = 0.025; Figure 7B) within the PVN relative to controls expressing only the AT1a flox gene. Similarly, PVN AT1a KO mice maintained on a HFD had a decreased number of Iba-1-positive cells relative to controls (p = 0.017; Figure 7C). These effects are likely not secondary to decreased levels of adiposity, since we previously determined that mice lacking PVN AT1a have an enhanced susceptibility to diet-induced obesity [29]. Conversely, when given HFD, the expression of CD11b and the size of microglia within the PVN were not different between mice that lack the PVN AT1a and controls (Figures 7B and 7D).
Figure 7.
(A) Relative gene expression in the PVN of the PVN AT1a KO mouse (n = 8) and controls (n = 9) after 8 wks of HFD consumption. (B) Quantification of the % Area of GFAP-positive staining in the PaPVN and MaPVN of PVN AT1a KO mice (n = 5) and controls (n = 5) given HFD. Quantification of the (C) number and (D) and cell body size of Iba-1 positive cells in the PaPVN and MaPVN in PVN AT1a KO mice (n = 5) and controls (n = 5) given HFD. Bars = SEM. * = (p < 0.05).
Discussion
Obesity and hypertension are accompanied by an increased activity of the RAS, as well as an accumulation of inflammatory factors and cells within specific brain regions, which are both hypothesized to exacerbate metabolic and cardiovascular dyshomeostasis [7, 10, 13, 39–42]. A principal finding of this study is that HFD-feeding initiates an inflammatory cascade within specific forebrain regions that regulate cardiovascular function and energy balance. Importantly, these studies reveal that in addition to the ARC, which is known to exhibit increases in both microglial activation and astrogliosis during HFD-induced obesity [13, 43], both the PVN and the SFO, two brain regions that are critical for the regulation of blood pressure and energy balance, initiate proinflammatory responses following the consumption of a diet high in fat. The results further indicate that some of these responses are reversed upon deletion of AT1a receptors specifically within the PVN. The implication is that HFD-feeding initiates an angiotensin-dependent increase in inflammatory cells that may then contribute to disturbances to the homeostatic systems regulating metabolism and cardiovascular function.
Astrocytes and microglia have important functions as the central nervous system’s resident immune cells that sense and respond to a variety of threats to the internal milieu of the brain, including obesity and hypertension. When activated, both microglia and astrocytes undergo a number of phenotypic changes that allow for quantitative and qualitative assessment of their activity. In the present studies, we chose to investigate the activity of these cell-types within specific brain regions by immunohistochemical staining of microglia (e.g., Iba-1) and astrocyte (e.g., GFAP) specific markers and then examining the morphology and number of these cells. Using such techniques, previous studies have ascertained that HFD-feeding induces elevations in microglial activation and in astrocytes within several brain regions, including the mediobasal hypothalamus (which includes the arcuate nucleus of the hypothalamus and the median eminence) [13, 43]. Our findings that HFD consumption leads to increases in the number and size of the cell bodies of Iba-1-positive microglia, as well as increases in gene expression and staining for the astrocytic marker, GFAP, within the ARC are consistent with these previously-published studies. Furthermore, there is also evidence that GFAP immunoreactivity becomes elevated within the other specific nuclei of the hypothalamus, including the PVN of genetically and diet-induced obese female mice [38], which is consistent with our findings that GFAP mRNA and immunoreactivity are also elevated within the PVN.
An important and novel finding of the current manuscript is that this observed increase in glial cells is localized also specifically to the parvocellular portion of the PVN and also within the SFO. Both of these brain regions express AT1a receptors [44, 45] and are known to play important roles not only in the regulation of cardiovascular function [46–49], but also in the maintenance of energy balance [29, 50]. In particular, the parvocellular portion of the PVN integrates signals from various brain regions to regulate many homeostatic functions, while the SFO is a circumventricular organ that impacts cardiovascular function and energy balance by sensing and responding to circulating factors. Within the PVN, an accumulation of active microglia has been associated with the onset of neurogenic hypertension and plays a fundamental role in its progression [10]. To this end, inhibition of microglial activation by the icv administration of minocycline (an anti-inflammatory antibiotic) or a reduction in the proinflammatory microenvironment specifically within the PVN via the adenoviral overexpression of the anti-inflammatory cytokine IL-10 prevents Ang-II-induced hypertension, supporting this causal relationship between hypertension and microglial activation [10].
There is also evidence that astrocyte-neuron interactions play an important role in neurohumoral and autonomic control, as reviewed in detail by Stern and Filosa [51]. Astrocytes are capable of sensing and responding to hypertensive and obesigenic stimuli [13, 37, 38]. They become activated during obesity [13, 38] and can then impact neuronal signaling within forebrain autonomic control centers through a variety of mechanisms [51]. For example, astrocytes can contribute to GABA tone in neural circuits that regulate sympathetic outflow and blood pressure via their GABA transporter 3, which removes GABA from the synapse thereby disinhibiting certain neural circuits [52].
Another finding of these studies is that changes in gene expression for certain proinflammatory factors and components of the RAS accompany the elevations in microglial and astrocytic activation. In particular, there was a substantial increase in IL-6 mRNA within the PVN. Acute stressors have previously been found to activate IL-6 neurons within the PVN and this activation is associated with elevated PVN and circulating IL-6 levels [53], which can then affect blood pressure and energy balance [54–58]. The current gene expression studies also indicate that diet-induced obesity leads to increased expression of AT1a within the SFO. This, to our knowledge, is the first study to determine that diet-induced obesity causes elevations in SFO AT1a receptor expression; however, earlier studies have revealed that AT1a receptors in the SFO are impacted by the levels of circulating factors that are regulated by levels of adiposity [59]. For example, leptin increases SFO AT1a expression, and interactions between leptin and angiotensin-II at the level of the SFO are thought to contribute to the regulation of blood pressure [59]. The SFO has direct neuronal projections to the PVN [60], and an increase in AT1a expression within the SFO could then enhance the activation of SFO-PVN projecting neurons when exposed to increased circulating Ang-II-levels as occurs during obesity [8].
These results additionally imply a role for the PVN AT1a receptor in the observed increase in GFAP immunoreactivity and mRNA expression within the parvocellular portion of the PVN. Although there were no observed increases in the level of AT1a mRNA in within the PVN of wild-type HFD-fed mice, relative to the LFD-fed controls, we have previously observed that a greater percentage of parvocellular neurons become responsive to Ang-II after 8 weeks of HFD-feeding, and this is associated with increases in the expression of TNFα, a proinflammatory cytokine and CD11b, a marker for microglia [29]. The implication is that PVN angiotensin receptor signaling is important for the generation of HFD-induced inflammation within the hypothalamus. In these experiments, we did not observe any differences in the size of the Iba-1-positive cell bodies; however the number of Iba-1-positive cells was increased within the PVN. These data suggest that the increased GFAP immunoreactivity and gene expression within the PVN during HFD are dependent on neuronal AT1a receptor signaling within the PVN and that PVN AT1a may contribute to the recruitment of microglia to PVN during obesity.
The aims of the current study were to determine the effect of HFD-induced obesity on glial cells and inflammatory factors within key forebrain regions that regulate cardiovascular function and to determine whether PVN AT1a are required for the immune cell activation in the PVN. Although from this study and from others it is clear that obesity and hypertension are associated with alterations in microglial and astrocyte activation, whether these alterations impact the neural control of energy balance and blood pressure is an active area of research. It is therefore of direct relevance to speculate on the potential impact of these obesity-related changes on cardiovascular and metabolic homeostasis. Previous studies support the hypothesis that the inflammatory responses within the ARC during obesity contributes to alterations in metabolic regulation [17], glucose homeostasis [61], and to cardiovascular regulation [27]. Furthermore, our research group and others have ascertained that inflammatory processes within the PVN are key contributors to several models of neurogenic hypertension [10]. Importantly, we have previously determined that in mice PVN AT1a deletion also leads to a resistance to increases in blood pressure that occur after long-term HFD consumption [29]. Associations between obesity and hypertension are well-established, and the present studies, in conjunction with these previously published studies [10, 29] bring to light the possibility that HFD consumption leads to an AT1a-dependent activation of glial cells within the PVN that ultimately leads to obesity-related hypertension. These studies thereby provide evidence that a RAS-dependent increase in inflammatory factors within the CNS may offer a causal link between obesity and hypertension.
Highlights.
High-fat diet intake causes proinflammatory responses in mouse forebrain nuclei.
Increased microglia size and number in forebrain nuclei follows high-fat diet intake.
High-fat diet consumption leads to increased GFAP expression and staining.
Some of these responses are reversed upon deletion of AT1a within the PVN.
Acknowledgments
This manuscript is based on work presented during the 2013 Annual Meeting of the Society for the Study of Ingestive Behavior, July 30 – August 3, 2013. This work was supported by an American Heart Association Postdoctoral Fellowship (12POST11550013), by an NIH T32 Training Grant (HL-083810), by an individual NIH postdoctoral fellowship (F32-HL-116074) and by the NIH grant HL-076803..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Timpson NJ, Harbord R, Davey Smith G, Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension. 2009;54(1):84–90. doi: 10.1161/HYPERTENSIONAHA.109.130005. [DOI] [PubMed] [Google Scholar]
- 3.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–19. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 4.Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Pediatr Diabetes. 2009;10(6):360–7. doi: 10.1111/j.1399-5448.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 5.Cassano PA, Segal MR, Vokonas PS, Weiss ST. Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Ann Epidemiol. 1990;1(1):33–48. doi: 10.1016/1047-2797(90)90017-m. [DOI] [PubMed] [Google Scholar]
- 6.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60(1):163–71. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 7.de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: A new therapeutic angle? Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper R, Forrester T, Ogunbiyi O, Muffinda J. Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J Hypertens. 1998;16(5):571–5. doi: 10.1097/00004872-199816050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10(6):464–9. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 10.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56(2):297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, et al. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71(9):774–82. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation. 2003;112(12):1821. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151(9):4109–15. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 16.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF- kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaB in the paraventricular nucleus. Hypertension. 2012;59(1):113–21. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82(3):503–12. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waki H, Gouraud SS, Maeda M, Paton JF. Specific inflammatory condition in nucleus tractus solitarii of the SHR: novel insight for neurogenic hypertension? Auton Neurosci. 2008;142(1–2):25–31. doi: 10.1016/j.autneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Waki H, Gouraud SS, Maeda M, Paton JF. Gene expression profiles of major cytokines in the nucleus tractus solitarii of the spontaneously hypertensive rat. Auton Neurosci. 2008;142(1–2):40–4. doi: 10.1016/j.autneu.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Waki H, Liu B, Miyake M, Katahira K, Murphy D, Kasparov S, et al. Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension. 2007;49(6):1321–7. doi: 10.1161/HYPERTENSIONAHA.106.085589. [DOI] [PubMed] [Google Scholar]
- 23.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisse BE, Ogimoto K, Morton GJ, Wilkinson CW, Frayo RS, Cummings DE, et al. Physiological regulation of hypothalamic IL-1beta gene expression by leptin and glucocorticoids: implications for energy homeostasis. Am J Physiol Endocrinol Metab. 2004;287(6):E1107–13. doi: 10.1152/ajpendo.00038.2004. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 26.Shi P, Grobe JL, Desland FA, Raizada MK, Sumners C. Microglial activation by the brain renin-angiotensin system. FASEB J. 2011;25:661.2. [Google Scholar]
- 27.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17(7):883–7. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–33. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, et al. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1067–77. doi: 10.1152/ajpregu.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Franklin KBJ, Paxinos G. The Mouse Brain: In Stereotaxic Coordinates. 3. New York, NY: Elsevier; 2008. [Google Scholar]
- 33.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 34.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130(2):169–75. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 36.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94(1):12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 37.Gerber AR, Bale TL. Antiinflammatory treatment ameliorates HPA stress axis dysfunction in a mouse model of stress sensitivity. Endocrinology. 2012;153(10):4830–7. doi: 10.1210/en.2012-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckman LB, Thompson MM, Moreno HN, Ellacott KLJ. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2012:n/a–n/a. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107(33):14875–80. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–9. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 42.Tomassoni D, Avola R, Di Tullio MA, Sabbatini M, Vitaioli L, Amenta F. Increased expression of glial fibrillary acidic protein in the brain of spontaneously hypertensive rats. Clin Exp Hypertens. 2004;26(4):335–50. doi: 10.1081/ceh-120034138. [DOI] [PubMed] [Google Scholar]
- 43.Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido MÁ, Sarman B, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012 doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of Angiotensin Type-1 (AT1) and Type-2 (AT2) Receptor mRNAs in the Adult Rat Brain: A Functional Neuroanatomical Review. Frontiers in Neuroendocrinology. 1997;18(4):383. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 45.Hauser W, Johren O, Saavedra JM. Characterization and distribution of angiotensin II receptor subtypes in the mouse brain. Eur J Pharmacol. 1998;348(1):101–14. doi: 10.1016/s0014-2999(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 46.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61(2):382–7. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, et al. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension. 2013;61(3):716–22. doi: 10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, et al. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122(11):3960–4. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, et al. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. Faseb J. 2008;22(9):3175–85. doi: 10.1096/fj.08-108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulman KJ, Fry WM, Cottrell GT, Ferguson AV. The subfornical organ: a central target for circulating feeding signals. J Neurosci. 2006;26(7):2022–30. doi: 10.1523/JNEUROSCI.3218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stern JE, Filosa JA. Bidirectional neuro-glial signaling modalities in the hypothalamus: role in neurohumoral regulation. Auton Neurosci. 2013;175(1–2):51–60. doi: 10.1016/j.autneu.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587(Pt 19):4645–60. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. Stress activation of IL-6 neurons in the hypothalamus. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;299(1):R343–R51. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, et al. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Infereron Cytokine Mediator Res. 2011;2011(3):65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension. 2012;59(1):136–44. doi: 10.1161/HYPERTENSIONAHA.111.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011;24(10):1143–8. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benrick A, Schéle E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, et al. Interleukin-6 Gene Knockout Influences Energy Balance Regulating Peptides in the Hypothalamic Paraventricular and Supraoptic Nuclei. J Neuroendocrinol. 2009;21(7):620–8. doi: 10.1111/j.1365-2826.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 58.Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obesity Reviews. 2008;9(1):20–9. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 59.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303(2):H197–206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, et al. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011;31(42):15009–15. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296(5):E1003–12. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]