Abstract
Background
Prior evidence indicates that predictors of weight loss outcomes after gastric bypass surgery fall within 5 domains: 1) presurgical factors; 2) postsurgical psychosocial variables (e.g., support group attendance); 3) postsurgical eating patterns; 4) postsurgical physical activity; and 5) follow-up at postsurgical clinic. However, little data exist on which specific behavioral predictors are most associated with successful outcomes (e.g., ≥50% excess weight loss) when considering the 5 domains simultaneously.
Objectives
Specify the behavioral variables, and their respective cutoff points, most associated with successful weight loss outcomes.
Setting
On-line survey.
Methods
Signal Detection Analysis evaluated associations between 84 pre-and postsurgical behavioral variables (within the 5 domains) and successful weight loss at ≥1 year in 274 post-gastric bypass surgery patients.
Results
Successful weight loss was highest (92.6%) among those reporting dietary adherence of >3 on a 9 point scale (median=5) who grazed no more than once-per-day. Among participants reporting dietary adherence <3 and grazing daily or less, success rates more than doubled when highest lifetime Body Mass Index was <53.7 kg/m2. Success rates also doubled for participants with dietary adherence =3 if attending support groups. No variables from the physical activity or postsurgical follow-up domains were significant, nor were years since surgery. The overall model’s sensitivity =.62, specificity =.92.
Conclusions
To our knowledge, this is the first study to simultaneously consider the relative contribution of behavioral variables within 5 domains and offer clinicians an assessment algorithm identifying cut-off points for behaviors most associated with successful postsurgical weight loss. Such data may inform prospective study designs and postsurgical interventions.
Keywords: bariatric surgery, postoperative eating behaviors, weight loss outcome, signal detection analysis
INTRODUCTION
Obesity is a global public health concern,(1) with bariatric surgery being the most effective treatment.(1,2) While the majority of bariatric patients experience successful postsurgical weight loss outcomes (commonly defined as ≥50% excess weight loss, (3–5) or %EWL) for the first 1–2 years post surgery, a significant minority (up to 30%) may experience unsuccessful postsurgical weight loss or show gradual weight regain along with the return of associated medical comorbidities.(6,7) Predicting which patients will lose ≥50% EWL is a challenge, with few identified modifiable risk factors. Available data suggest that certain pre- and postsurgical variables are associated with postsurgical weight loss success. These can be grouped into 5 domains including 1) presurgical variables;(8–14) 2) postsurgical psychosocial (e.g., support group attendance) variables;(12, 15, 16) 3) postsurgical adherence to recommendations regarding intake and eating behavior;(4, 8, 11, 17–22) 4) postsurgical adherence to recommendations for physical activity;(23–25) and 5) postsurgical adherence to surgery clinic follow-up appointments.(26,27)
Examples of presurgical variables positively associated with weight loss include female gender, Caucasian race, higher socio- economic status, lower baseline Body Mass Index (BMI),(8,9) absence of preoperative disordered eating behaviors (e.g., binge eating, emotional eating, loss of control (LOC),(10,11) lower levels of psychopathology(12) and compliance with presurgical guidelines.(13) In a recent meta-analysis of 115 studies, 3 of 16 presurgical variables were found to have evidentiary support as predictors of less successful outcomes: higher presurgical BMI (particularly super-obesity, BMI >50 kg/m2), lack of achievement of mandatory presurgical weight loss (i.e., the requirement that patients lose a certain percentage of excess weight (most commonly greater than 10% EWL) over the weeks immediately preceding surgery), and certain personality traits (e.g., lower self-directedness, higher grievance).(14)
Increasingly, surgical outcome is being connected with postsurgical behaviors. For example, postsurgical psychosocial variables associated with less successful postoperative weight outcome include reported decreased well-being, addictive behaviors,(15) general (non-eating related) psychopathology (e.g., depression),(12) and lower bariatric support group meeting attendance.(16)
Another postsurgical domain associated with weight loss outcome includes adherence to the surgical team’s recommendations for food intake and for eating behaviors. For example, Sarwer et al.(8) found that patients’ self-reported ratings of their overall adherence to nutritional guidelines at 20 weeks after surgery predicted weight outcome at 92 weeks. In addition, numerous studies have linked specific postsurgical disordered eating behaviors (e.g., binge eating, grazing, a subjective sense of LOC overeating, emotional eating) to less favorable postsurgical weight outcomes.(4,11, 17–22)
Postsurgical physical activity has, in some studies, also been predictive of postsurgical weight loss outcome. Although increased levels of physical activity have been significantly associated with the degree of weight loss,(23,24) one intervention study targeting improvement of postsurgical physical activity did not demonstrate significant effects upon weight loss.(25) Finally, though many patients fail to attend their postsurgical follow-up appointments,(26) adherence to recommendations for attendance at postsurgical follow-up appointments has been shown to be a significant predictor of weight loss outcome.(27)
Notably, several gaps in the literature remain. To begin, studies identifying behaviors associated with postsurgical weight loss outcomes rarely include quantitative descriptions for at-risk behaviors, such specifying the frequency at which a maladaptive eating behavior such as grazing (often defined as nibbling, snacking, or eating small amounts of food in an unplanned and repetitious way, although consensus has yet to be reached (e.g., 4, 18, 19, 22) on a standard definition) is likely to be problematic. In addition, studies often focus on only 1 of the 5 domains, such as the impact of adherence to a physical activity program on postsurgical weight loss. Hence, research is not yet able to offer clinicians an assessment algorithm for better identifying which patient variables within the 5 domains, when considered simultaneously, are most important for obtaining postsurgical weight outcome success.
Signal Detection Analysis (SDA) is used in medical decision making to evaluate the performance of diagnostic tests(28) or to identify characteristics of subgroups at risk for disease or other binary health outcomes. (28,29) The signal is the binary health outcome (e.g., successful postsurgical weight loss outcome), and the detection is the set of predictor variables. Signal detection employs recursive partitioning, an empirically driven iterative nonparametric process, to produce a series of “and/or” (Boolean) rules, based on a priori identified predictor variables, which specify subgroups of individuals who are more or less likely to have a particular binary health outcome according to a selected criterion. (28) For instance, SDA can identify the combination of demographic characteristics of distinct subgroups of individuals who are more or less likely to have successful postsurgical weight outcome. SDA uses an iterative forward procedure to specify successive cut-off points for each a priori identified predictor variable entered, incorporating specific stopping rules such as p<.001 and/or a sample size too small to further divide. Thus, a specific and optimal cut-off point for each predictor variable that significantly partitions the sample is identified. While it is important to stress that this exploratory analytical procedure does not test a hypothesis and no causal relationships can be concluded, the decision tree output provided by the SDA may form the basis of hypotheses that can later be examined empirically. The aims of the present study were to employ SDA to 1) identify which pre- and postsurgical variables, within the 5 domains and when considered together, significantly predict postsurgical weight loss outcome success; and 2) specify the optimal level at which a pre- and/or postsurgical variable distinguishes likelihood of weight outcome success (e.g., cutoff points).
METHODS
Data Collection
The study’s sample was gathered from the general membership of an online bariatric support website which sought out our team for assistance with refining a previously administered survey. The survey had been emailed to all website members to assess whether and/or how much they complied with website provided postsurgical recommendations (e.g., weighing oneself daily, drink at least 64 ounces fluid daily, etc.). The revised 100 item self-report survey was derived from several sources of information and has not yet been validated. Survey item included questions from the original survey, modified questions from the Weight and Lifestyle Inventory(30) (a comprehensive, standardized self-report instrument assessing weight and dieting histories, eating and activity habits, and social/psychological status), and items previously found to be predictive of weight loss outcome (such as that of Sarwer et al.(8) regarding overall dietary adherence, which asks “How well are you following the diet plan given to you by the dietician/nutritionist/surgeon?” with anchors 1=not very well, 5=moderately well, and 9=very well; see Table 1). Criteria regarding anxiety disorders, depressive disorders, and eating disorders were pulled from the DSM-IV-TR (31) in order to capture important psychological variables that might impact long-term success.
Table 1.
Description of survey domains and representative survey items
| Domain | Description | Response Format | Number of items |
|---|---|---|---|
|
| |||
| Presurgical factors | Demographic information; eating habits, presurgical BMI, presurgical weight, highest lifetime BMI, time since surgery | 18 | |
|
| |||
| Postsurgical intake and eating behaviors | Frequency of eating behaviors (e.g. grazing), information about over-eating episodes and digressions from planned meals. For example: | 44 | |
| (1) During the past 3 months, there have been times when I felt I had eaten what other people would regard as an unusually large amount of food given the circumstances? | Yes – No | ||
| (2) During the past 3 months when I ate an unusually large amount of food, I simultaneously experienced a loss of control (e.g., felt I couldn’t stop eating or control what or how much I was eating)? | Yes – No | ||
| (3) How often do you graze (defined as nibbling, snacking, or eating small amounts of food in an unplanned and repetitious way) over an extended period of time? | Never(1) – More than once per day(7) | ||
|
| |||
| Postsurgical physical activity | Frequency and intensity of exercise For example: |
8 | |
| (1) I do purposeful vigorous intensity cardio-aerobic physical activity an average of ___ times per week for an average of ____ minutes each time. | 0–7 days 0–120 minutes for each time |
||
|
| |||
| Attendance at surgical follow-up appointments | Regularity of attendance, timing of discontinuation For example: |
2 | |
| (1) Have you regularly attended all of your recommended Bariatric Surgery Clinic Follow-up appointments (i.e., routine post-surgical appointments with your surgeon/surgical team)? | Yes – No | ||
| (2) I stopped regularly attending my post-surgical follow-up appointments at ____ (weeks/months/years) post-surgery. | 2weeks–5+years | ||
|
| |||
| Postsurgical psychosocial factors | Behaviors (e.g. alcohol consumption, support group attendance) and emotional state post-surgery (e.g. depression symptoms). For example: | 12 | |
| (1) Over the past two weeks, how often have you been bothered by having little interest or pleasure in doing things? | Not at all(1) – Nearly every day(4) | ||
| (2) Which of the following responses best describes your attendance at SUPPORT GROUP meetings for patients who have had bariatric surgery? | Regularly(1), Sometimes/irregularly(2), Rarely or never(3), Actively participate online(4), Regularly attend in-person & active online(5) | ||
The survey was emailed to paying website members, made available to non-member website visitors, and forwarded by professional provider website members (e.g., surgeons, bariatric support group leaders) to their patients. The Stanford University Medical Center Institutional Review Board deemed the study exempt due to its de-identified external dataset analysis.
Target variables
Postsurgical weight loss outcome success, commonly defined as attaining at least 50% EWL (see Livhits et al., 2010;(3) Kofman et al.,(4) Snyder et al.(5)) was the primary outcome. The following formula was used to calculate % EBWL = [(pre-operative BMI – follow-up BMI)/(pre-operative BMI – Ideal Body Weight (IBW))] × 100. Ideal body weight was based on a BMI of 25 kg/m2. Eighty-four variables derived from the self-report survey were hypothesized to be related to %EWL. These were categorized into the 5 (1 presurgical and 4 postsurgical) domains. As described in more detail in Table 1, they included: 18 presurgical variables, 12 variables assessing psychosocial factors, 44 variables assessing a wide array of current intake and eating behaviors, 8 physical activity variables, and 2 variables assessing attendance at surgical follow-up appointments. Responses relating to frequency ranged from 1 (never) to 7 (more than once/day) and answers indicating degree utilized Likert scales ranging from 1 (not at all) to 9 (very). Please see Table 1 for additional information regarding the format of responses. Though validation of the survey was not the aim of this study, Cronbach’s alphas were calculated on all of the continuous variables within each domain and were as follows; .846; .808; and .634 for Postsurgical intake and eating behaviors; Postsurgical physical activity; and Postsurgical psychosocial factors, respectively.
Statistical Analysis
SDA was used to assess the strength of association between the a priori identified 84 variables and postsurgical weight loss outcome success. Recursive partitioning based on the QROC program (available at http://www.stanford.edu/~yesavage/ROC.html) specifies the optimal cut-off point for each predictor variable entered based on weighted kappa values until stopping rules apply including p <.001 and/or sample size n<10.
RESULTS
Data Selection
Of the 539 participants who originally began the survey, 48 who did not report surgery type were excluded. Of the 491 remaining, 72% (n=353) reported gastric bypass, 15% (n=74) lap band, 9% (n=44) gastric sleeve, and 4% (n=20) other (e.g., revision surgery) as their surgery type. In order to maintain as homogenous a sample as possible, only gastric bypass patients were included. Thirty-two were excluded due to having had surgery less than 1 year prior (leaving insufficient time to achieve postsurgical outcome success) or more than 12 years prior (given their low frequency (n=15 patients spanning years 13–23) and erratic nature lowered the reliability of statistical inferences for this group). Of the 321 remaining, 47 were excluded because of incomplete survey responses, which prevented calculation of outcome data, leaving a final sample size of 274.
Demographics
Overall, 79.10% (n=214) of the sample met criteria for postsurgical weight loss outcome success and 21.90% (n=60) for failure. The sample (N=274) was 96% female and 89% white, with a mean age of 51.14±8.39 years and average number of years since surgery of 5.8±3.12. Mean presurgical BMI was 47.41±8.39, highest lifetime BMI prior to surgery was 49.35±9.52, current BMI was 32.55±7.95, and mean %EWL was 72.20%±39.01%. The majority of the sample self-identified as white (89%) (4% of whom were Hispanic), followed by black (6%), Native American or Alaskan Native (1%), other (2%), or of mixed race (3%). At the time of surgery, participants indicated that they were married (65%), single (20%), divorced (8%), partnered/engaged (6%), or widowed (1%). Descriptive statistics for the 84 a priori identified variables are available upon request.
Signal Detection Analysis (SDA)
Table 2 displays the sensitivity, specificity, predictive value positive (PVP), predictive value negative (PVN), and efficiency used to evaluate the SDA’s overall model classification accuracy. Kraemer classifies >.8=almost perfect, .6–.8= substantial, .4–.6=moderate, .2–.4=fair, and < .2 = slight or poor.(28) Tests of sensitivity best identify participants with the desired outcome (true positives), while tests of specificity best identify participants without the desired outcome (true negatives). Efficiency tests balance the two extremes of true positives and negatives. The overall model yielded a sensitivity =.62, a specificity=.92, and an efficiency=.84. A PVP=.72 represents the proportion of respondents correctly classified as being surgical outcome failures. A PVN=.88 represents the proportion of respondents correctly classified as not being surgical outcome failures. The SDA, by definition, is an exploratory, hypothesis generating approach to data analysis and thus does not require control for Type 1 error.
Table 2.
Specific SDA Selected Variables with Efficiency Results
| Run # | Variable identified by SDA | Description | SDA Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Cut Point | PVP | PVP n | PVN | PVN n | Kappa | χ2 | ||
| 1 | Adherence to dietary recommendations | 4.50 (2.51) | 5 | 1–9 | >3 | .49 | 70 | .87 | 204 | .38 | 39.11*** |
| 2 | Grazing frequency | 4.83 (1.65) | 5 | 1–7 | <7 | .72 | 29 | .68 | 41 | .40 | 11.27*** |
| 3 | Adherence to dietary recommendations | 4.50 (2.51) | 5 | 1–9 | ≥4 | .32 | 31 | .91 | 173 | .25 | 12.52*** |
| 5 | Highest lifetime presurgical BMI | 47.90 (9.52) | 47.90 | 21.30–91.98 | <53.7 | .64 | 14 | .85 | 27 | .50 | 10.42** |
| 6 | Attendance at support groups | 0 | Yes/no | ≥1 | .56 | 15 | .93 | 15 | .49 | 8.71** | |
| 7 | Grazing frequency | 4.83 (1.65) | 5 | 1–7 | <7 | .36 | 11 | .93 | 162 | .24 | 10.29** |
Note: PVP=predictive value positive; PVN=predictive
SDA Output
The SDA decision tree output delineates graphically how the selected predictors were combined to identify a participant’s relative chance for postsurgical weight loss outcome success (see Figure 1). At the first level, dietary adherence was selected as the optimal variable for all respondents (N=274; Level 1, Figure 1) with a cut-point of 3. It divided the full sample into two sub-samples. Surgical success among those with “low moderate” or higher dietary adherence (i.e., ≥3 of 9) was 87.3% compared to 41.6% among those who endorsed <3. Among those with dietary adherence ≥3 (left hand side of Level 2, Figure 1), the next optimal variable to divide this subgroup was again dietary adherence with a score of ≥4. Specifically, those who rated their adherence as “nearly moderate” or higher (i.e., ≥4) had a 91.8% weight loss outcome success rate compared to 67.7% among those who endorsed <4 (or exactly =3). The group endorsing “nearly moderate” or higher ratings for dietary adherence (≥4) was further modified by the frequency of grazing (Level 3, Figure 1). Specifically, postsurgical success was 92.6% among those who did not graze more than once per day. Conversely, those who endorsed grazing more than once per day had a postsurgical success rate of 63.6%. Also on Level 3, the group reporting a dietary adherence of exactly =3, or “low moderate,” was further modified by the frequency of attendance at postsurgical support group meetings. Postsurgical weight loss outcome success dropped from 92.3% among those who endorsed regularly attending either in-person or online to 40% among non-regular attenders.
Figure 1.
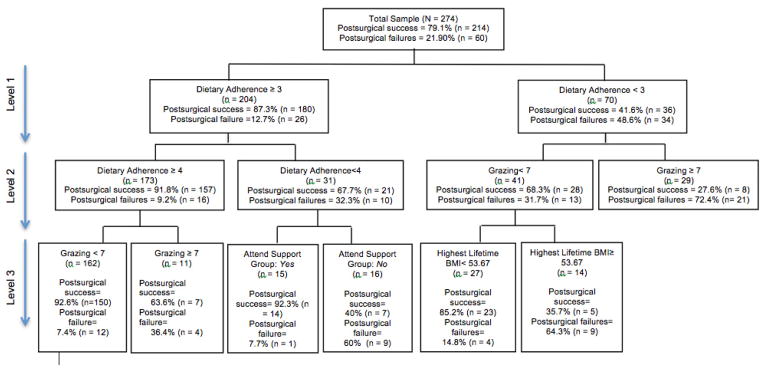
Signal Detection Analysis Decision Tree Output
Returning to the subgroup whose dietary adherence was <3 (right hand side of Level 1, Figure 1), the frequency of grazing subdivided this group. Specifically, those who endorsed grazing no more than once per day had a postsurgical success rate of 68.3% compared to 27.6% among those who grazed more than once per day (Level 2, Figure 1). Highest lifetime BMI during years prior to surgery subdivided the less frequent grazing group (Level 3, Figure 1). Here, postsurgical weight loss outcome success dropped from 85.2% among those with a highest lifetime BMI <53.67 to 35.7% among those with ≥53.67.
The SDA identified 4 core variables with 6 total cut-points as having robust associations with the primary outcome of interest, postsurgical weight loss outcome success (defined as ≥50% EWL): 1) Dietary adherence: greater to or equal to 3 (Level 1) and greater to or equal to 4 (Level 2); 2) Grazing: daily or less (Levels 2 and 3); 3) Highest lifetime BMI prior to surgery: <53.7 kg/m2 (Level 3); 4) Regular attendance at in-person or online support group: yes (Level 3). No variables from the physical activity or surgical follow-up domains were identified by the SDA, nor were number of years since surgery.
DISCUSSION
Bariatric surgery is currently considered the most successful treatment for refractory morbid obesity.(1,2) The present study employed signal detection analysis (SDA), a nonparametric technique used to identify characteristics of subgroups at risk for a clinically relevant binary outcome such as a successful versus unsuccessful outcome after bariatric surgery. Unlike linear models, the SDA does not rely on assumptions of a normal distribution and instead, iteratively tests hypothesized variables until either a stringent minimum significance level (p< .001) is identified and/or a minimum sample size (n<10) is reached. Although this type of analysis does not test a hypothesis and no causal relationships can be concluded, it does allow multiple predictors to be evaluated simultaneously and independently.(28)
Four core variables representing 3 of these 5 domains, along with their respective cut-off points, were identified by the SDA as increasing the likelihood of postsurgical success (defined as at least 50% excess weight loss, or % EWL). These variables were: a postsurgical global dietary adherence rating of at least “low moderate” (i.e., ≥3 on a 9 point scale following Sarwer et al.);(8) postsurgical grazing frequency (of no more than once per day); highest lifetime BMI prior to surgery (≤53.7 kg/m2); and regular attendance at postsurgical bariatric support groups. Taken together, the overall model yielded a modest to high level of sensitivity, specificity, and efficiency (.62, .92, .84, respectively).
Domains relating to physical activity or postsurgical follow-up appointment attendance were not identified, implying that their impact on outcome is relatively less. While other studies have found positive associations between postsurgical success and variables within the physical activity and postsurgical follow-up domains,(23, 24) our study is the only one to our knowledge to consider the relative contribution of factors within these domains alongside factors within the other 3 domains. Of note, while years since surgery has been associated with gradual decreases in %EWL,(32) this variable was not identified as a significant predictor of a successful postsurgical outcome.
The SDA’s identification of postsurgical global dietary adherence and grazing as core variables associated with postsurgical success support the increasing recognition that variability in surgical weight loss outcomes is largely a result of patient behavior, particularly adherence to dietary recommendations regarding food intake and eating behaviors.(4, 8, 9) To our knowledge, this study is the only one to also offer cut-off points for these modifiable risk factors. Our results support prior research linking attendance at postsurgical support group meetings with surgical outcome.(16) For example, participants who reported regular attendance at postsurgical bariatric support group meetings (see Table 1 for survey question format) had more than double the likelihood of a successful postsurgical weight outcome (92% compared to 40%) within the subdivision of participants reporting “low moderate” (i.e., a 3 out of 9) adherence to the postsurgical dietary recommendations. A clinical implication is the importance of assessing a patient’s level of dietary adherence. Especially for those who report “low moderate” adherence, regular attendance at a support group (either in-person or online) would be highly encouraged. Though the specific beneficial components of these support groups as well as optimal attendance frequency remain to be clarified, it is possible that patients with “low moderate” adherence particularly benefit from the social support, accountability, and sharing of informational “tips” that promote adherence (i.e., which protein shakes taste best).
Previous data have similarly linked the presence of presurgical super-obesity (BMI > 50 kg/m2) to worsened postsurgical outcomes.(14) In this study, the absence of a BMI ≥ 53.7 kg/m2 at any lifetime point prior to surgery improved chances of surgical success among a particular subgroup of participants with lower dietary adherence (< 3). Interestingly, nearly all research efforts to date have focused on BMI immediately preceding surgery and not the highest lifetime BMI ever. However our data suggest that it may be prudent to inquire about both.
Through the specification of the strongest risk factor variables and their corresponding degree of severity (e.g., cut-off points), our findings have implications for a better understanding of both postsurgical success and failure. Postsurgical outcome failure is important to consider because of the significant minority of patients (20–30%) who do not lose expected amounts of weight and/or subsequently regain weight previously lost. In providing a decision tree, the SDA offers clues to how and when these risk factors relate to each other, thus providing information that is clinically relevant and useful. For example, asking postsurgical patients to rate their level of global adherence to postsurgical dietary recommendations and the frequency of grazing allows identification of the level of patient risk and the need for intervention on these modifiable behaviors. The provider, based on a patient’s responses, might recommend additional dietary assistance, make a referral for behavior therapy, and/or strongly recommend attendance at bariatric support groups.
Though it is premature to presume that intervention upon risk factors identified within this study would result in greater overall postsurgical success rates, the current findings provide a valuable starting point for further study. However, limitations of the current study must be noted. 1) Data analyzed were cross-sectional, limiting the ability to make predictive prospective inferences. Similarly, replication of these results with prospective data is important. 2) The sample was selective, given recruitment through membership in an online bariatric support website, and thus was not entirely representative of the postsurgical population (although 21% were deemed postsurgical failures, a rate consistent with estimates in the current literature). 3) The survey has not yet been validated and thus cannot be used as a definitive guide, particularly for the Postsurgical psychosocial factors domain which demonstrated slightly lower reliability.4) Data (i.e., current BMI, physical activity) were self-reported and not directly observed. 5) Neither biological or anatomical variables that may be associated with weight regain were assessed, nor was potentially useful information regarding pertinent demographic variables (such accessibility to fitness center/gym, a walking city versus more rural area, accessibility of food). 6) Moreover, we used a definition of grazing (nibbling, snacking, or eating small amounts of food in an unplanned and repetitious way over an extended period of time) yet realize that a firm consensus on the definition of grazing has not yet been formed in the field. Reaching consensus on a standardized definition will allow for clarity, consistency, and cross-study comparisons in future works.
As noted, an analytic strength of the study is the use of SDA. While exploratory, SDA offers advantages over traditional linear methods by providing information about hierarchical relationships among variables. It offers a level of granularity regarding which patients have the highest likelihood of postsurgical weight loss success.
Future research is encouraged to employ prospective study designs to longitudinally track the identified risk factors and their respective cut-off points to: 1) verify their impact upon the likelihood of postsurgical success and failure; 2) substantiate critical time points wherein the risk factors first attain cut-off levels; and 3) specify potentially tractable precursors to the risk factors. Moreover, additional investigation into both the delineation of the specific behavioral components of global self-ratings of dietary adherence and how the presence and frequency of grazing impacts postsurgical weight loss outcome will be valuable. Ongoing research efforts such as these are necessary to ultimately derive a comprehensive yet specific understanding of postsurgical outcome success and failure, and provide tailored yet standardized interventions accordingly.
Acknowledgments
This research was funded in part by a grant from the National Institute of Mental Health K23MH085732 awarded to Athena Robinson, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Athena H. Robinson, Email: athenar@stanford.edu.
Sarah Adler, Email: sadler1@stanford.edu.
Helen B. Stevens, Email: helen.stevens@stanford.edu.
Alison M. Darcy, Email: adarcy@stanford.edu.
John M. Morton, Email: morton@stanford.edu.
Debra L. Safer, Email: dlsafer@stanford.edu.
References
- 1.World Health Organization. Obesity and overweight. 2013 August 27, 2013 Available from: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: Surgical treatment of obesity. Ann Intern Med. 2005;142:547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 3.Livhits M, Mercado C, Yermilov I, et al. Behavioral factors associated with successful weight loss after gastric bypass. Am Surg. 2010;76:1139–42. [PubMed] [Google Scholar]
- 4.Kofman MD, Lent MR, Swencionis C. Maladaptive eating patterns, quality of life, and weight outcomes following gastric bypass: results of an Internet survey. Obesity (Silver Spring) 2010;18:1938–43. doi: 10.1038/oby.2010.27. [DOI] [PubMed] [Google Scholar]
- 5.Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc. 2009;23:2302–6. doi: 10.1007/s00464-008-0322-1. [DOI] [PubMed] [Google Scholar]
- 6.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31:1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 8.Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:4640–6. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toussi R, Fujioka K, Coleman KJ. Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obesity. 2009;17:996–1002. doi: 10.1038/oby.2008.628. [DOI] [PubMed] [Google Scholar]
- 10.Crowley N, Budak A, Karl Byrne T, Thomas S. Patients who endorse more binge eating triggers before gastric bypass lose less weight at 6 months. Surg Obes Relat Dis. 2011;7:55–9. doi: 10.1016/j.soard.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Niego SH, Kofman MD, Weiss JJ, Geliebter A. Binge eating in the bariatric surgery population: A review of the literature. Int J Eat Disord. 2007;40:349–59. doi: 10.1002/eat.20376. [DOI] [PubMed] [Google Scholar]
- 12.de Zwaan M, Enderle J, Wagner S, et al. Anxiety and depression in bariatric surgery patients: A prospective, follow-up study using structured clinical interviews. J Affect Disord. 2011;133:61–8. doi: 10.1016/j.jad.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 13.El Chaar M, McDeavitt K, Richardson S, Gersin KS, Kuwada TS, Stefanidis D. Does patient compliance with preoperative bariatric office visits affect postoperative excess weight loss? Surg Obes Relat Dis. 2011;7:743–8. doi: 10.1016/j.soard.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Livhits M, Mercado C, Yermilov I, Parikh J, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: Systematic review. Obes Surg. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 15.Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser KA, Franks SF, Smith AB. Positive relationship between support group attendance and one-year postoperative weight loss in gastric banding patients. Surg Obes Relat Dis. 2011;7:89–93. doi: 10.1016/j.soard.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 17.White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of contol over eating predicts outcomes in bariatric surgery patients: A prospective, 24-month follow-up study. J Clin Psychiatry. 2010;71:175–84. doi: 10.4088/JCP.08m04328blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colles SL, Dixon JB, O’Brien PE. Grazing and loss of control related to eating: Two high-risk factors following bariatric surgery. Obesity. 2008;16:615–22. doi: 10.1038/oby.2007.101. [DOI] [PubMed] [Google Scholar]
- 19.de Zwaan M, Hilbert A, Swan-Kremeier L, et al. Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2010;6:79–85. doi: 10.1016/j.soard.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Canetti L, Berry EM, Elizur Y. Psychosocial predictors of weight loss and psychological adjustment following bariatric surgery and a weight loss program: The mediating role of emotional eating. Int J Eat Disord. 2009;42:109–17. doi: 10.1002/eat.20592. [DOI] [PubMed] [Google Scholar]
- 21.Chesler BE. Emotional eating: A virtually untreated risk factor for outcome following bariatric surgery. ScientificWorldJournal. 2012;2012:365961. doi: 10.1100/2012/365961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders R. “Grazing”: A high-risk behavior. Obes Surg. 2004;14:98–102. doi: 10.1381/096089204772787374. [DOI] [PubMed] [Google Scholar]
- 23.Egberts K, Brown W, Brennan L, O’Brien P. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg. 2011;22:335–41. doi: 10.1007/s11695-011-0544-5. [DOI] [PubMed] [Google Scholar]
- 24.Welch G, Wesolowski C, Zagarins S, et al. Evaluation of clinical outcomes for gastric bypass surgery: Results from a comprehensive follow-up study. Obes Surg. 2011;21:18–28. doi: 10.1007/s11695-009-0069-3. [DOI] [PubMed] [Google Scholar]
- 25.Shah M, Snell PG, Rao S, et al. High-volume exercise program in obese bariatric surgery patients: A randomized, controlled trial. Obesity. 2011;19:1826–34. doi: 10.1038/oby.2011.172. [DOI] [PubMed] [Google Scholar]
- 26.Moroshko I, Brennan L, O’Brien P. Predictors of attrition in bariatric aftercare: A systematic review of the literature. Obes Surg. 2012;22:1640–7. doi: 10.1007/s11695-012-0691-3. [DOI] [PubMed] [Google Scholar]
- 27.Compher C, Hanlon A, Kang Y, Elkin L, Williams N. Attendance at clinical visits predicts weight loss after gastric bypass surgery. Obes Surg. 2012;22:927–34. doi: 10.1007/s11695-011-0577-9. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer HC. Objective and Quantitative Guidelines. California: Sage Publications; 1992. [Google Scholar]
- 29.Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods. 2001;6:35–48. doi: 10.1037/1082-989x.6.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Foster GD. Weight and Lifestyle Inventory (WALI) Surg Obes Relat Dis. 2006;2:180–99. doi: 10.1016/j.soard.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 32.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–24. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


