Abstract
Proteins bound to nanoparticle surfaces are known to affect particle clearance by influencing immune cell uptake and distribution to the organs of the mononuclear phagocytic system. The composition of the protein corona has been described for several types of nanomaterials, but the role of the corona in nanoparticle biocompatibility is not well established. In this study we investigate the role of nanoparticle surface properties (PEGylation) and incubation times on the protein coronas of colloidal gold nanoparticles. While neither incubation time nor PEG molecular weight affected the specific proteins in the protein corona, the total amount of protein binding was governed by the molecular weight of PEG coating. Furthermore, the composition of the protein corona did not correlate with nanoparticle hematocompatibility. Specialized hematological tests should be used to deduce nanoparticle hematotoxicity.
Keywords: nanoparticles, protein corona, complement, coagulation, hematocompatibility
Introduction
Increasing use of nanotechnology in biology and medicine accentuates the importance of understanding nanomaterial interactions with cells and biological fluids. Nanoparticle safety and effective performance as drug-delivery platforms are influenced by their disposition and clearance in the body. The latter is determined by nanoparticle physicochemical properties such as size, charge, hydrophobicity and shape (1, 2). Proteins bind to a nanoparticle surface almost immediately upon entry into the blood stream. Some of these proteins stay on the surface as long as particles circulate in the blood stream and “target” the particle to certain cells and tissues. For example, ApoE binding to the surface of polysorbate 80 coated nanoparticles is believed to deliver these particles to the brain, while fetuin is thought to “mark” the particle for macrophage uptake through a specific scavenger-receptor mediated route (3-6). Protein binding affects nanoparticle hydrodynamic size and charge (7) and thus may influence the way cells and tissues interact with and process the particles, ultimately guiding cellular uptake, clearance route and organ accumulation. Recent studies have also demonstrated that proteins adsorbed onto a nanoparticle's surface change after cellular uptake and intracellular distribution (8).
The human blood plasma protein repertoire is very dynamic and is sensitive to physiological changes. The reason(s) why some proteins are present in plasma permanently and others reside there only temporarily can be manifold. For example, some proteins are present in plasma only during infection or stress (e.g. C reactive protein). Other proteins are always present, but levels can fluctuate depending on diet (e.g. lipoproteins), infection (e.g. cytokines), or stress (e.g. serum deprivation protein, heat shock proteins). Also, some “house-keeping” protein levels (e.g. albumin, globulin, and fibrinogen) fluctuate in response to environmental changes, while a variety of other proteins (e.g. blood group antigens, Rhesus factor) are only present in the blood of individuals with certain genetic backgrounds. In addition, infections and diseases, contribute foreign and aberrant proteins, which otherwise are not present in plasma. Consequently, even when using blood from healthy donor volunteers, the composition of plasma proteins can vary greatly between individuals. Intuitively, these facts suggest that in order to link protein corona composition to nanoparticle toxicity one would have to study the corona for each individual patient at a given time. The complexity of such an approach is further convoluted by the fact that phlebotomy, blood handling and storage conditions of plasma can also affect the protein repertoire.
Formation of protein corona is also a dynamic process, however opinions about the mechanism of protein binding diverge (9-13). While some studies clinch in favor of protein displacement (9, 13), others conclude there is no typical Vroman effect in nanoparticle-protein interaction (12). Moreover, a range of opinions exist regarding the role plasma protein binding plays in defining the toxicity of nanoparticles (14-20). The differences in conclusions between these studies are not surprising because nanoparticles (gold colloids, silver colloids, iron oxides, polystyrene and acrylamide), the source of protein (human plasma, culture medium containing diluted fetal bovine serum, isolated individual proteins), and methods of analysis (gel-electrophoresis, mass spectrometry, fluorescent correlation spectroscopy, or a combination thereof) widely vary between studies. Therefore, questions about composition and dynamics of the protein corona and its role in nanoparticle biocompatibility still require careful examination.
Colloidal gold particles are considered a versatile biomedical platform. Their applications include image enhancing contrast agents, biosensors and drug-delivery vehicles (21-24). Although experimental use of colloidal gold in vivo was described in the early 1970s, the main apprehension for their clinical in vivo application is rapid particle clearance from the blood stream and accumulation in organs of the mononuclear phagocytic system (MPS) (21, 25-28). To prolong particle circulation and eliminate potential long term toxicity, surface modification of colloidal gold with hydrophilic polymers such as poly(ethylene glycol) (PEG) has proven a reliable shield, provided these coatings are stable and not displaced by proteins in the blood stream.
Previously it has been shown that colloidal gold particles adsorb serum proteins and that protein binding influences their clearance by immune cells (25). Paciotti et al reported that 30 nm gold colloids rapidly accumulate in the liver and spleen, while their 20 kDa PEG-functionalized counterparts remained in circulation, allowing these particles to effectively deliver drugs into tumor tissue (23). The molecular identity of proteins bound to the surface of anionic colloidal gold nanoparticles has been reported (7); however, the role of incubation time and PEG surface coating on the formation and composition of the protein corona has not been explored.
In the present study we used 30 nm citrate stabilized gold nanoparticles from a commercial source to prepare several formulations bearing PEG of various molecular weights (2 kDa, 5 kDa, 10 kDa and 20 kDa). These PEG coatings were selected because of their common use in nanomedicines involving gold colloids and gold nanoshells (23, 29, 30). Both uncoated and PEGylated particles were incubated with pooled human plasma for various time periods: 5 min, 30 min, 1 hr, 6 hr and 24 hr. The amount of bound protein, as well as the composition of the protein corona, was then studied by mass spectrometry. The most abundant proteins in the corona were fibrinogen and complement, central in plasma coagulation and complement activation, respectively; therefore, we also performed in vitro biological assessments to ascertain whether nanoparticle binding to these proteins would alter their function.
Methods
Reagents
Colloidal gold nanoparticles were purchased from TedPella (Redding, CA, US). Cobra venom factor and veronal buffer were purchased from Quidel Corporation (San Diego, CA, US) and Boston Bioproducts (Boston, MA, US), respectively. Normal and abnormal coagulation controls, buffers, and activators for coagulation assays were purchased from Diagnostica Stago (Parsippany, NJ, US).
Synthesis of PEGylated gold colloids
MethoxyPEG gold nanoparticles were synthesized following a modified procedure by Mirkin et al.(31). Procedural details are outlined in the Supplemental Section.
Research donor blood
Healthy human volunteer blood specimens were drawn under NCI-Frederick Protocol OH99-C-N046. Details about sample handling and processing are provided in supplemental materials.
Protein binding
Gold nanoparticles were used at equivalent gold concentrations. To achieve this, PEGylated gold nanoparticles were diluted to a final concentration of 42 μg of gold per mL, i.e. the lowest concentration of all test samples. Next, 250 μL of each stock solution (10.5 μg of gold) was placed into sterile, pyrogen-free, low-retention eppendorf tubes, and the volume was adjusted to 1 mL with sterile water. Pooled human plasma (1 mL) was then added to each tube and samples were incubated on a rotary mixer for 5 min, 30 min, 1 hr, 6 hr and 24 hr at 37°C. Following incubation, the tubes were centrifuged 10 min at 4°C in a microcentrifuge. The pellets were washed 3 times with 2 mL of sterile PBS and the final pellets were processed for analysis by mass spectrometry. Water (1 mL) was used as a control for non-specific adherence of proteins to the tubes.
Mass spectrometry
Particles with bound proteins were treated with trypsin prior to mass spectrometry analysis. Trypsinized samples were analyzed by a nano-ESI reversed-phase liquid chromatography using an Agilent 1100 nano-flow LC system (Agilent Technologies, Inc., Santa Clara, CA, US) coupled online to a LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, US). Detailed protocol is provided in supplementary materials section.
Data analysis
The total number of peptides identified for each protein by mass spectrometry was normalized by subtracting the number of peptides identified for the corresponding individual protein at the corresponding incubation time in the control (no nanoparticle) sample. Proteins with a peptide number of 2 or less were excluded from analysis as insignificant (32).
Complement activation
Platelet poor plasma (PPP) was prepared by centrifugation of freshly drawn whole blood for 10 min at 2500×g. Activation of complement was assessed by the detection of the iC3b component of complement in test plasma samples using the commercial Quidel EIA kit (Santa Clara, CA, US). Detailed protocol is provided in supplemental materials.
Plasma coagulation (PT, APTT and thrombin time)
To test whether gold colloids initiated plasma coagulation, normal human plasma pooled from 3 donors was treated with gold colloids and coagulation time was measured using the STArt4 coagulometer (Diagnostica Stago, Parsippany, NJ, US). Detailed protocol is provided in supplemental materials.
Results
Physicochemical characterization
Physicochemical characterization included analysis of nanoparticle hydrodynamic size by dynamic light scattering (DLS), zeta potential by laser doppler velocimetry, and quantification of gold by inductively coupled plasma-mass spectrometry (ICP-MS). The data is summarized in Table 1. As expected, the hydrodynamic size increased and zeta potential shifted towards neutral as increasing molecular weights of PEG were applied to the particle surface. (Further details on the characterization can be found in the Supplemental Section.). Presence of equal amounts of PEG on the surface of PEGylated samples was confirmed by HPLC-CAD (data not shown).
Table 1. Physicochemical characterization of gold colloids.
Nanoparticle hydrodynamic size, charge and concentration of gold was measured by dynamic light scattering, zeta potential analyzer and ICM-MS, respectively as described in materials and methods. Shown is mean result ± standard deviation (N=12).
| Nanoparticles | DLS (Intensity-Peak) (nm) | Zeta potential (mV) | [Au] by ICP-MS (μg/mL) |
|---|---|---|---|
| 30 nm | 35.1 ± 0.3 | -30.4 ± 2.0 | 42.02 ± 4.35 |
| 30 nm + 2kDa PEG | 58.5 ± 0.7 | -8.7 ± 0.8 | 80.87 ± 3.86 |
| 30 nm + 5 kDa PEG | 74.5 ± 0.9 | -7.9 ± 0.5 | 60.68 ± 2.96 |
| 30 nm + 10 kDa PEG | 83.9 ± 1.1 | -6.7 ± 1.2 | 78.30 ± 3.56 |
| 30 nm + 20 kDa PEG | 107 ± 1.1 | -6.0 ± 1.1 | 91.07 ± 4.32 |
Make-up of protein corona is consistent
For the purpose of the paper we will use “composition” to refer to the “make-up” of protein corona, i.e. the proteins, not the quantity of each individual protein. To evaluate inter-assay variability in protein corona composition, experiments were conducted at different times, each using plasma pooled from different donors. To assess intra-assay variability, a third experiment was conducted in which two independent sets of samples were run using the same pooled plasma. Regardless of the incubation time or plasma donors, the composition of the protein corona was fairly consistent. Proteins from the same functional categories – cell adhesion, complement, coagulation, immunoglobulins, cytoskeleton, and transport proteins – were identified in all experiments (Table 2). Furthermore, the majority of individual proteins identified within each functional category were also consistent between experiments.
Table 2. Individual proteins in the protein corona.
30 nm colloidal gold nanoparticles were incubated with pooled human plasma and proteins in the corona were identified by mass spectrometry. Presented is the summary of all proteins identified over 5 min, 30 min, 1 hr, 6 hr and 24 hr incubations, grouped by their function. ND- none detected.
| Protein Category | Inter-Assay Variability | Intra-Assay Variability | ||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3A | Experiment 3B | |
| Cell adhesion | Fibronectin, vitronectin, thrombospondin | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein | fibronectin | fibronectin |
| Complement | C1q, C1r, C1s, C4a, C4b, C5, C8, C9, B, H | C1q, C3, C4a | C1q, C1r, C1s, C4a, C4b, C3, H | C1q, C1r, C1s, C4a, C4b, C3, H |
| Coagulation | Fibrinogen, vWF, FXII, plasminogen, antithrombin, PF4, plekstrin, pGP, CD9, histidine-rich glycoprotein | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin | Fibrinogen, FXII, vit K dependent protein | Fibrinogen, FXII, vit K dependent protein |
| Immunoglobulins | IgG and IgM light chains | IgG and IgM light chains | IgG and IgM light chains | IgG and IgM light chains |
| Cytoskeleton | Actin, myosin, tubulin, gelsolin, filamin, talin | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin | Actin, myosin, gelsolin, talin | Actin, myosin, gelsolin, talin |
| Transport | Hemoglobin, albumin, anion transport protein | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein | Hemoglobin, hemopexin, serum amyloid A | Hemoglobin, hemopexin, serum amyloid A |
| Extracellular Matrix | EMP, hyaluronan | Tenascin | ND | EMP |
| Cellular signal transduction | ND | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin | ND | ND |
| Enzymes | ND | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase | ND | ND |
| Stress-response | ND | Heat shock protein, serum-deprivation protein | ND | ND |
| Other | LBP, serum amyloid P, secreted phosphoprotein 24, erythrocyte band 7 integral membrane protein, pigment epithelium derived factor, plasma protease C1 inhibitor, histone H2B and H4, glyceraldehyde-3-phosphate dehydrogenase, estrogen receptor | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 inhegral membrane protein | Estrogen receptor, a2-macroglobulin | Estrogen receptor |
In spite of consistency in the protein composition of the corona, the kinetics of protein binding varied greatly. Variability was observed at both the level of protein functional categories (Figure 1) and at the level of individual proteins within each category (Figure 2). For example, in the inter-assay variability assessment, cell adhesion proteins made up the bulk of the corona in Experiment 1, while cytoskeleton proteins made up the bulk of the corona in Experiment 2. Across the various time points however, most protein levels remained fairly constant, generally fluctuating less than 10%. The largest exception to this was a 28% decrease in the number of cell adhesion protein between the 6 and 24 hr time points of Experiment 1. In the intra-assay variability assessment, cell adhesion proteins comprised the bulk of the corona. Furthermore, there was significantly more variability in protein levels across the various time points. Cell adhesion proteins decreased 37% between 1 and 24 hr, and complement proteins decreased 29% between 6 and 24 hr in Experiment 3A. In Experiment 3B, coagulation proteins increased 27% between 5 min and 24 hr, while complement and cell adhesion proteins both decreased approximately 15% across all time points (Figure 1).
Figure 1. composition of protein corona over time.
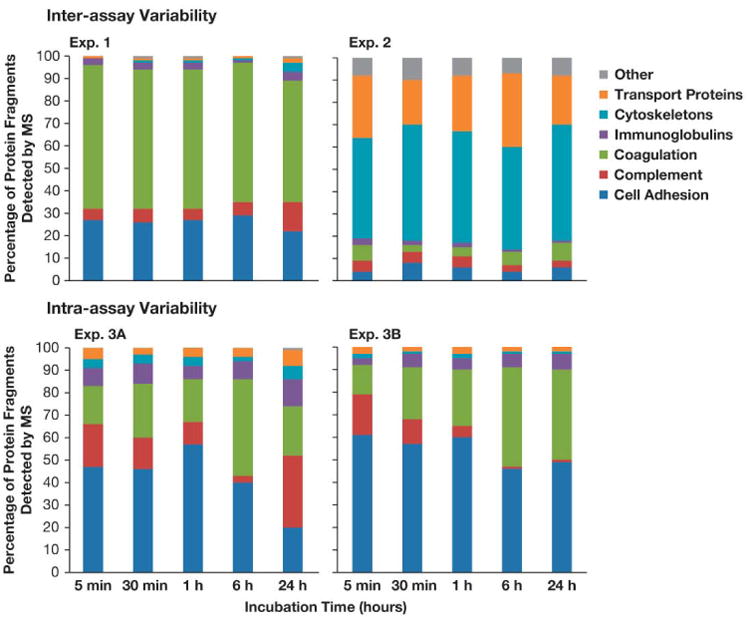
30 nm gold colloids were incubated with pooled human plasma for 5 min, 30 min, 1 hr, 6 hr and 24 hr. After several washes with PBS to remove excess plasma, the particle was pelleted and the particle pellet with bound proteins was analyzed by mass spectrometry. Proteins were grouped based on their function. Each bar shows the percentage of bound protein in each category for a given time point.
Figure 2. Kinetics of individual protein binding over time.
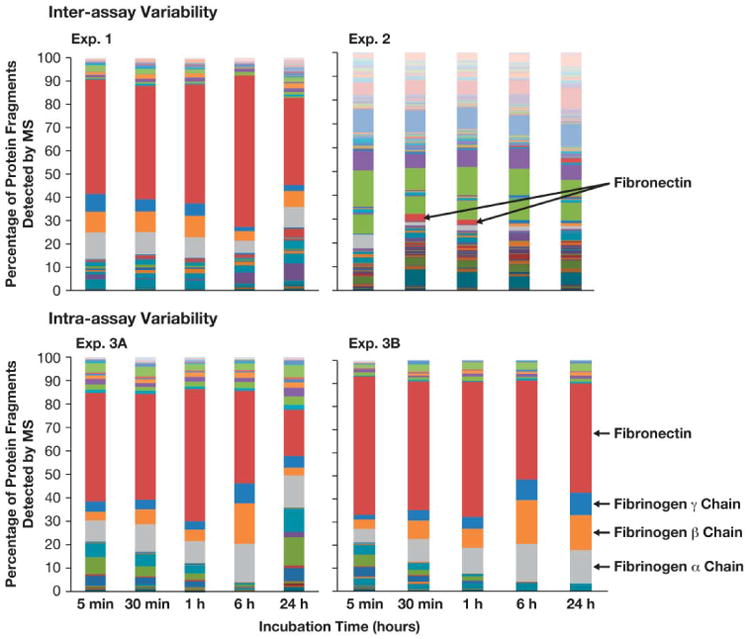
30 nm colloidal gold nanoparticles were incubated with pooled human plasma and proteins in the corona were identified by mass spectrometry. Presented is the summary of individual proteins identified over 5 min, 30 min, 1 hr, 6 hr and 24 hr incubations.
Similarly, when analysis is done at the level of the individual protein, little consistency in kinetics was observed. For example, fibronectin was the most abundant protein in Experiment 1 at all time points, but was only detected in small quantities at 30 min and 1 hr time points in Experiment 2. In the intra-assay variability assessment, fibronectin was the major protein in both experiments, but levels of other proteins varied between the replicates. For example, complement proteins were a significant component of the corona in Experiment 3A, but were almost nonexistent in Experiment 3B, and fibrinogen proteins comprised almost 40% of the corona in Experiment 3B, but only 22% in Experiment 3A at the 24 hr time point.
The total amount of bound protein varied with each experiment. Again, there is little consistency in the kinetics of binding. Linear trend line analysis revealed a slight decrease in total protein in three of the four experiments over the entire timespan studied (Figure 3). However, three of the four experiments show an increase in total bound protein from the 6 hr to 24 hr time points. In Experiment 1, the 5 min time point has the most protein, and in Experiments 2, 3A and 3B the 30 min, 1 hr and 6 hr time points have the greatest protein content, respectively.
Figure 3. Kinetics of protein corona over time.
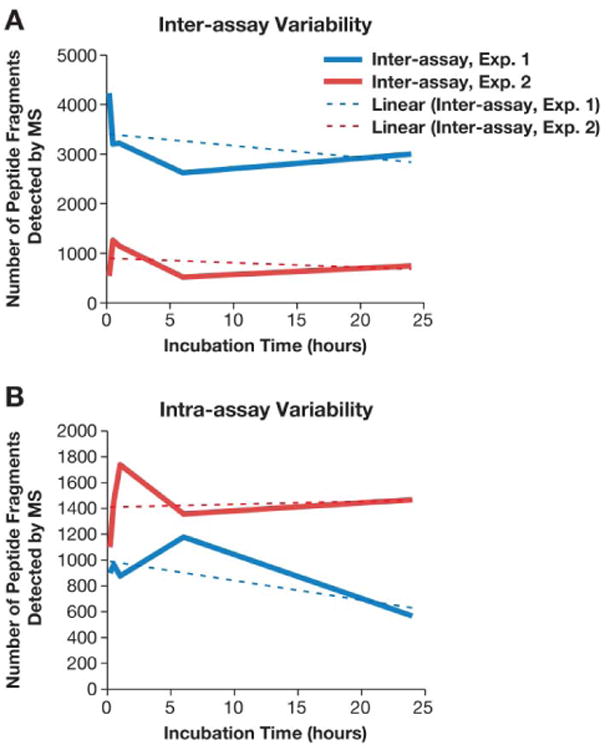
30 nm colloidal gold nanoparticles were incubated with pooled human plasma and proteins in the corona were identified by mass spectrometry. The total amount of bound protein identified over 5 min, 30 min, 1 hr, 6 hr and 24 hr time points is shown. The linear trend line is shown as the color-coordinated dashed line, and was used to estimate the general trend in total bound protein between individual time points.
PEGylation decreases total protein, but does not dramatically change protein corona composition
The total amount of protein detected on the surface of gold nanoparticles was generally highest in uncoated gold colloids and decreased as the molecular weight of PEG increased (Figure 4). Also, the amount of bound protein generally decreased over time in all tested samples. The lowest amount of protein was detected on gold colloids coated with 20 kDa PEG; while some protein binding was observed at the 5 and 30 min time points, the amount of total protein was significantly diminished at the 6 hr and 24 hr time points (Figure 4). A decrease in total protein binding with increased PEG molecular weight was also confirmed by 1D PAGE with densitometry analysis (see Supplemental Section).
Figure 4. Kinetics of protein binding to gold colloids with various surface coatings.
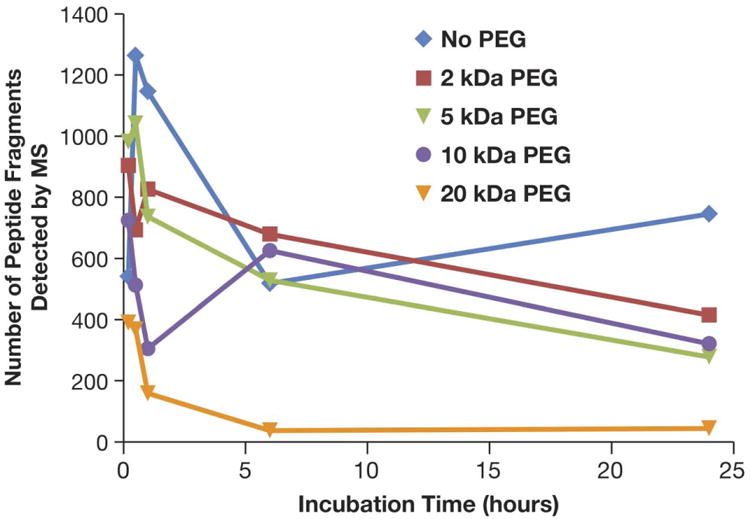
30 nm core colloidal gold nanoparticles uncoated or coated with PEG of various molecular weights (2 kDa, 5 kDa, 10 kDa and 20 kDa) were incubated with pooled human plasma and proteins in the corona were identified by mass spectrometry. Presented is the total amount of bound protein identified over 5 min, 30 min, 1 hr, 6 hr and 24 hr incubation periods.
In spite of the differences noted in the amount of total bound protein, the protein composition of the corona was not dramatically affected. Proteins detected in the corona of 30 nm uncoated gold colloids were also detected in corona of PEGylated gold nanoparticles (Table 3). The noted exceptions to this were extracellular matrix proteins, which were undetected in all PEGylated samples, and immunoglobulins, which were undetected in the corona of 20 kDa PEGylated gold colloids (Table 3, red font).
Table 3. Individual proteins identified by mass spectrometry in the protein corona surrounding 30 nm core gold colloids with various surface coatings.
30 nm colloidal gold nanoparticles, un-PEGylated or coated with 2 kDa, 5 kDa, 10 kDa or 20 kDa PEG, were incubated with pooled human plasma and proteins in the corona were identified by mass spectrometry. Below is the summary of all proteins identified in each time point studied (5 min, 30 min, 1 hr, 6 hr and 24 hr), grouped by their function. ND -not detected. The bold red font is used to highlight samples and proteins which were different between PEGylated and unPEGylated particles.
| Protein Category | un PEGylated | 2 kDa PEG | 5 kDa PEG | 10 kDa PEG | 20 kDa PEG |
|---|---|---|---|---|---|
| Cell adhesion | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein | Fibronectin, vitronectin, thrombospondin, integrins, beta parvin, vasodilator-stimulated phosphoprotein |
| Complement | C1q, C3, C4a | C1q, C3, C4a | C1q, C3, C4a | C1q, C3, C4a | C1q, C3, C4a |
| Coagulation | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin | Fibrinogen, FXIII, pGP, kininogen, CD9, histidine-rich glycoprotein, multimerin |
| Immunoglobulins | IgG and IgM light chains | IgG and IgM light chains | IgG and IgM light chains | IgG and IgM light chains | ND |
| Cytoskeleton | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin | Actin, myosin, tropomyosin, tubulin, filamin, gelsolin, F-actin, vinculin, coronin, cofilin, transgelin, ezrin, zyxin, profiling, caldesmon, PDZ and LIM domain protein 1, multimerin |
| Transport | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein | Albumin, anion transport protein, Apo C, Apo E, Apo A, Apo J, hemoglobin, 78kD glucose regulated protein |
| Extracellular Matrix | Tenascin | ND | ND | ND | ND |
| Cellular signal transduction | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin | Ras, Rab, 14-3-3 protein Z, protein S100-A9, fermitin |
| Enzymes | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase | Glyceraldehyde phosphate dehydrogenase, adenylyl cyclase, aldolase, isocitrate dehydrogenase, protein-disulfide isomerase, triosephosphate isomerase, ATP synthase, lactade dehydrogenase, enolase, pyruvate kinase, frusctose-biphosphate aldolase, peptidyl-prolyl cis-trans isomerase, carbonic anhydrase, glyceraldehyde-3 phosphate dehydrogenase |
| Stress-response | Heat shock protein, serum-deprivation protein | Heat shock protein, serum-deprivation protein | Heat shock protein, serum-deprivation protein | Heat shock protein, serum-deprivation protein | Heat shock protein, serum-deprivation protein |
| Other | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 integral membrane protein | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 integral membrane protein | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 integral membrane protein | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 integral membrane protein | Histone H4, Hermansky-Pudlock syndrome 1 protein, thymosin, erythrocyte band 7 integral membrane protein |
Plasma coagulation and complement system are unaffected
Since both complement components and plasma coagulation factors were among the most abundant proteins identified in the corona, we assessed whether the binding of these proteins to the nanoparticle surface would alter their physiological function. Complement and plasma coagulation functions were assessed in vitro using normal human plasma pooled from several donors. Nanoparticles per se did not activate plasma coagulation (Figure 5A) and did not activate the complement system (Figure 6A). Interestingly, low activation of the alternative pathway was detected as seen by weak elevation of Bb levels (Figure 6A, Bb bar). However, this activation was not strong enough to proceed along the cascade to generate substantial levels of C3 split products (Figure 6A, iC3b bar). Cobra venom factor (CVF) activates complement through the alternative pathway, and was not expected to generate a positive response in C4d assay. The assay performance was monitored by quality controls supplied with the kit.
Figure 5. Nanoparticles and plasma coagulation.
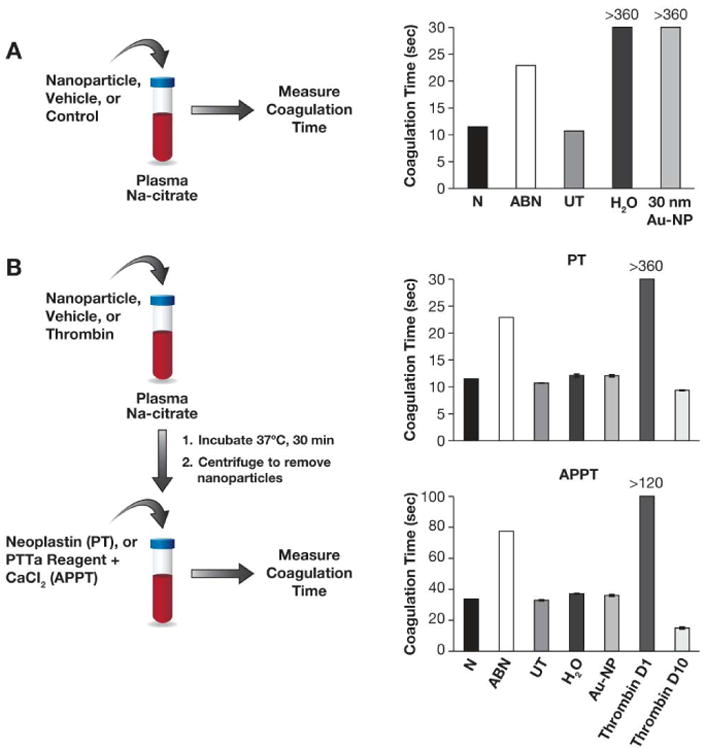
(A) 30 nm colloidal gold nanoparticles were studied in vitro to evaluate their pro-coagulant properties. Coagulation in normal control (N), abnormal control (ABN) and untreated healthy donor plasma (UT) was initiated by the addition of Neoplastin reagent. Water (H2O) and gold colloids (30 nm Au-NP) were also tested for initiation of coagulation in samples of untreated healthy donor plasma. (B) Pooled human plasma was either untreated (UT) or treated with 30 nm gold colloids (Au-NP), high concentration (0.15NIHU/mL) of thrombin (thrombin D1) or low (0.015NIH U/mL) concentration of thrombin (thrombin D10) for 30 min at 37°C. Plasma coagulation was initiated in all samples by the addition of Neoplastin reagent (PT) or PTTa reagent and calcium chloride (APPT); >360 refers to the absence of detectable coagulation within 6 min; each bar shows the mean of duplicate results (%CV < 5).
Figure 6. Nanoparticles and complement activation.
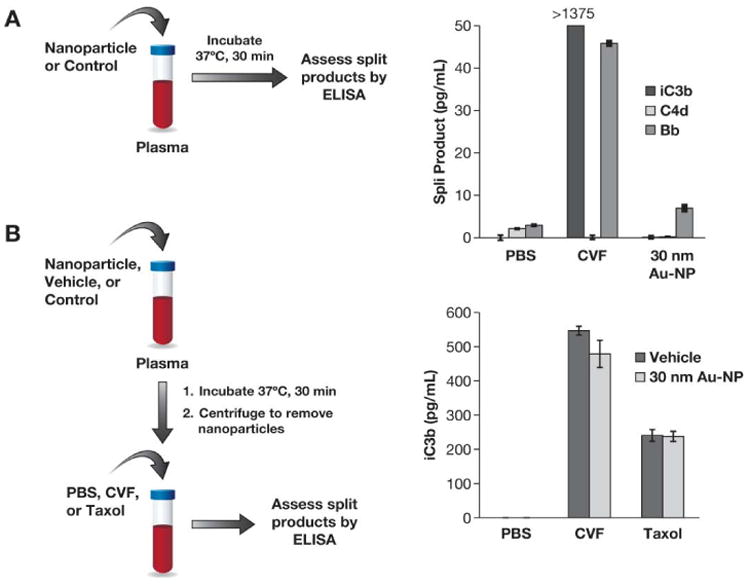
(A) 30 nm colloidal gold nanoparticles were studied in vitro to evaluate their ability to activate the human complement system. Pooled normal human plasma was treated with PBS, cobra venom factor (CVF) or nanoparticles for 30 min at 37°C, and complement split products iC3b, C4d and Bb were measured by ELISA. (B) Pooled human plasma was treated with 30 nm gold colloids (30 nm Au-NP) or vehicle (water) for 30 min at 37°C. Following incubation, plasma was spun down to remove particles, and supernatants were treated with PBS, CVF or Taxol. Complement split products were monitored by ELISA. Each bar shows the mean ± SD (N=3).
We also assessed whether nanoparticles would affect plasma coagulation or complement functions initiated by a known activator. To assess particle effects on plasma coagulation, plasma was pretreated with gold colloids, and after nanoparticles and nanoparticle-bound proteins were removed, plasma was then subjected to a known plasma coagulation initiator. Gold nanoparticles did not interfere with plasma coagulation time in either PT or APTT assays with known initiators (Figure 5B, compare UT bars with H2O [vehicle] and 30 nm Au-NP bars). Plasma was also pre-treated with two concentrations of thrombin, which is known to deplete fibrinogen, one of the major components of the protein corona of gold nanoparticles. If the depletion of fibrinogen as a result of nanoparticle binding is physiologically significant, we expected to see a delay in plasma coagulation. Plasma coagulation in samples pre-treated with the standard concentration (0.15 NIH U/mL) of thrombin was delayed in both PT and APTT assays, as was expected (Figure 5B APTT and PT panels, compare D1 bars with UT bars). However, when a lower concentration (0.015NIH U/mL) of thrombin (to assess potentially lower potency of the gold nanoparticles) was used, a slight acceleration of plasma coagulation was observed (compare D10 bars with UT bars on Figure 5B).
To assess effects on the complement system, plasma was incubated with gold colloids or water (vehicle control) for 30 min; after centrifugation to remove the gold colloids and any complement proteins bound to the particles, plasma supernatants of each sample were split into 3 parts and tested against PBS (negative control), Taxol (nanoparticle positive control) and CVF (positive control). Gold nanoparticles did not affect complement activation by Taxol and CVF (Figure 6B).
Discussion
Gold colloids are increasingly used for drug delivery, and the role of their surface coating in biodistribution has been previously established (21, 23, 27). However, the composition of the protein corona, and its role in the hematocompatibility of gold colloids has not been fully explored. Therefore, we examined the kinetics of plasma protein binding to the surface of citrate stabilized gold colloids with a nominal size of 30 nm, as well as derivatives bearing PEG coatings of different molecular weights (2 kDa, 5 kDa, 10 kDa and 20 kDa). To avoid variability in the protein repertoire due to storage conditions and inter-donor variability, we have used fresh whole blood drawn from at least 3 healthy donor volunteers, and processed within 1 hour to obtain plasma; the plasma was then pooled and used immediately for each experiment. To avoid donor-specific bias in repeated experiments, pooled plasma from different donors was used for each experiment. Our data demonstrate that the protein composition of the corona is fairly consistent in both inter- and intra-assay variability assessments (Table 2), and is also consistent with our previous study using 2D PAGE to separate and identify nanoparticle bound proteins (7). However, the amount of bound protein and the kinetics of binding varied between experiments (Figures 1 - 3).
The so called “Vroman's effect”, initially reported in 1962 and related to competitive adsorption of plasma proteins to surfaces with different hydrophobicity, was expected to apply to engineered nanomaterials (33). Several research groups have attempted to verify this hypothesis. Vroman's effect was confirmed for polymeric and solid lipid nanoparticles, while studies with oil-in-water emulsions and iron oxide nanoparticles concluded there was no displacement of previously adsorbed proteins (12, 34-36). Results of our data are in agreement with that reported for nanoemulsions and iron oxide particles, i.e. there is no competitive displacement; proteins detected at 5 min are also detected at 24 hr time point (Figure 1). One possible explanation for this difference could be that gold colloids are more similar to iron oxide nanoparticles in terms of lower hydrophobicity than that of solid lipid nanoparticles. Another possible explanation is that the studies suggesting the existence of Vroman's effect for nanomaterials used diluted plasma, which changes the ratio between the nanoparticle and certain plasma proteins. Lastly, these studies all use different incubation periods for protein binding. According to Vroman et al, displacement takes place within the first seconds (33); the shortest incubation time in our study was 5 min.
Another interesting observation from our study is that regardless of the method (2D PAGE followed by mass spectrometry (7) vs. direct processing for MS analysis), and in spite of the fact that we used plasma from different donors for each experiment, the functional categories of proteins detected in the protein corona are consistent (Figure 1, Table 1). It is our understanding that all previous studies used the same plasma or animal serum (individual or pooled) for experiments. Since our data suggest that protein binding is not specific, prediction of toxicities based on the protein corona determined from a single donor or single pool of plasma is less likely to be accurate. It is also worthwhile to note the composition of the protein corona may be affected based on the matrix used in the study. For example, fibrinogen is abundant in plasma but not in serum; absence of this protein in serum will certainly influence the composition of the corona.
Nanoparticle surface modification with PEG is widely recognized as an efficient means of protection from opsonization and subsequent immune recognition (23). Here we studied how nanoparticle surface modification with PEG of various molecular weights influences the kinetics of protein binding over 24 hours and the composition of the protein corona. During the first 5 minutes of incubation there was no clear trend in the total protein binding between unPEGylated gold and its PEGylated counterparts (Figure 4). A likely explanation for this observation is difference in incubation temperature. All plasma samples were handled at room temperature to avoid spontaneous complement activation; incubation on ice was not used as it can alter the plasma proteome by triggering activation of the coagulation cascade. After sample preparation, incubation was performed at 37°C; however, it takes about 5 min for the sample to equilibrate to 37°C. Thus, during the initial 5 min of incubation sample was likely subjected to a range of temperatures from 22°C to 37°C. After 30 and 60 minutes incubation there was a clear difference in total protein binding between the samples. Total protein in the corona generally decreased with increased molecular weight of PEG. At the 6 and 24 hr time points there was no significant difference in total protein binding between gold colloids with 2 kDa, 5 kDa and 10 kDa PEG. At time points between 30 min and 24 hr there was dramatic difference in total bound protein between the unPEGylated gold and gold coated with 20 kDa PEG (Figure 4). This data is in agreement with biological studies showing a dramatic difference in opsonization and biodistribution to MPS organs of animals injected with 30 nm gold colloids with and without 20 kDa PEG (23). Interestingly, in spite of the difference in total protein binding between unPEGylated and PEGylated gold colloids, the composition of protein corona did not change dramatically (Table 3). All proteins detected in the corona of the unPEGylated 30 nm gold colloids were also detected, although at much lower quantity, in the corona of the PEGylated nanoparticles with two exceptions: 1) extracellular matrix proteins were not detected on all PEGylated particles, and 2) immunoglobulins were not detected on gold colloids coated with 20 kDa PEG. This data is consistent with that reported by Gref, et al. who also detected a decrease in the total amount of protein binding but no substantial change in the composition of the protein corona for solid lipid nanoparticles (SLN) (37). Similar to our study, light chain immunoglobulins were not detected in the corona of PEGylated SLN in contrast to their unPEGylated counterparts (37). This phenomenon seems to be common for nanoparticles of different composition and size (our study used small [<100nm] anionic core particles, while Gref et al used large [>200nm] cationic core particles) when coated with large PEG moieties (20 kDa in our study and 45 kDa in the SLN study). We hypothesize the mechanism for immunoglobulin exclusion may be related to steric hindrance. The polymer coating does not cover the entire particle surface, leaving some sites for capture of proteins; however, due to their size and conformation not all proteins can reach the particle surface, which in turn leads to exclusion of these proteins.
To understand whether knowing the protein corona composition could be used to predict hematological toxicity, we performed routine hematology tests focusing on complement and coagulation, since proteins representing these two systems were consistently abundant in the corona of these gold nanoparticles. Fibrinogen binding to gold colloids does not change the function of this protein. Neither induction of plasma coagulation by particles per se, nor disturbance of plasma coagulation induced by known agonists of both extrinsic and intrinsic pathways was detected in plasma pretreated with gold nanoparticles (Figure 5). We also showed that gold colloids do not activate the complement system, and that activation of complement by some known stimuli, such as CVF and Taxol, was unaffected (Figure 6). These data suggest that binding of complement proteins and proteins involved in plasma coagulation to the surface of gold colloids does not lead to the depletion of these proteins to levels which would become insufficient for normal function. Our data is consistent with previous studies utilizing carbon nanotubes and functionalized gold and metal oxide nanoparticles, reporting that binding of the complement protein or fibrinogen per se does not always cause activation of the protein and/or a change in the protein function (14-16, 38-40). Since both complement and fibrinogen are highly abundant proteins in plasma, the particle concentration required to deplete these proteins to a level affecting their function must be very high. Achieving such high concentration in vitro is possible for some but not all nanoparticles. While protein concentration is constant under in vitro conditions, it is dynamic in vivo due to homeostasis. Thus, a particle concentration affecting protein function in vivo presumably should be even higher. This further emphasizes the importance of hematocompatibility tests in establishing nanoparticle safety profiles and suggests that detection and identification of the composition of a nanoparticle's protein corona cannot reliably serve this function.
In summary, we demonstrated that surface properties (i.e. PEGylation) of colloidal gold nanoparticles determine the amount of total bound protein, but only slightly affect the composition of the protein corona. The overall protein corona composition fluctuates with time but is generally consistent between experiments utilizing plasma from different donors. Identification of proteins in the corona cannot be used to substitute relevant biocompatibility tests, as binding per se does not directly correlate to a change in protein function.
Supplementary Material
Acknowledgments
The study was supported in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Footnotes
Conflict of Interest: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Treuel L, Nienhaus GU. Nanoparticle interaction with plasma proteins as it relates to biodistribution. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials 1. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2013. pp. 151–64. [Google Scholar]
- 2.Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl. 2007;46:5754–6. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 3.Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–87. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- 4.Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–53. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- 5.Nagayama S, Ogawara K, Minato K, Fukuoka Y, Takakura Y, Hashida M, et al. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int J Pharm. 2007;329:192–8. doi: 10.1016/j.ijpharm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137:78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5:106–17. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundqvist M, Stigler J, Cedervall T, Berggard T, Flanagan MB, Lynch I, et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5:7503–9. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- 9.Casals E, Puntes VF. Inorganic nanoparticle biomolecular corona: formation, evolution and biological impact. Nanomedicine (Lond) 2012;7:1917–30. doi: 10.2217/nnm.12.169. [DOI] [PubMed] [Google Scholar]
- 10.Gebauer JS, Malissek M, Simon S, Knauer SK, Maskos M, Stauber RH, et al. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir. 2012;28:9673–9. doi: 10.1021/la301104a. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino Y, Nakamoto M, Miura Y. Control of protein-binding kinetics on synthetic polymer nanoparticles by tuning flexibility and inducing conformation changes of polymer chains. J Am Chem Soc. 2012;134:15209–12. doi: 10.1021/ja306053s. [DOI] [PubMed] [Google Scholar]
- 12.Jansch M, Stumpf P, Graf C, Ruhl E, Muller RH. Adsorption kinetics of plasma proteins on ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles. Int J Pharm. 2012;428:125–33. doi: 10.1016/j.ijpharm.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Milani S, Bombelli FB, Pitek AS, Dawson KA, Radler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona. ACS Nano. 2012;6:2532–41. doi: 10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- 14.Deng ZJ, Liang M, Toth I, Monteiro M, Minchin RF. Plasma protein binding of positively and negatively charged polymer-coated gold nanoparticles elicits different biological responses. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.655342. [DOI] [PubMed] [Google Scholar]
- 15.Deng ZJ, Liang M, Toth I, Monteiro MJ, Minchin RF. Molecular interaction of poly(acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano. 2012;6:8962–9. doi: 10.1021/nn3029953. [DOI] [PubMed] [Google Scholar]
- 16.Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20:455101. doi: 10.1088/0957-4484/20/45/455101. [DOI] [PubMed] [Google Scholar]
- 17.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105:14265–70. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–86. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 19.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. J Am Chem Soc. 2010;132:5761–8. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoudi M, Saeedi-Eslami SN, Shokrgozar MA, Azadmanesh K, Hassanlou M, Kalhor HR, et al. Cell “vision”: complementary factor of protein corona in nanotoxicology. Nanoscale. 2012;4:5461–8. doi: 10.1039/c2nr31185b. [DOI] [PubMed] [Google Scholar]
- 21.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79:248–53. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Lu Y. Colorimetric biosensors based on DNAzyme-assembled gold nanoparticles. J Fluoresc. 2004;14:343–54. doi: 10.1023/b:jofl.0000031816.06134.d3. [DOI] [PubMed] [Google Scholar]
- 23.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–83. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 24.Thaxton CS, Georganopoulou DG, Mirkin CA. Gold nanoparticle probes for the detection of nucleic acid targets. Clin Chim Acta. 2006;363:120–6. doi: 10.1016/j.cccn.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Diluzio NR, Zilversmit DB. Influence of exogenous proteins on blood clearance and tissue distribution of colloidal gold. Am J Physiol. 1955;180:563–5. doi: 10.1152/ajplegacy.1955.180.3.563. [DOI] [PubMed] [Google Scholar]
- 26.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine (Lond) 2009;4:401–10. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rygard J. Mechanism of blood clearance of colloidal gold in mice. An atoxic clinical investigation using activation analysis. Acta Radiol Suppl. 1971;308:1–240. [PubMed] [Google Scholar]
- 28.Terentyuk GS, Maslyakova GN, Suleymanova LV, Khlebtsov BN, Kogan BY, Akchurin GG, et al. Circulation and distribution of gold nanoparticles and induced alterations of tissue morphology at intravenous particle delivery. J Biophotonics. 2009;2:292–302. doi: 10.1002/jbio.200910005. [DOI] [PubMed] [Google Scholar]
- 29.Kah JC, Wong KY, Neoh KG, Song JH, Fu JW, Mhaisalkar S, et al. Critical parameters in the pegylation of gold nanoshells for biomedical applications: an in vitro macrophage study. J Drug Target. 2009;17:181–93. doi: 10.1080/10611860802582442. [DOI] [PubMed] [Google Scholar]
- 30.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Mirkin CA, Lytton-Jean AKR, Hurst SJ. USAUS Patent US2010/0099858. inventorsMaximizing Oligonucleotide Loading on Gold Nanoparticle. 2009 Nov 9; publication date.
- 32.Issaq HJ, Chan KC, Blonder J, Ye X, Veenstra TD. Separation, detection and quantitation of peptides by liquid chromatography and capillary electrochromatography. J Chromatogr A. 2009;1216:1825–37. doi: 10.1016/j.chroma.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 33.Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55:156–9. [PubMed] [Google Scholar]
- 34.Blunk T, Luck M, Calvor A, Hochstrasser DF, Sanchez JC, Muller BW, et al. Kinetics of plasma protein adsorption on model particles for controlled drug delivery and drug targetting. Eur J Pharm Biopharm. 1996;42:262–8. [Google Scholar]
- 35.Goppert TM, Muller RH. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Int J Pharm. 2005;302:172–86. doi: 10.1016/j.ijpharm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Harnisch S, Muller RH. Adsorption kinetics of plasma proteins on oil-in-water emulsions for parenteral nutrition. Eur J Pharm Biopharm. 2000;49:41–6. doi: 10.1016/s0939-6411(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 37.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–13. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 38.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 39.Salvador-Morales C, Basiuk EV, Basiuk VA, Green ML, Sim RB. Effects of covalent functionalization on the biocompatibility characteristics of multi-walled carbon nanotubes. J Nanosci Nanotechnol. 2008;8:2347–56. doi: 10.1166/jnn.2008.090. [DOI] [PubMed] [Google Scholar]
- 40.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


